Susceptibility to Predation Varies with Body Mass, Foraging Niche, and Anti-Predator Responses among Bird Species
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
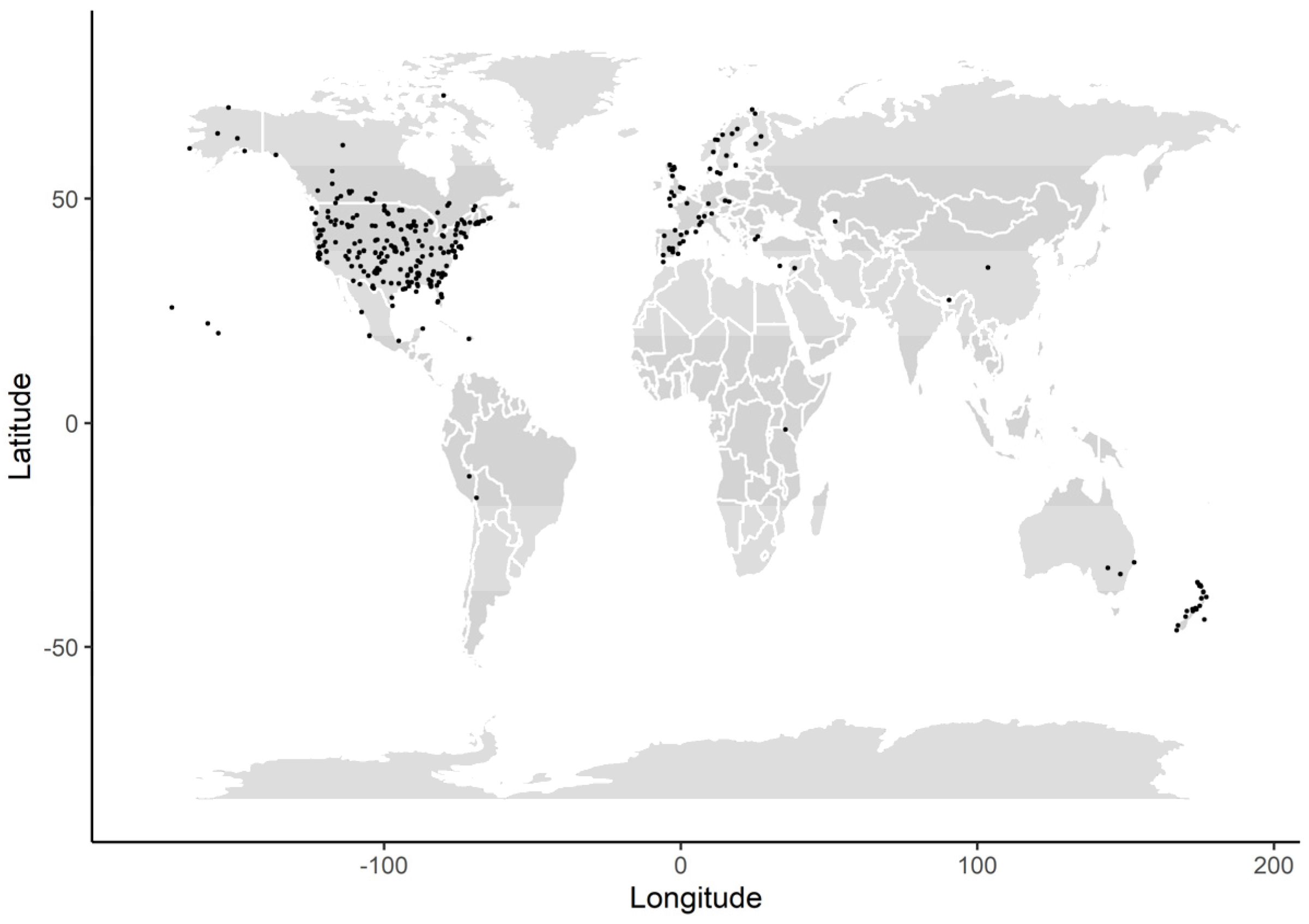
2.1. Data Collection
2.2. Data Analysis
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, J.E.; DeVault, T.L.; Belant, J.L. Cause-specific mortality of the world’s terrestrial vertebrates. Glob. Ecol. Biogeogr. 2019, 28, 680–689. [Google Scholar] [CrossRef]
- Sinclair, A.R.E.; Mduma, S.A.R.; Brashares, J.S. Patterns of predation in a diverse predator–prey system. Nature 2003, 425, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.L.; Dill, L.M. Behavioral decisions made under the risk of predation: A review and prospectus. Can. J. Zool. 1990, 68, 619–640. [Google Scholar] [CrossRef]
- Creel, S.; Christianson, D. Relationships between direct predation and risk effects. Trends Ecol. Evol. 2008, 23, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Reznick, D.A.; Bryga, H.; Endler, J.A. Experimentally induced life-history evolution in a natural population. Nature 1990, 346, 357–359. [Google Scholar] [CrossRef]
- Bliard, L.; Paquet, M.; Robert, A.; Dufour, P.; Renoult, J.P.; Grégoire, A.; Crochet, P.-A.; Covas, R.; Doutrelant, C. Examining the link between relaxed predation and bird coloration on islands. Biol. Lett. 2020, 16, 20200002. [Google Scholar] [CrossRef]
- Chapa-Vargas, L.; Ceballos, G.; Tinajero, R.; Torres-Romero, E.J. Latitudinal effects of anthropogenic factors driving raptor species richness across the American continent. J. Biogeogr. 2019, 46, 1948–1958. [Google Scholar] [CrossRef]
- Shattuck, M.R.; Williams, S.A. Arboreality has allowed for the evolution of increased longevity in mammals. Proc. Natl. Acad. Sci. USA 2010, 107, 4635–4639. [Google Scholar] [CrossRef]
- Healy, K.; Guillerme, T.; Finlay, S.; Kane, A.; Kelly, S.B.A.; McClean, D.; Kelly, D.J.; Donohue, I.; Jackson, A.L.; Cooper, N. Ecology and mode-of-life explain lifespan variation in birds and mammals. Proc. R. Soc. Lond B Biol. Sci. 2014, 281, 20140298. [Google Scholar] [CrossRef]
- Magnhagen, C. Predation risk as a cost of reproduction. Trends Ecol. Evol. 1991, 6, 183–186. [Google Scholar] [CrossRef]
- Lima, S.L. Predators and the breeding bird: Behavioral and reproductive flexibility under the risk of predation. Biol. Rev. 2009, 84, 485–513. [Google Scholar] [CrossRef]
