Simple Summary
Birds of a whole range of species are housed in zoological collections globally; they are some of the most frequently seen of species in animal populations kept under human care. Research output on birds can provide valuable information on how to advance husbandry and care for particular species, which may further feed into conservation planning. Linking birds housed in human care to those in the wild adds value to these zoo-housed populations; this paper provides areas of research that could be conducted to add value to these zoo-housed birds and suggests increasing the conservation focus and conservation relevance of birds housed by humans.
Abstract
Birds are the most speciose of all taxonomic groups currently housed in zoos, but this species diversity is not always matched by their inclusion in research output in the peer-reviewed literature. This large and diverse captive population is an excellent tool for research investigation, the findings of which can be relevant to conservation and population sustainability aims. The One Plan Approach to conservation aims to foster tangible conservation relevance of ex situ populations to those animals living in situ. The use of birds in zoo aviculture as proxies for wild-dwelling counterparts is considered from this integrated conservation approach. This paper considers the relevance of ex situ bird populations to field-based conservation action and it illustrates how “added value” to captive populations can be gained from their inclusion in conservation efforts. Current trends in scientific publications that focus on birds are provided to identify patterns in species focus and identification of areas of study that could be relevant to advancing avicultural practices, bird husbandry standards, animal welfare and conservation relevance of such populations. Research into wild birds is extremely useful for furthering how birds are managed in zoological collections. Collaboration between field-based projects that have involved zoo professionals are reviewed, to showcase information transfer from the field to the zoo and vice versa, and the ultimate benefits to aviculture and the added value that can be brought to zoo bird populations. Suggested ideas for research into specific areas of ex situ population management and conservation, and avicultural practices are provided to guide future researchers in their endeavors to ensure we have the evidence needed to care for and conserve birds as appropriately and as viably as possible.
1. Introduction
Birds are the most speciose of vertebrate taxa housed in zoos currently [1,2]; the diverse array of avian species within animal collections provides an excellent tool for directed research to be used to evidence conservation action, development of welfare measures and the advancement of husbandry to ensure population sustainability. Collection planning for birds in zoos should consider the conservation needs of species (i.e., species of high Red List threat category) as well as the requirements to manage sustainable populations under human care to fulfil the “Ark Paradigm” of zoological collections [3]; the idea that zoological collections, globally, collaborate to ensure viable and self-sustaining populations of species are managed in ex situ populations [4]. Whilst still relevant, such preservation ideas have been augmented and enhanced by new ways of engaging with a wider range of stakeholders (those in the field and in the zoo) to provide long term but adaptable, species-specific plans for conservation action, for example the IUCN One Plan Approach [5], which can bring tangible links to field conservation for captive populations [6].
In spite of this large captive population, diversity of species and potential for palpable conservation relevance of zoo-housed birds, research has documented that the majority of bird species held in large accredited zoos around the world are less likely to be of conservation concern and are less likely to originate from threatened ecosystems, more likely to have a wide-ranging distribution, [7]. However, these “common-to-the-zoo and not-yet-of-conservation-concern species” are still relevant to the development of avicultural practice, as they can serve as excellent models for testing and trialing key aspects of animal care needed for similar species that do require conservation planning and action. For example, trialing the resilience of, or illustrating the physiological changes that may occur in, captively housed populations of threatened species destined for wild reintroduction, but in a commonly housed experimental population [8], provides useful details on developing conservation-promoting husbandry routines for threatened species. These conservation surrogates can also be used in public relations efforts [9], to promote the reasons for the conservation work, the relevance of conservation action and to explain how management of ex situ individuals helps those in the wild.
Numerous anthropogenic threats, specifically pertaining to wild birds, are evident and these are the focus of current conservation action involving collaboration between a range of zoological organizations, conservation NGOs, government agencies and industry partners. Identifying research priorities can be helpful to filling knowledge gaps that are essential to the advancement of husbandry for individuals of species in populations that are housed in zoo-focused programs (i.e., managing a population for long-term sustainability outside of its range states), as well as for supporting the likelihood of successful conservation action for populations in reintroduction and re-release programs out in the field. Several papers show the impact of zoo-themed research and how the knowledge from such publication is embedded into management practices that promote population sustainability.
Review of conservation action for threatened bird species from 1993 to 2020 has identified that 21–32 (since 1993) and 9–18 (since 2010) bird extinctions have been prevented due to coordinated conservation efforts [10]. This review of Target 12, which sits under Strategic Goal C of the Convention on Biological Diversity (CBD) 20 “Aichi Biodiversity Targets” [11] by Bolam, Mair [10] shows that whilst the full premise of this Target has not been realized (Strategic Goal C: To improve the status of biodiversity by safeguarding ecosystems, species and genetic diversity. Target 12: By 2020 the extinction of known threatened species has been prevented and their conservation status, particularly of those most in decline, has been improved and sustained) without conservation action for highly threatened birds, extinction rates would have been up to 4.2 times higher than actually documented. Zoo-housed bird populations, alongside the development of avicultural practices that promote sustainability and reproductive success of these populations, are a useful tool for helping meet these Aichi Targets. By linking together zoo expertise with field based projects, and by constructing directed research questions that can provide answer relating to species management, population viability and conservation needs, knowledge gaps are filled and challenges around implementation of effective conservation action (between the zoo and the wild) are removed.
As such, this article aims to provide information on the usefulness of zoo-housed birds to the direction of conservation planning and the implementation of conservation action. It suggests areas of research that could add important data to how we manage captive bird populations, as well as suggesting research themes that could result in evidence-based husbandry approaches, which in turn could feed into conservation planning. When discussing “zoo-housed birds”, the paper refers to populations of species managed in accredited zoological organizations, i.e., the British and Irish Association of Zoos and Aquariums (BIAZA) [12], the European Association of Zoos and Aquaria (EAZA) [13], the Association of Zoos and Aquariums (AZA) [14], and other such global zoo bodies that expect higher standards of animal welfare and care from their members. Not all zoological organizations will hold the birds that are used for conservation work (e.g., reintroduction efforts or conservation breeding specimens) in the public facing side of the animal collection and may have dedicated facilities for these individuals [15,16,17,18], but they are still part of that specific institution’s ex situ conservation strategy. Dissemination of avicultural knowledge and expertise will occur between those managing the “public collection” and those in charge of the “conservation collection”, enhancing the role of the zoo bird to conservation objectives.