- Cheney, D.L.; Wrangham, R.W. Predation. In Primate societies; Smuts, B.B., Cheney, D.L., Seyfarth, R.M., Wrangham, R.W., Eds.; University of Chicago Press: Chicago, IL, USA, 1987; pp. 227–239. [Google Scholar]
- Clark, C.W.; Mangel, M. The evolutionary advantages of group foraging. Theor. Popul. Biol. 1986, 30, 45–75. [Google Scholar] [CrossRef]
- Ioannou, C.C.; Bartumeus, F.; Krause, J.; Ruxton, G.D. Unified effects of aggregation reveal larger prey groups take longer to find. Proc. R. Soc. Lond B Biol. Sci. 2011, 278, 2985–2990. [Google Scholar] [CrossRef] [PubMed]
- Isbell, L.A. Predation on primates: Ecological patterns and evolutionary consequences. Evol. Anthropol. 1994, 3, 61–71. [Google Scholar] [CrossRef]
- Møller, A.P.; Nielsen, J.T. Malaria and risk of predation: A comparative study of birds. Ecology 2007, 88, 871–881. [Google Scholar] [CrossRef]
- Millspaugh, J.J.; Marzluff, J.M. Radio-tracking and animal populations: Past trends and future needs. In Radio Tracking and Animal Populations; Millspaugh, J.J., Marzluff, J.M., Eds.; Academic Press: Cambridge, MA, USA, 2001; pp. 383–393. [Google Scholar]
- Díaz, M.; Møller, A.P.; Flensted-Jensen, E.; Grim, T.; Ibáñez-Álamo, J.D.; Jokimäki, J.; Markó, G.; Tryjanowski, P. The geography of fear: A latitudinal gradient in anti-predator escape distances of birds across Europe. PLoS ONE 2013, 8, e64634. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.E. Avian life history evolution in relation to nest sites, nest predation, and food. Ecology 1995, 65, 101–127. [Google Scholar] [CrossRef]
- Rolland, C.; Danchin, E.; de Fraipont, M. The evolution of coloniality in birds in relation to food, habitat, predation, and life-history traits: A comparative analysis. Am. Nat. 1998, 151, 514–529. [Google Scholar] [CrossRef]
- Beauchamp, G. Group-foraging is not associated with longevity in North American birds. Biol. Lett. 2010, 6, 42–44. [Google Scholar] [CrossRef]
- Bird, J.P.; Martin, R.; Akçakaya, H.R.; Gilroy, J.; Burfield, I.J.; Garnett, S.T.; Symes, A.; Taylor, J.; Şekercioğlu, Ç.H.; Butchart, S.H.M. Generation lengths of the world’s birds and their implications for extinction risk. Conserv. Biol. 2020, 34, 1252–1261. [Google Scholar] [CrossRef]
- Götmark, F.; Post, P. Prey selection by sparrowhawks, Accipiter nisus: Relative predation risk for breeding passerine birds in relation to their size, ecology and behaviour. Phil. Trans. R. Soc. Lond. B Biol. Sci. 1996, 351, 1559–1577. [Google Scholar]
- Hughes, T.W.; Tapley, J.L.; Kennamer, J.E.; Lehman, C.P. The imapct of predation on wild turkeys. Proc. Natl. Wild Turk. Symp. 1997, 9, 117–126. [Google Scholar]
- Blumstein, D.T. Developing an evolutionary ecology of fear: How life history and natural history traits affect disturbance tolerance in birds. Anim. Behav. 2006, 71, 389–399. [Google Scholar] [CrossRef]
- Beauchamp, G. Flocking in birds increases annual adult survival in a global analysis. Oecologia 2021, 197, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Leighton, P.A.; Horrocks, J.A.; Kramer, D.L. Conservation and the scarecrow effect: Can human activity benefit threatened species by displacing predators? . Biol. Conserv. 2010, 143, 2156–2163. [Google Scholar] [CrossRef]
- Ciuti, S.; Northrup, J.M.; Muhly, T.B.; Simi, S.; Musiani, M.; Pitt, J.A.; Boyce, M.S. Effects of humans on behaviour of wildlife exceed those of natural predators in a landscape of fear. PLoS ONE 2012, 7, e50611. [Google Scholar] [CrossRef]
- Crosmary, W.G.; Makumbe, P.; Côté, S.D.; Fritz, H. Vulnerability to predation and water constraints limit behavioural adjustments of ungulates in response to hunting risk. Anim. Behav. 2012, 83, 1367–1376. [Google Scholar] [CrossRef]
- Suraci, J.P.; Clinchy, M.; Zanette, L.Y.; Wilmers, C.C. Fear of humans as apex predators has landscape-scale impacts from mountain lions to mice. Ecol. Lett. 2019, 22, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.A. Predation and starvation: Age-specific mortality in juvenile juncos (Junco phaenotus). J. Anim. Ecol. 1989, 58, 275–286. [Google Scholar] [CrossRef]
- Barron, D.G.; Brawn, J.D.; Weatherhead, P.J. Meta-analysis of transmitter effects on avian behaviour and ecology. Methods Ecol. Evol. 2010, 1, 180–187. [Google Scholar] [CrossRef]
- Estes, J.A.; Terborgh, J.S.; Brashares, J.S.; Power, M.E.; Berger, J.; Bond, W.J.; Carpenter, S.R.; Essington, T.E.; Holt, R.D.; Jackson, J.B.C.; et al. Trophic downgrading of planet Earth. Science 2011, 333, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Meriggi, A.; Brangi, A.; Cuccus, P.; Della Stella, R.M. High mortality rate in a re-introduced grey partridge population in central Italy. Ital. J. Zool. 2002, 69, 19–24. [Google Scholar] [CrossRef]