2. Collaborative Approaches and Conservation Action
Sharing information from the zoo to the wild can enhance the relevance of captive bird populations, not only by enabling zoo-housed birds to play a more direct role in field-based conservation action, but also by encouraging dialogue between those working in the range states of the birds that the zoo personnel work with. This builds capacity (within tzoo personnel) to learn and develop new skills and techniques, applicable to in-zoo population management, which have come directly from experts who work with the species in its native range. Moreover, likewise, field biologists can be enthused about the role of zoo bird populations to modern day conservation planning and action—how information from ex situ facilities can feed into conservation action by trialing methods of identification and individual recognition [19], or by testing technology before it goes into the field [20], or by understanding behavior patterns and validating ethograms for use in field studies [21,22]. The One Plan Approach to conservation encourages such dialogue and discussion [5]; work on threatened mammalian carnivores shows that using a One Plan Approach, involving experts with in situ and ex situ experiences, can help guide collection planning (within and across captive facilities) so that species housed have real education, research and conservation potential [6]. Such tangible conservation value (of ex situ individuals) is the core of the One Plan Approach; developing avicultural practices to enhance their species-specific focus and aiming for best practices across facilities, embeds conservation value into zoo-based collection planning. Collaboration between zoos and international conservation organizations, such as the Specialists Groups of the IUCN further pinpoints focus on how captive populations can be integral to relevant and species-specific conservation action [23].
Linking the Captive Population to the Wild: An Example
To direct where avicultural practice can be developed and to further support conservation initiatives, identification of research areas that would fill gaps in knowledge is a viable and relevant way of utilizing resources and expertise in the most efficient and effective manner. This approach is further expanded upon in Section 5. Identifying available information from the wild (i.e., structured literature reviews, meta-analyses or interviews with field biologists) provides a foundation for how ex situ individuals can be of value to free-living birds. Commonly seen populations of zoo birds, that are of conservation concern in the wild, can therefore be included in research projects whose question has been constructed using valid information from wild ecology, behavior or evolutionary information.
Hornbills (Bucerotidae) are essential to the long viability and sustainability of biodiversity in the rainforest, the Helmeted Hornbill (Rhinoplax vigil), a critically endangered species [24], plays an important role in the dispersal of seeds within pristine, undisturbed areas of south-east Asian rainforests [25,26]. The precipitous population decline of the Helmeted Hornbill (Rhinoplax vigil) has been caused by poaching of the birds for their “ivory” [27,28], the large casque on the bird’s head and bill that can be up to 10% of its overall body mass [29]. Whilst the Helmeted Hornbill is not found in captivity, other species of large hornbill are; knowledge of the behavior of these birds and their management requirements can be shared with field conservation initiatives. For example, nest box schemes to enhance the quality of habitats for hornbills to breed in, as well as providing a wider choice of nesting locations, have been instigated in areas of the Helmeted Hornbill’s range in Borneo [30]. The avicultural knowledge available across the ex situ facilities housing hornbills (of related species or ecology) is essential to determining what nest boxes are likely to be successful (in design and structure). Zoos with Hornbill experience have helped with such nest box erection schemes; expertise and financial support have been provided by several large zoological collections in European Association of Zoos and Aquaria (EAZA) and North American Association of Zoos and Aquariums (AZA) that have successfully seen wild Rhinoceros Hornbills (Buceros rhinoceros), listed as vulnerable [31], fledge a chick from an artificial nest box in the Bornean rainforest [32].
Continued research into the design of nest boxes for hornbills, species-specific features that increase the likelihood of their usage (e.g., hole size and location, perching and accessibility into the cavity), as well as ensuring that cavity microclimate (temperature and humidity) replicating that of natural nesting cavities has been performed [33]. Collection of data on the temperature and humidity range of wild hornbill nesting sites not only benefits in situ conservation efforts (as birds using such nesting sites are more likely to hatch eggs and rear chicks), but also can be used by those managing hornbills in zoos to allow them to match the nest box’s environmental parameters as closely as possible to what is experienced by wild breeding pairs. Using data loggers to record such parameters could be used to refine a nest box design that would be of benefit to the helmeted hornbill in the wild, as well as to populations of particular species of hornbills that can prove challenging to breed readily, and successfully, such as the Great Hornbill, Buceros bicornis [34], when held in captivity. In a similar manner to nest box temperature evaluation for hornbills, comparison of the ambient temperature of captive housing with that of the wild has been shown to be important to deciphering differences in reproductive output in the Micronesian Kingfisher, Todiramphus cinnamominus [35], a species that is extinct in the wild and reliant on captive breeding for its survival [36]. Data from the nesting locations of the closely related Pohnpei Kingfisher (Todiramphus reichenbachii), found on a neighboring island, showed that temperatures were hotter than those sometimes provided for captive Guam Kingfishers and that, when Guam Kingfishers had nested it was in higher ambient temperature conditions [35]. This research provided valid and applicable evidence for raising the temperature of housing for Guam Kingfishers in ex situ facilities based on the ecology of a wild bird in a manner likely to improve nesting success.
Figure 1 provides an example, using the Great Indian Hornbill, of how wild data collection and free-living birds can yield information useful to population management in the zoo and ex situ conservation goals; and how birds under human care can be useful to developing methods or approaches that can facilitate the successful completion of conservation objectives out in the wild. By identifying a standard set of “unknowns” (i.e., information on species housing, husbandry or management that is lacking an evidence base), a longitudinal research program can be initiated to gather this relevant information, to identify what wild evidence is required and to provide researchers a set of questions that, if answered, can have real application to bird conservation, welfare and ex situ management [37].
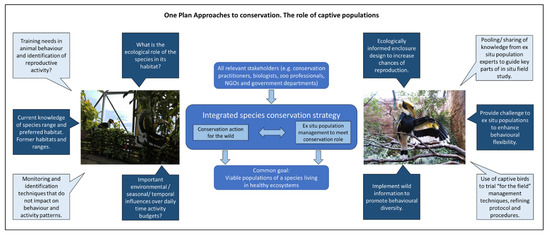
Figure 1.
An example of how the One Plan Approach to conservation (as described by the Conservation Planning Specialist Group [5], middle diagram) integrates knowledge from the field (left) to enhance and inform ex situ husbandry and management (dark blue squares) that may improve captive population sustainability, alongside how ex situ bird populations (right) can assist with the success of field-based conservation schemes (light blue squares) by being used to model or trial potential in situ solutions.
3. Sustainability Goals: Data from the Field for Success in Captivity
Data from the wild are helpful in planning the facilities required for the successful breeding of birds in captive settings. Information pertaining to spatial requirements, territory and home range size, as well as information on habitat features that may need replication within aviaries, can be evaluated to provide solutions to challenges faced when attempting to encourage nesting (for example). Southern Ground Hornbills (Bucorvus leadbeateri) are listed as vulnerable on the IUCN Red List, with a decreasing population trend [38]. Wild Southern Ground Hornbills have a complex multi-generational social system, a cooperative breeding strategy and specific features within a habitat (large trees and cliffs to roost in and to provide shade to combat heat stress, short basal vegetation that enables walking and foraging, and mature trees with suitable nesting cavities within their trunks [39]). Conservation action plans to reverse the population decline of this species of hornbill in South Africa have been in part written and coordinated by the Johannesburg Zoo [40] and for Zimbabwe lists, the importance of collaborative conservation, the seeking out of advice and expertise from all stakeholders, when assigning conservation action goals [41]. Both of these action planning documents, as well as research on reintroductions by Kemp, Kotze [39], identify the large home range (80 km2 to 250 km2) of ground hornbills as a positive factor in their reproductive success. Given that we can predict how challenging captive management can be for a specific mammalian species using measurements of their home range size [42,43,44], perhaps it is possible to unpick problems with captive population viability and sustainability for birds by critically evaluating ecological data on habitat choices, ranging patterns and movement, and resources used within their natural systems.
Consideration of the ecological and evolutionary relevance of such wild data should be conducted in light of what behaviors are being conserved and what the long-term plan is for that population in captivity, i.e., as an “ark” population for long term viability within ex situ facilities, or for more immediate reintroduction and release conservation projects. Ensuring that behavioral traits are conserved and that populations can perform key adaptative behavior when and if required in the future is something that avicultural experts, zoo biologists and bird keepers can assist with. The knowledge that such personnel have about enclosure design—how to evaluate and modify it, environmental enrichment, how to promote diversity within time-activity budgets, and on providing the most appropriate nutrition, encouraging natural feeding and foraging as well as the giving access to important pigments, supplements and other dietary stimulants for breeding—supports the behavioral and genetic diversity and viability within ex situ populations.
Developing zoo bird exhibits to theme them around specific conservation messages can be used to promote wider understanding of the threats faced by wild birds specifically and, hopefully, encourage human behavior change that benefits ecosystem health and integrity more generally. It is understood that humans are more likely to support conservation initiatives for species with features that are valued [45], by understanding species familiarity and what drives concepts of caring [46]; information on conservation needs and an awareness of these needs can be specified to species that are on show in the zoo. Birds are some of the most popular of species [47], in terms of characteristics that people find attractive [45], and both physical features (such as body size) as well as local familiarity within the bird’s range correlate with how identifiable and noticeable people find specific bird species [46]. Building on well-known bird behaviors (for example, familiar-to-many species such as swallows (Hirundinidae) and their wide-ranging journeys to and from breeding and wintering grounds) may be a way of promoting conservation needs to zoo visitors. Migratory birds pose a particular challenge to the implementation of conservation action [48]. Movement across various range states proves a challenge for consistent conservation measures to be applied [49], due to the changing threats within each territory that the birds cross. As zoo aviaries change focus, the species displayed within them can be chosen to present specific aspects of the human-bird relationship. For example, the work to redevelop the Bird House at the National Zoo in Washington, USA focusses on promoting the migration patterns of North, central and South American birds and the habitats that they travel between [50]. This new exhibit has conservation education as a central theme, expanding on the work of citizen scientists alongside of ornithological research by scientists and providing opportunities for longer term behavior change in visitors by promoting the needs for bird conservation using apps and electronic media [51].
Adding Value to the Zoo Bird—A Proxy for Species in the Wild
The example provided above shows how the development of a bird exhibit, using information provided by field conservation biologists plus husbandry practice based on ecological information, allows the display of zoo-housed birds to be conducted in an increasing naturalistic settings, which integrate conservation relevance into all areas of the visitor experience, both whilst at the zoo and after they have left. In areas of the world where zoo regions have specified aims as outlined in legislation or policy [52], holding specific species within the animal collection can successfully promote these aims to the organization’s visitors and members. This approach is illustrated and explained by Figure 2 using a common and (relatively) easy to breed flamingo, the Chilean Flamingo (Phoenicopterus chilensis), as the ambassador species for the more threatened Andean Flamingo (Phoenicoparrus andinus).
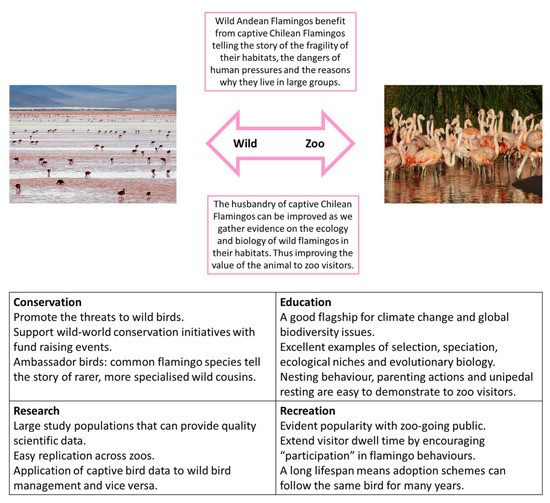
Figure 2.
Adapted from Rose [23], the relationship between species where conservation efforts may be better focused out in the wild range states of the bird integrated with the promotion of that species’ story (evolution, ecology, relationship to humans, conservation needs) using a surrogate or example species in a captive collection. In this case, the Vulnerable Andean Flamingo, with focused in situ conservation, is promoted by the easier-to-keep and much commoner (in captivity) Chilean Flamingo, a Near Threatened species.