- Leighton, K.; Chilvers, D.; Charles, A.; Kelly, A. Post-release survival of hand-reared tawny owls (Strix aluco) based on radio-tracking and leg-band return data. Anim. Welf. 2008, 17, 207–214. [Google Scholar] [CrossRef]
- Lislevand, T.; Figuerola, J.; Székely, T. Avian body sizes in relation to fecundity, mating system, display behaviour, and resource sharing. Ecology 2007, 88, 1605. [Google Scholar] [CrossRef]
- Dunning, J.B. CRC Handbook of Avian Body Masses, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Wilman, H.; Belmaker, J.; Simpson, J.; de la Rosa, C.; Rivadeneira, M.M.; Jetz, W. EltonTraits 1.0: Species-level foraging attributes of the world’s birds and mammals. Ecology 2014, 95, 2027. [Google Scholar]
- Billerman, S.M.; Keeney, B.K.; Rodewald, P.G.; Schulenberg, T.S. (Eds.) Birds of the World; Cornell Laboratory of Ornithology: Ithaca, NY, USA, 2022. [Google Scholar]
- Heisey, D.M.; Fuller, T.K. Evaluation of survival and cause-specific mortality rates using telemetry data. J. Wildl. Manage. 1985, 49, 668–674. [Google Scholar] [CrossRef]
- Li, D.; Dinnage, R.; Nell, L.A.; Helmus, M.R.; Ives, A.R. Phyr: An R package for phylogenetic species-distribution modelling in ecological communities. Methods Ecol. Evol. 2020, 11, 1455–1463. [Google Scholar] [CrossRef]
- Scholer, M.N.; Strimas-Mackey, M.; Jankowski, J.E. A meta-analysis of global avian survival across species and latitude. Ecol. Lett. 2020, 23, 1537–1549. [Google Scholar] [CrossRef]
- Hackett, S.J.; Kimball, R.T.; Reddy, S.; Bowie, R.C.K.; Braun, E.L.; Braun, M.J.; Chojnowski, J.L.; Cox, W.A.; Han, K.L.; Harshman, J.; et al. A phylogenomic study of birds reveals their evolutionary history. Science 2008, 320, 1763–1768. [Google Scholar] [CrossRef]
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef]
- Cohen, J.E.; Pimm, S.L.; Yodzis, P.; Sadana, J. Body sizes of animal predators and animal prey in food webs. J. Anim. Ecol. 1993, 62, 67–78. [Google Scholar] [CrossRef]
- Thiollay, J.-M.; Jullien, M. Flocking behaviour of foraging birds in a neotropical rain forest and the antipredator defence hypothesis. Ibis 1998, 140, 382–394. [Google Scholar] [CrossRef]
- Shultz, S.; Noë, R.; McGraw, W.S.; Dunbar, R.I.M. A community-level evaluation of the impact of prey behavioural and ecological characteristics on predator diet composition. Proc. R. Soc. Lond. B Biol. Sci. 2004, 271, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.; Kays, R. Causes of mortality in North American populations of large and medium-sized mammals. Anim. Conserv 2011, 14, 474–483. [Google Scholar] [CrossRef]