Adding value to birds within ex situ populations can be easily undertaken by providing stronger or enhanced links to wild individuals and wild population that may (i) not be present in captive populations or (ii) is less frequently seen in captive populations. In this instance, one species with a frequent captive presence, can be used to “tell the story” of another species that is less common in captivity. This story can relate to the ecosystem or habitat that the two species are evolved to live in, or it may relate to animal behavior and ecology, or a specific conservation focus. Such proxy species can be useful for husbandry and training models, the results of which can then be applied to in situ conservation action; e.g., Madagascar Pochard (Aythya innotata) and Baer’s Pochard (Aythya baeri) conservation action utilizing skills and experience gained with related species in aviculture [53,54]. The conservation action used for some species, e.g., reintroduction initiatives for ground hornbills, has never been based on a proxy [39], so the role that zoo specimens play in the field-based conservation of various birds should be considered on a species by species basis; in some situations, individuals of that actual species are directly integral to the implementation of conservation plans whereas in other cases, such as the Greater Adjutant Stork (Leptoptilos dubius), the zoo-housed population of a related species (in this case the Marabou, Leptoptilos crumeniferus [55]), or the example of the Andean Flamingo (proxy would be the Chilean Flamingo) in Figure 2, can be the most effective way of advocating for conservation action and engaging audiences with the threat to the species, as well as trialing husbandry or management techniques before they are used in the wild.
4. Behavior and Welfare, Conserving Adaptive Traits
A key area of evidence needed for successful development and implementation of One Plan Approaches to show how to evaluate the relevance of zoo enclosures to bird activity patterns is that from wild behavioral ecology. Comparing the activity patterns of wild animals to best understand the behavior of individuals under human care, their behavioral normality and welfare experience are not new [42,56,57,58,59]. Critical evaluation of the relevance (from an ecological standpoint) of captive provision, e.g., husbandry, management protocols and environmental provision, against wild time-activity budgets should be thoughtfully considered to ensure that they are valid when all limitations and variation (i.e., with individual animals, locations, quality of data and environmental heterogeneity) associated with where these wild data came from are considered. Figure 3 shows the usefulness of comparison of captive flamingo behavior with that published on wild flamingo behavior patterns [56]; sampling the published literature and creating standardized comparatives for data mined in this way (i.e., converting raw data to percentages), allowing for a review of what that bird is likely to be doing in captivity, and just how “natural” this may be. Further consideration of un-needed behavior in captivity (e.g., anti-predatory responses) or behavior being promoted by a secure and safer environment (e.g., play) should be included in such reflection of captive activity patterns.
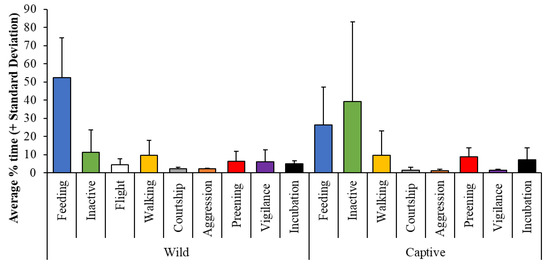
Figure 3.
Taken from Brereton and Rose [56], how information on wild activity patterns available within the published literature can be used to compare data from captive birds of the same species, in this case, wild (left) and captive (right) flamingo state (i.e., long duration) behaviors.
Consideration of “behavioral normality” for birds in zoos can only be judged against that performed by individuals within other captive facilities alongside of information gathered on free-living individuals (to ascertain what behavior is a product of the captive environment and what is commonplace within a species’ behavioral repertoire). More data from the wild on more species, especially those with known behavioral issues in captivity (e.g., the performance of abnormal repetitive behaviors) and/or those of a population sustainability need, is the best way of refining avicultural practice in line with evolutionary and ecological traits of the birds that we are trying to conserve. Keeping a record of such data is useful to comparing behavioral normality over time: when new information on bird ecology becomes available, evaluation of enclosures can be changed in light of this information and then data on the bird’s behavior measured and compared to past records. This allows for the assessment of positive changes to behavior (e.g., increasing time socializing or foraging), as well as changes in visitor interest or engagement (e.g., increased dwell time at the exhibit).
Multiple comparison within the same animal collection provides data at the individual bird and flock-level on responses to environmental variables, as well as information on trends in behavioral performance over time. For example, the longitudinal study of social behavior and aggression within one flock of Lesser Flamingos (Phoeniconaias minor) or the study of different groups of flamingos with one animal collection to provide evidence on social networks, enclosure usage and behavioral diversity [60,61,62], provides a means of evaluating animal, temporal, seasonal and environmental influences on behavior patterns and their potential normality for that species. Collection of data across multiple individuals across multiple facilities should be the next step to such studies, encouraging the comparison and evaluation of data that shows how birds are responding to the environment provided.
Enthusing zoo and aquarium visitors about birds might sometimes be challenging, as surveys about popular or “must see” species at the zoo frequently do not show birds in the highest scoring categories [63,64] and research into species that people deem as charismatic does not feature birds either [65]. In spite of this, birds are engrained in human culture and some taxonomic groups, such as the wildfowl (Anseriformes), form the basis of the first interactions we can have with the natural world [47]. Tapping into this deep-seated interest, displaying the bird in a manner that is representative of the free-living individual could promote conservation outputs further. Given that zoo visitors express a wish to see more information about behavior, habitat choices and conservation action on zoo signage [66], there is a clear way of promoting the amazing diversity of birds, their ecology and adaptations, by engaging visitors with interesting information about the avian species that they are watching and observing at close quarters.
The coordinated efforts of government, NGOs, zoos, scientists and other experts are essential to the prevention of extinction and the reversal of population decline [10]. Clear messaging to the zoo visitor explains the importance of these links and why the zoo is directly connected to the wild. Avicultural evidence also emphasizes the strength of this message—well-kept birds, displaying natural behavior, feeling more comfortable in their managed environment, will be more likely to be on display (i.e., seen by the visitor). Zoo and conservation scientists can therefore use rational decision making processes to determine what information is needed to advance husbandry and management of ex situ populations that will directly impact, positively, on conservation outcomes. An example of this rational decision-making process, using the Lesser Flamingo (one of the more challenging flamingo species to care for), is provided in Figure 4. In this flamingo example, the selection of the best option may be increased collaboration between zoos to enable the collection of data on flocks that breed well and that breed frequently, to understand the social and environmental parameters that positively impact on the Lesser Flamingo breeding behavior in the zoo. Given that this species is near threatened in the wild with a decreasing population trend [67], directed research to further inform husbandry can have realistic ex situ conservation outcomes.