- Hayward, M.W.; Henschel, P.; O’Brien, J.; Hofmeyr, M.; Balme, G.; Kerley, G.I.H. Prey preferences of the leopard (Panthera pardus). J. Zool. 2006, 270, 298–313. [Google Scholar] [CrossRef]
- Kinlock, N.L.; Prowant, L.; Herstoff, E.M.; Foley, C.M.; Akin-Fajiye, M.; Bender, N.; Umarani, M.; Ryu, H.-Y.; Şen, B.; Gurevitch, J. Explaining global variation in the latitudinal diversity gradient: Meta-analysis confirms known patterns and uncovers new ones. Glob. Ecol. Biogeogr. 2018, 27, 125–141. [Google Scholar] [CrossRef]
- Caraco, T.; Martindale, S.; Pulliam, H.R. Avian flocking in the presence of a predator. Nature 1980, 285, 400–401. [Google Scholar] [CrossRef]
- Cresswell, W. Flocking is an effective anti-predation strategy in redshanks, Tringa totanus. Anim. Behav. 1994, 47, 433–442. [Google Scholar] [CrossRef]
- Jullien, M.; Clobert, J. The survival value of flocking in neotropical birds: Reality or fiction? Ecology 2000, 81, 3416–3430. [Google Scholar] [CrossRef]
- Srinivasan, U. Morphological and behavioral correlates of long-term bird survival in selectively logged forest. Front. Ecol. Evol. 2019, 7, 17. [Google Scholar] [CrossRef]
- Brashares, J.S.; Garland, T.; Arcese, P. Phylogenetic analysis of coadaptation in behavior, diet, and body size in the African antelope. Behav. Ecol. 2000, 11, 452–463. [Google Scholar] [CrossRef]
- Ebensperger, L.A.; Bozinovic, F. Communal burrowing in the hystricognath rodent, Octogon degus: A benefit of sociality? Behav. Ecol. Sociobiol. 2000, 47, 365–369. [Google Scholar] [CrossRef]
- Stankowich, T.; Haverkamp, P.J.; Caro, T. Ecological drivers of antipredator defenses in Carnivores. Evolution 2014, 68, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Liker, A.; Liu, Y.; Székely, T. Evolution of social organization: Phylogenetic analyses of ecology and sexual selection in Weavers. Am. Nat. 2022, 200, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, G. Flocking in birds is associated with diet, foraging substrate, timing of activity, and life history. Behav. Ecol. Sociobiol. 2022, 76, 74. [Google Scholar] [CrossRef]
- Weston, M.A.; Radkovic, A.; Kirao, L.; Guay, P.-J.; Van Dongen, W.F.D.; Malaki, P.; Blumstein, D.T.; Symonds, M.R.E. Differences in flight initiation distances between African and Australian birds. Anim. Behav. 2021, 179, 235–245. [Google Scholar] [CrossRef]