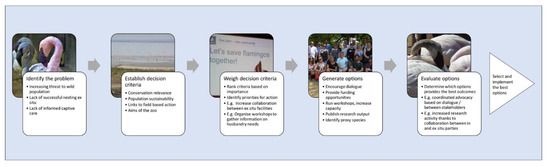
Figure 4.
An example of a rational decision making process to increase evidence gathering for best practice husbandry for Lesser Flamingos. Identification of a challenge or problem (in the case of this species, poor ex situ breeding results) leads to the establishment of decision criteria that can guide future action (for example, this species has a declining wild population and is increasingly under pressure from anthropogenic threats, therefore a viable zoo-housed population is a relevant conservation outcome). Ranking of these decision criteria identifies the best course of action for the species.
5. What Do We Investigate and What Should We Investigate?
An example of how to understand potential gaps in avicultural knowledge can come from meta-analysis studies and a critical review of the existing literature to identify what is known and what is still poorly understood. Evaluation of what questions have been posed, the species investigated, and the outcomes provided by the research identifies how to advance best practice ex situ management practices and illuminates what data are still needed to provide solutions to conservation challenges. A review of 10 years of zoo-focused research output identified that species bias is apparent in publications that feature zoo and aquarium housed animals and diversity in species holding is not replicated in the diversity of species that is seen in the literature [1]. These data were collected from the journal repository Web of Science®, searching across all collections within the repository using the key search term zoo* combined with either behavior*/behavior* or welfare or nutrition and research for each type of taxa (mammal, bird, reptile, amphibian, fish), for example, “zoo* bird behavior*, and the abstract of each article were read to ensure that the paper was focused on zoo and aquarium housed individuals [1]. Searching the repository for all articles across all listed journals took place in 2018, with a final search in January 2019 for any December 2018 articles that may have been published. There was no restriction for English-only articles. The database was checked for duplicate references and these were removed, and only references that met the key requirement of pertaining to zoo- and aquarium-housed animals were included in the final dataset. A total of 1063 publications were included in the resulting analyses from this Web of Science review. The aim of each paper was categorized as either: Behavior, Cognition, Conservation and breeding programs, Husbandry and training, Methods, Nutrition, Physiology and reproductive technologies, Veterinary medicine and animal health, Visitor studies, Welfare. The outcome of each paper was classed as either: Animal and ecosystem health, (human) Behavior change, Conservation and sustainability, Husbandry and welfare, Pure biology. Moreover, the gain of each paper’s outcome (i.e., what could be taken from the paper and applied to zoo management) was classed as either: (specific or general) Advancement of knowledge, (specific or general) Advancement of practical application, Data deficient (i.e., more data needed to be conclusive). Full explanation of the classification of each aim, outcome and gain can be found in Rose, Brereton [1].
By taking the subsection of this dataset that focused specifically on articles related to birds and extending the analysis and evaluation to look at what was investigated using these species it can be seen that of 232 records of papers solely or partly featured birds. Key findings from this review are provided in Figure 5. Twelve papers had a Breeding programs aim (and the majority of these papers were a multiple taxa review, 67%), 45 papers had a Husbandry and training aim (62% were covering multiple species of bird or multiple taxonomic classes that included birds), 24 papers focused on visitor studies, but only two of these papers focused on a specific type of bird (one on condors and one on penguins)—all other papers had birds included as part of a wider research population.
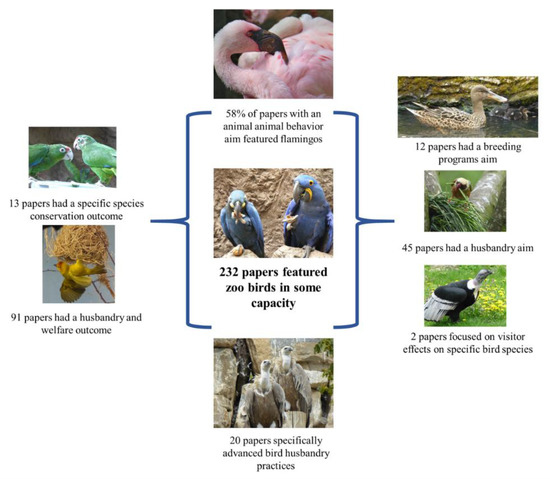
Figure 5.
Key findings from the subset of data pertaining to zoo birds from the wider review of zoo research conducted by Rose, Brereton [1].
Thirty-three articles focused on Behavior, with 58% focused on flamingos. Twenty-six papers had a Conservation and sustainability outcome, of which 13 focused on a specific species of bird. Endangered and critically endangered species were apparent in this subsample; for example, papers that feature conservation action for Gunnison Sage Grouse, Centrocercus minimus [68], Bali Myna (Leucopsar rothschildi) population sustainability and management in the Species Survival Program (SSP) [69] and Puerto Rican Parrot (Amazona vittata) management to ensure the success of a reintroduction program [70]. All have a clear conservation element to the research and show the relevance of captive populations to integrated conservation action. Linkage to the wild and to populations of locally threatened taxa are also evident in this sample of literature; research on genetic diversity of Eastern Sarus Cranes (Grus antigone sharpii) at two breeding centers, using microsatellite markers, showed high levels of diversity in the sampled birds [71], making them suitable populations for reintroduction into habitats where they had been extirpated. This article used microsatellite loci isolated from captive Whooping (Grus americana) and Blue (Grus paradise) Cranes for screening in the Sarus Cranes; again, illustrating useful areas of future, directly applicable to conservation work that zoo-housed birds are relevant to.
Ninety-one papers had a Husbandry and welfare outcome, showing that most articles (40%) on zoo birds (as a single species or included with other taxa) were interested in providing evidence for improved husbandry or development of more relevant management. Themes can be developed in a similar way across related species to add to the body of research on their care in the zoo. For example, research on the influence of weather and visitors on the stress levels of Jackass Penguins (Spheniscus demersus) is applicable to other Spheniscus species that have shown stress-like responses to ecotourist visitors in the wild [72]. Twenty of these 232 bird-focused zoo-themed articles provided a specific advancement to practice, 67 papers provided a specific advancement in knowledge and 23 provided a general advancement in knowledge. Four papers were considered as needing an extension to the research area to provide a definitive advancement in knowledge or practice. To further advance practice, rather than just subject knowledge, we can continue to look at those areas of husbandry practice that are in need of review and reflection.