- Ekanayake, K.B.; Gnanapragasam, J.J.; Ranawana, K.; Vidanapathirana, D.R.; Abeyawardhana, U.T.; Fernando, C.H.; McQueen, A.; Weston, M.A.; Symonds, M.R.E. Ecological and environmental predictors of escape among birds on a large tropical island. Behav. Ecol. Sociobiol. 2022, 76, 31. [Google Scholar] [CrossRef]
- Morelli, F.; Benedetti, Y.; Díaz, M.; Grim, T.; Ibáñez-Álamo, J.D.; Jokimäki, J.; Kaisanlahti-Jokimäki, M.-L.; Tätte, K.; Markó, G.; Jiang, Y.; et al. Contagious fear: Escape behavior increases with flock size in European gregarious birds. Ecol. Evol. 2019, 9, 6096–6104. [Google Scholar] [CrossRef]
- Willis, E.O. Do birds flock in Hawaii, a land without predators? Calif. Birds 1972, 3, 1–9. [Google Scholar]
- Blumstein, D.T.; Daniel, J.C. The loss of anti-predator behaviour following isolation on islands. Proc. R. Soc. Lond. B Biol. Sci. 2005, 272, 1663–1668. [Google Scholar] [CrossRef]
- Beauchamp, G. Do avian species survive better on islands? Biol. Lett. 2021, 16, 20200643. [Google Scholar] [CrossRef] [PubMed]
- Courchamp, F.; Chapuis, J.-L.; Pascal, M. Mammal invaders on islands: Impact, control and control impact. Biol. Rev. 2003, 78, 347–383. [Google Scholar] [CrossRef] [PubMed]
- Péron, G. Compensation and additivity of anthropogenic mortality: Life-history effects and review of methods. J. Anim. Ecol. 2013, 82, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Riecke, T.V.; Lohman, M.G.; Sedinger, B.S.; Arnold, T.W.; Feldheim, C.L.; Koons, D.N.; Rohwer, F.C.; Schaub, M.; Williams, P.J.; Sedinger, J.S. Density-dependence produces spurious relationships among demographic parameters in a harvested species. J. Anim. Ecol. 2022, 91, 2261–2272. [Google Scholar] [CrossRef] [PubMed]
- Suhonen, J.; Norrdahl, K.; Korpimaki, E. Avian predation risk modifies breeding bird community on a farmland area. Ecology 1994, 75, 1626–1634. [Google Scholar] [CrossRef]
- Groom, M.J. Sand-colored nighthawks parasitize the antipredator behavior of three nesting bird species. Ecology 1992, 73, 785–793. [Google Scholar] [CrossRef]
- Sæther, B.-E. Survival rates in relation to body weight in European birds. Ornis Scand. 1989, 20, 13–21. [Google Scholar] [CrossRef]
- Muñoz, A.P.; Kéry, M.; Martins, P.V.; Ferraz, G. Age effects on survival of Amazon forest birds and the latitudinal gradient in bird survival. Auk 2018, 135, 299–313. [Google Scholar] [CrossRef]
- Conroy, M.J.; Costanzo, G.R.; Stotts, D.B. Winter survival of female American Black Ducks on the Atlantic Coast. J. Wildl. Manage. 1989, 53, 99–109. [Google Scholar] [CrossRef]
- Maness, T.J.; Anderson, D.J. Predictors of juvenile survival in birds. Ornithol. Monogr. 2013, 78, 1–55. [Google Scholar] [CrossRef]
- Naef-Daenzer, B.; Grüebler, M.U. Post-fledging survival of altricial birds: Ecological determinants and adaptation. J. Field Ornithol. 2016, 87, 227–250. [Google Scholar] [CrossRef]
- Møller, A.P. Flight distance of urban birds, predation, and selection for urban life. Behav. Ecol. Sociobiol. 2008, 63, 63–75. [Google Scholar] [CrossRef]
- Balmori, A. Radiotelemetry and wildlife: Highlighting a gap in the knowledge on radiofrequency radiation effects. Sci. Tot. Environ. 2016, 543, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Thirgood, S.J.; Redpath, S.M.; Hudson, P.J.; Hurley, M.M.; Aebischer, N.J. Effects of necklace radio transmitters on survival and breeding success of red grouse Lagopus lagopus scoticus. Wildl. Biol. 1995, 1, 121–126. [Google Scholar] [CrossRef]
- Naef-Daenzer, B.; Widmer, F.; Nuber, M. A test for effects of radio-tagging on survival and movements of small birds. Avian Sci. 2001, 1, 15–23. [Google Scholar]
- Biro, P.A.; Dingemanse, N.J. Sampling bias resulting from animal personality. Trends Ecol. Evol. 2009, 24, 66–67. [Google Scholar] [CrossRef]
| Variable | Prediction | Rationale | Source |
|---|---|---|---|
| Body mass | Predation rate decreases with body mass | Large body mass makes prey more difficult to capture and subdue | [2,12] |
| Latitude | Predation rate increases at lower latitudes | Latitudinal gradient in predator diversity and abundance | [7,18] |
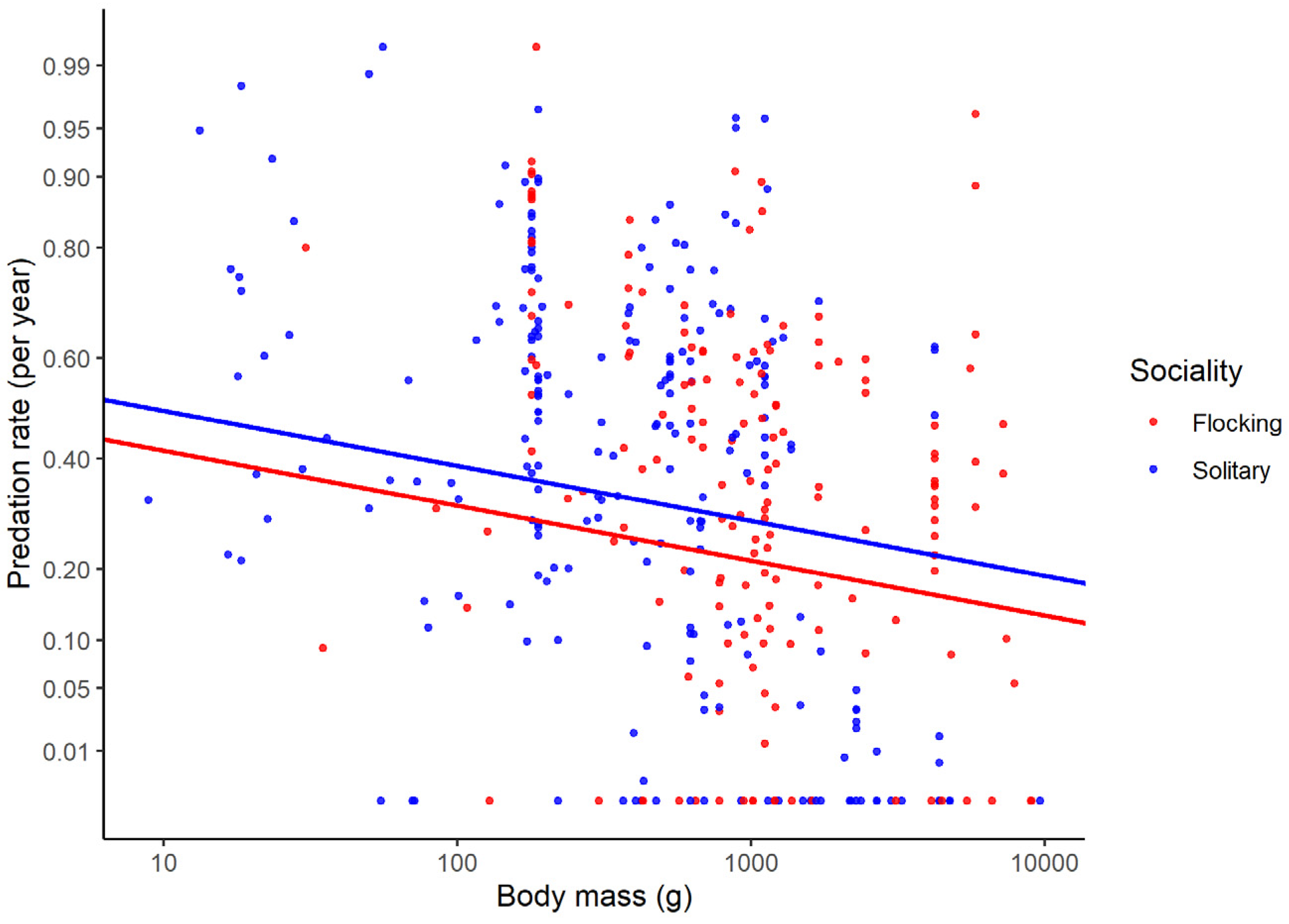
| Flocking | Predation rate decreases in flocking species | Living in groups increases the ability to detect predators and dilutes risk | [13] |
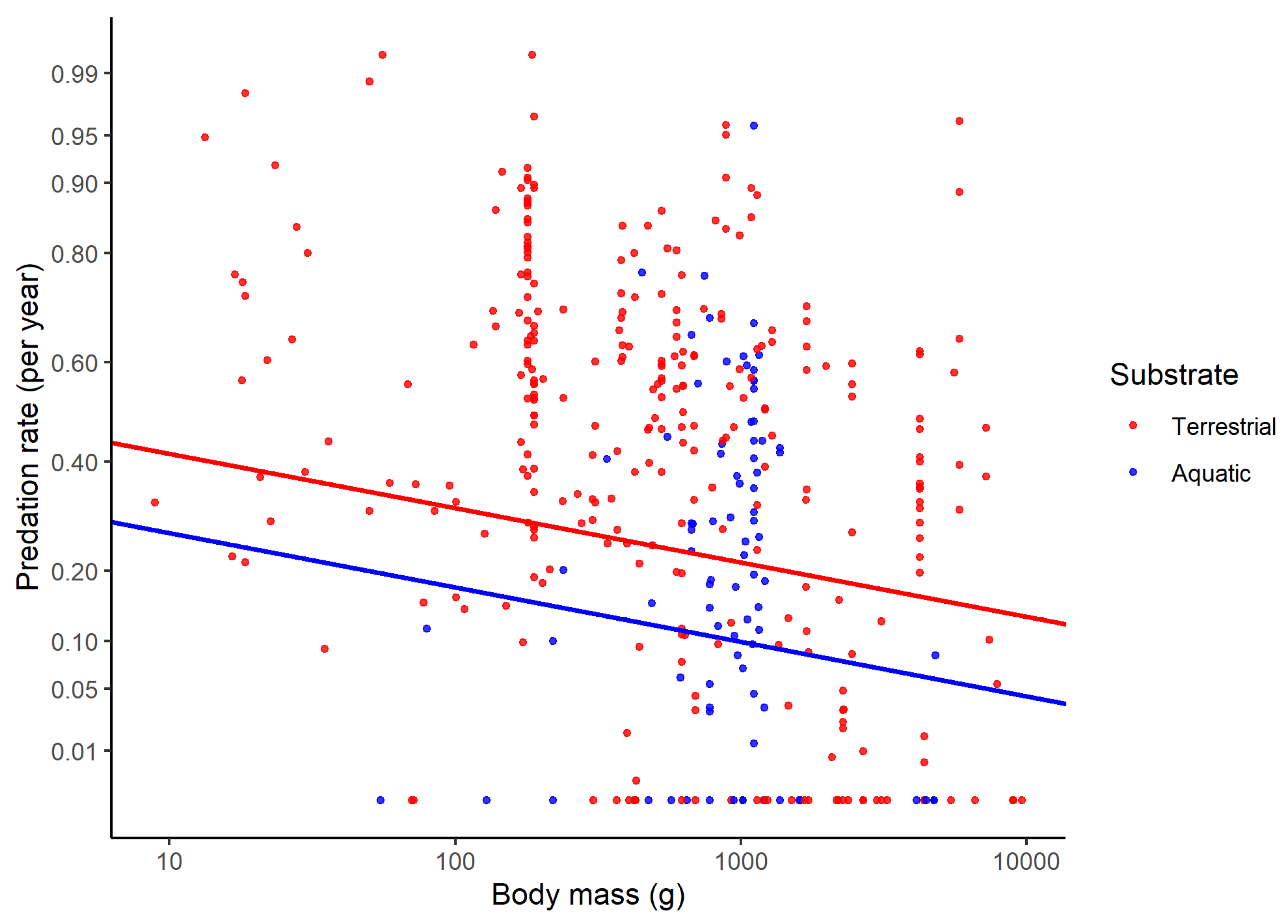
| Foraging substrate | Predation rate is lower for aquatic than terrestrial species | Fewer predators in aquatic habitats | [19,20,21,22] |
| Foraging strata | Predation rate increases closer to the ground | Greater diversity of predators closer to the ground | [23,24] |
| Diet | Predation rate is higher for herbivores than carnivores | Better visual detection abilities in carnivores | [25,26] |