Moving Forwards with Research
Modern day ex situ aviculture can be of real help to the writing, implementation, trialing and evaluation of conservation action. Precedent is available in the literature that shows how captive birds can support field-based conservation [73,74,75,76,77,78]. The increased uptake of the One Plan Approach to conservation and the rationalizing of regional collection plans across zoos, to further justify species kept to enhance the chances of maintaining genetically viable and sustainable bird populations, increases the need for directed research and action research to consistently evaluate avicultural practice to ensure that it is fit for purpose. The evaluation of husbandry for specific species, from a welfare perspective for example, can be undertaken using a standardized method [79] and as this approach can be shared amongst all animal care staff, directly comparable data on bird responses to captive care can be reliably undertaken. Given the need to check methods for consistent approaches when comparing information from the wild to the zoo [59], the development of data collection methods that involve all experts on that species seems to be the best way of credibly improving aviculture to ensure it remains specific and relevant.
Table 1 provides examples of where directed research could run in the future to answer questions relating to holes in our avicultural knowledge, as well as horizon scanning for the future to forewarn and forearm against potential threats to the viability and sustainability of ex situ populations. The aim of this table is to encourage researchers to consider how to answer these questions, as well as to stimulate dialogue and exchange between bird keepers and ornithologists as the best ways of making ex situ populations relevant to wider conservation action for as many, or indeed all, of the bird species we house in captive populations. This approach has been identified as being successful in other papers on captive birds, where there has been a need to provide evidence for ex situ population management. For example, a review of captive flamingo welfare identified a set of questions that, once answered, could reliably inform how to advance husbandry in the future and provide evidence for how to assess the welfare of these birds in captivity [37]. Directing research activity in such a way can increase the publication activity that features specific species; in this case, zoo-housed flamingos featured in very few research outputs in 2009 [80], but by 2019, were the commonest order of zoo birds being investigated [1].

Table 1.
Future research questions to assist the gathering of evidence for best practice avicultural practices. “Theme” includes the direction of the question and what data could be collected. “Focal species” provides an example of research subjects suitable for that specific theme. “Potential output” provides ideas for how this research theme might advance practice and inform conservation actions.
6. Conclusions
The use of field data and research from the wild develops best practice husbandry approaches and enhances avicultural techniques to ensure that they remain species relevant. Advances in the training of zoo professionals; capacity building to expand knowledge and insight into species biology, ecology and therefore captive care [81]; and the writing of conservation action plans where specific emphasis is given to zoo-housed individuals [82] increases the relevance of ex situ populations to global conservation work. Conservation of highly endangered birds is not without struggle. The captive population of Guam Kingfishers, for example, continues to pose a challenge regarding its long-term viability and sustainability [83], due to the sudden extirpation of the species and resulting small founding population brought into human care [84]. Use of ecological information, including changes to diet and nesting log characteristics has been responsible for a growth in this population [83], and the key role of field biologists and zoo conservation and aviculture was outlined in the species recovery plan for this bird [85]. This species recovery plan explains the importance of a managed population in the kingfisher’s natural home alongside of that held in ex situ facilities and it points to the need for growth in all of these managed populations to ensure eventual reintroduction success. The scientific expertise within zoo research departments that can run specific testing relevant to increasing conservation success, e.g., endocrinology assessments and pair compatibility, may hopefully bring the likelihood of reintroduction more swiftly [86].
The example of the Guam Kingfisher shows the need for continued research into the wild ecology of the species housed in zoo and use of evidence for all aspects of husbandry. Measurement of environmental parameters should be undertaken using data available from the species’ natural range states. Given the physiological stresses involved in reproduction, temperature is likely to be a key influence over the probability of successful nesting, particularly in a captive environment where such parameters are under human control. Alongside biologically relevant environmental parameters and their maintenance within ex situ housing, consideration of mate choice into conservation planning [87] and the role of the social environment on successful breeding also needs more emphasis across a wider range of avian species. Continued collaboration with field partners, as evaluated in the examples provided in this article, will keep adding to our knowledge on the wild lives of the birds housed under human care that will ultimately result in abiotic, biotic, physical and social environments that more closely match a species’ requirements. Such collaborative approaches have been integral to the successful reassessment of the Guam Rail (Hypotaenidia owstoni) from Extinct in the Wild to Critically Endangered [88] with a now increasing population trajectory, as well as for the reintroduction of the Common Crane (Grus grus) into areas of its former range in the UK [89]. The relevance of ex situ approaches is evidenced by Bolam, Mair [10], who showed them to be the most important conservation factors for preventing extinction in 63% of the 32 species of highly threatened bird analyzed.
Even populations of species of individuals that are not likely to go back into the wild have a role to play in informing, promoting and advancing conservation action. The use of proxy species to encourage research into conservation or management unknowns, applicable to a related threatened species elsewhere in the wild, can be a key aim of many zoo housed populations. Research helps fill knowledge gaps apparent in our avicultural knowledge and practice; directing scientists to questions where an answer is needed provides the biggest return from the limited time and resources that may be available. Application and dissemination of research findings is integral to the continued assessment of housing, husbandry and captive care; and this provides further knowledge of how to promote the performance of key adaptive behaviors and how to remove a need in the individual bird to display abnormal repetitive behaviors, and it informs on any potential influences of uncontrollable variables, such as visitors or caregivers, on the bird’s welfare state when housed under human care. Zoological collections should have a zero-tolerance approach to abnormal repetitive behavior [90] and evidence-based approaches, such as biologically relevant enclosures and provision of suitable environmental enrichment, help achieve this [91]. The researcher’s impact also benefits by increasing the applicability and accessibility of their science. Thought, creativity and imagination in the review of regional collection plans to add value to the species being held in ex situ facilities, based on meaningful dialogue between all stakeholders (zoo-based or field-based), will ensure that the role of zoo aviculture to modern day bird conservation can continue to be realized.