| Island living | Predation rate decreases in island-living species | Lower diversity and abundance of predators on islands | [6] |
| Hunting | Predation rate varies for harvested species | Changes in habitat and prey behaviour increases predation risk but hunting might shield prey species from predators | [27,28,29,30] |
| Season | Predation rate is higher in the breeding season | Parental care increases encounters with predators | [11] |
| Age | Predation rate is higher in juveniles than in adults | Relative lack of experience of juveniles with predators | [31] |
| Time | Predation rate decreases over time | Technological improvements over time reduce the negative impact of transmitters or general decrease in predator populations over the years | [32,33] |
| Variable | β (SE) | p |
|---|---|---|
| Fixed effects | ||
| Body mass in log10 scale | −0.34 (0.040) | <0.0001 |
| Absolute latitude | 0.0013 (0.0017) | 0.44 |
| Solitary vs. flocking | 0.12 (0.047) | 0.013 |
| Aquatic vs. terrestrial | −0.18 (0.062) | 0.003 |
| Foraging strata | 0.0029 (0.00092) | 0.001 |
| Herbivore vs. carnivore | 0.31 (0.060) | <0.0001 |
| Omnivore vs. carnivore | 0.098 (0.075) | 0.19 |
| Island vs. continent | −0.16 (0.079) | 0.047 |
| Hunting vs. non-hunting | 0.067 (0.035) | 0.058 |
| Breeding vs. non-breeding | 0.053 (0.047) | 0.26 |
| Juvenile vs. non-juvenile | 0.077 (0.036) | 0.03 |
| Time | −0.0042 (0.0014) | 0.002 |
| Random effects | Variance | |
| Species | 0.040 | |
| Methodology | 0.000000076 | |
| Residual variance | 0.051 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beauchamp, G. Susceptibility to Predation Varies with Body Mass, Foraging Niche, and Anti-Predator Responses among Bird Species. Birds 2023, 4, 73-84. https://doi.org/10.3390/birds4010006
Beauchamp G. Susceptibility to Predation Varies with Body Mass, Foraging Niche, and Anti-Predator Responses among Bird Species. Birds. 2023; 4(1):73-84. https://doi.org/10.3390/birds4010006
Chicago/Turabian StyleBeauchamp, Guy. 2023. "Susceptibility to Predation Varies with Body Mass, Foraging Niche, and Anti-Predator Responses among Bird Species" Birds 4, no. 1: 73-84. https://doi.org/10.3390/birds4010006
APA StyleBeauchamp, G. (2023). Susceptibility to Predation Varies with Body Mass, Foraging Niche, and Anti-Predator Responses among Bird Species. Birds, 4(1), 73-84. https://doi.org/10.3390/birds4010006