Funding
No funding was received.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data used in the example in Section 5 are freely available at https://ore.exeter.ac.uk/repository/handle/10871/39092.
Conflicts of Interest
The author declares no conflict of interest.
References
- Rose, P.E.; Brereton, J.E.; Rowden, L.J.; Lemos de Figueiredo, R.; Riley, L.M. What’s new from the zoo? An analysis of ten years of zoo-themed research output. Palgrave Commun. 2019, 5, 1–10. [Google Scholar] [CrossRef]
- Brereton, S.R.; Brereton, J.E. Sixty years of collection planning: What species do zoos and aquariums keep? Int. Zoo Yearb. 2020, 54, 1–15. [Google Scholar] [CrossRef]
- Lees, C.M.; Wilcken, J. Sustaining the Ark: The challenges faced by zoos in maintaining viable populations. Int. Zoo Yearb. 2009, 43, 6–18. [Google Scholar] [CrossRef]
- Soulé, M.; Gilpin, M.; Conway, W.G.; Foose, T. The millenium ark: How long a voyage, how many staterooms, how many passengers? Zoo Biol. 1986, 5, 101–113. [Google Scholar] [CrossRef]
- Conservation Planning Specialist Group. The One Plan Approach to Conservation. Available online: http://www.cbsg.org/our-approach/one-plan-approach-conservation (accessed on 15 January 2021).
- Traylor-Holzer, K.; Leus, K.; Bauman, K. Integrated Collection Assessment and Planning (ICAP) workshop: Helping zoos move toward the One Plan Approach. Zoo Biol. 2019, 38, 95–105. [Google Scholar] [CrossRef]
- Martin, T.E.; Lurbiecki, H.; Joy, J.B.; Mooers, A.O. Mammal and bird species held in zoos are less endemic and less threatened than their close relatives not held in zoos. Anim. Conserv. 2014, 17, 89–96. [Google Scholar] [CrossRef]
- Angelier, F.; Parenteau, C.; Trouvé, C.; Angelier, N. Does the stress response predict the ability of wild birds to adjust to short-term captivity? A study of the rock pigeon (Columbia livia). R. Soc. Open Sci. 2016, 3, 160840. [Google Scholar] [CrossRef]
- Caro, T. Conservation by Proxy: Indicator, Umbrella, Keystone, Flagship, and Other Surrogate Species; Island Press: Washington DC, USA, 2010. [Google Scholar]
- Bolam, F.C.; Mair, L.; Angelico, M.; Brooks, T.M.; Burgman, M.; Hermes, C.; Hoffmann, M.; Martin, R.W.; McGowan, P.J.K.; Rodrigues, A.S.L.; et al. How many bird and mammal extinctions has recent conservation action prevented? Conserv. Lett. 2020, e12762. [Google Scholar] [CrossRef]
- Convention on Biological Diversity. Aichi Biodiversity Targets. Available online: https://www.cbd.int/sp/targets/ (accessed on 15 January 2021).
- British & Irish Association of Zoos & Aquariums. Our Association. Available online: https://biaza.org.uk/our-association (accessed on 15 January 2021).
- European Association of Zoos & Aquaria. About Us. Available online: https://www.eaza.net/about-us/ (accessed on 15 January 2021).
- Association of Zoos & Aquariums. About AZA Accreditation. Available online: https://www.aza.org/what-is-accreditation?locale=en (accessed on 15 January 2021).
- Soriano Redondo, A. Reintroduction Ecology of the Eurasian Crane Grus Grus. Ph.D. Thesis, University of Exeter, Exeter, UK, 2017. Available online: https://ore.exeter.ac.uk/repository/handle/10871/28381 (accessed on 15 January 2021).
- Pain, D.; Hughes, B.; Syroechkovskiy, E.; Zöckler, C.; Chowdhury, S.; Anderson, G.; Clark, N. Saving the spoon-billed sandpiper: A conservation update. Br. Birds 2018, 111, 323–333. [Google Scholar]
- Low, R. The breeding centre of Loro Parque Foundation. AFA Watchb. 2005, 32, 25–31. [Google Scholar]
- San Diego Zoo Safari Park. San Diego Zoo Safari Park Condor Cam. Available online: https://www.sdzsafaripark.org/condor-cam (accessed on 15 January 2021).
- Burghardt, T.; Thomas, B.; Barham, P.J.; Calic, J. Automated visual recognition of individual african penguins. In Proceedings of the Fifth International Penguin Conference, Ushuaia, Argentina, 6–10 September 2004. [Google Scholar]
- Nuijten, R.; Prins, E.F.; Lammers, J.; Mager, C.; Nolet, B.A. Calibrating tri-axial accelerometers for remote behavioural observations in Bewick’s swans. J. Anim. Ecol. 2020, 8, 231–238. [Google Scholar]
- King, C.E. Captive flamingo populations and opportunities for research in zoos. Waterbirds 2000, 23, 142–149. [Google Scholar] [CrossRef]
- King, C.E. The potential contribution of captive flamingos to research. Flamingo Bull. Flamingo Spec. Group 2008, 16, 61–64. [Google Scholar]
- Rose, P.E. The relevance of captive flamingos to meeting the four aims of the modern zoo. Flamingo 2018, e1, 23–32. [Google Scholar]
- BirdLife International. Rhinoplax Vigil (Amended Version of 2018 Assessment). Available online: https://dx.doi.org/10.2305/IUCN.UK.2019-3.RLTS.T22682464A155467793.en (accessed on 15 January 2021).
- Savini, T.; Kanwatanakid-Savini, C. Feeding overlap and seed dispersal efficiency between sympatric hornbills and gibbons in Thailand. Raffles Bull. Zool. 2011, 24, 115–122. [Google Scholar]
- Kitamura, S.; Thong-Aree, S.; Madsri, S.; Poonswad, P. Characteristics of hornbill-dispersed fruits in lowland dipterocarp forests of southern Thailand. Raffles Bull. Zool. 2011, 24, 137–147. [Google Scholar]
- Beastall, C.; Shepherd, C.R.; Hadiprakarsa, Y.; Martyr, D. Trade in the helmeted hornbill Rhinoplax vigil: The ‘ivory hornbill’. Bird Conserv. Int. 2016, 26, 137–146. [Google Scholar] [CrossRef]
- BirdLife International Asia. The Helmeted Hornbill Crisis and BirdLife’s Conservation Efforts. Available online: https://www.birdlife.org/asia/projects/helmeted-hornbill (accessed on 15 January 2021).
- BirdLife International. How We’re Helping the Helmeted Hornbill to Nest in Safety Again. Available online: https://www.birdlife.org/worldwide/news/how-were-helping-helmeted-hornbill-nest-safety-again (accessed on 15 January 2021).
- Fauna & Flora Interrnational. Helmeted Hornbill. Available online: https://www.fauna-flora.org/species/helmeted-hornbill (accessed on 15 January 2021).
- BirdLife International. Buceros Rhinoceros. Available online: https://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22682450A132376232.en (accessed on 15 January 2021).
- BirdLife International. World First: Rhinoceros Hornbills Give Artificial Nest Box Seal of Approval. Available online: https://www.birdlife.org/worldwide/news/world-first-rhinoceros-hornbills-give-artificial-nest-box-seal-approval (accessed on 15 January 2021).
- Kaur, R. Conservation Leadership Programme: Final Report. 3276 the Conservation of Bornean Hornbills in Malaysia. Conservation Leadership Programme; 2019. pp. 1–12. Available online: https://www.conservationleadershipprogramme.org/media/2019/10/CLP15032019FinalReportBorneoHornbills.pdf (accessed on 15 January 2021).
- Crofoot, M.; Mace, M.; Azua, J.; MacDonald, E.; Czekala, N.M. Reproductive assessment of the great hornbill (Buceros bicornis) by fecal hormone analysis. Zoo Biol. 2003, 22, 135–145. [Google Scholar] [CrossRef]
- Kesler, D.C.; Haig, S.M. Thermal characteristics of wild and captive Micronesian kingfisher nesting habitats. Zoo Biol. 2004, 23, 301–308. [Google Scholar] [CrossRef]
- BirdLife International. Todiramphus Cinnamominus (Amended Version of 2016 Assessment). Available online: https://dx.doi.org/10.2305/IUCN.UK.2017-3.RLTS.T22725862A117372355.en (accessed on 15 January 2021).
- Rose, P.E.; Croft, D.P.; Lee, R. A review of captive flamingo (Phoenicopteridae) welfare: A synthesis of current knowledge and future directions. Int. Zoo Yearb. 2014, 48, 139–155. [Google Scholar] [CrossRef]
- BirdLife International. Bucorvus Leadbeateri. Available online: https://www.iucnredlist.org/species/22682638/92955067 (accessed on 15 January 2021).
- Kemp, L.V.; Kotze, A.; Jansen, R.; Dalton, D.L.; Grobler, P.; Little, R.M. Review of trial reintroductions of the long-lived, cooperative breeding Southern Ground-hornbill. Bird Conserv. Int. 2020, 1–26. [Google Scholar] [CrossRef]
- Jordan, M. Southern Ground Hornbill (Bucorvus Leadbeateri) Species Recovery Plan for South Africa; Conservation Breeding Specialist Group (SSC/IUCN): Johannesburg Zoo, South Africa, 2011. [Google Scholar]
- Shito, P.; Karimanzira, A.; Kemp, L.V.; Mundava, J.; Nkomo, M.N.; Pierini, J. (Eds.) Conservation Strategy and Action Plan 2020–2021: Southern Ground Hornbill, Bucorvus Leadbeateri; IUCN SSC Conservation Specialist Group: Gland, Switzerland, 2020; Available online: http://www.cbsg.org/sites/cbsg.org/files/documents/Southern_Ground_Hornbill_Bucorvus_Leadbeateri_Species_Conservation_Strategy_Action_Plan_Zimbabwe_2020_2021.pdf (accessed on 15 January 2021).
- Mason, G.J. Species differences in responses to captivity: Stress, welfare and the comparative method. Trends Ecol. Evol. 2010, 25, 713–721. [Google Scholar] [CrossRef]
- Clubb, R.; Mason, G.J. Natural behavioural biology as a risk factor in carnivore welfare: How analysing species differences could help zoos improve enclosures. Appl. Anim. Behav. Sci. 2007, 102, 303–328. [Google Scholar] [CrossRef]
- Kroshko, J.; Clubb, R.; Harper, L.; Mellor, E.; Moehrenschlager, A.; Mason, G. Stereotypic route tracing in captive Carnivora is predicted by species-typical home range sizes and hunting styles. Anim. Behav. 2016, 117, 197–209. [Google Scholar] [CrossRef]
- Prokop, P.; Fančovičová, J. Does colour matter? The influence of animal warning coloration on human emotions and willingness to protect them. Anim. Conserv. 2013, 16, 458–466. [Google Scholar] [CrossRef]
- Correia, R.A.; Jepson, P.R.; Malhado, A.C.M.; Ladle, R.J. Familiarity breeds content: Assessing bird species popularity with culturomics. PeerJ 2016, 4, e1728. [Google Scholar] [CrossRef] [PubMed]
- Kear, J. Man and Wildfowl; A&C Black: London, UK, 1990. [Google Scholar]
- Lin, H.-Y.; Schuster, R.; Wilson, S.; Cooke, S.J.; Rodewald, A.D.; Bennett, J.R. Integrating season-specific needs of migratory and resident birds in conservation planning. Biol. Conserv. 2020, 252, 108826. [Google Scholar] [CrossRef]
- Yong, D.L.; Jain, A.; Liu, Y.; Iqbal, M.; Choi, C.Y.; Crockford, N.J.; Millington, S.; Provencher, J. Challenges and opportunities for transboundary conservation of migratory birds in the East Asian-Australasian flyway. Conserv. Biol. 2018, 32, 740–743. [Google Scholar] [CrossRef]
- Smithsonian’s National Zoo & Conservation Biology Institute. Bird House Exhibit. Available online: https://nationalzoo.si.edu/animals/exhibits/bird-house-0 (accessed on 15 January 2021).
- Smithsonian’s National Zoo & Conservation Biology Institute. Bird House Preview. Available online: https://nationalzoo.si.edu/animals/bird-house-preview (accessed on 15 January 2021).
- European Association of Zoos & Aquaria. The Modern Zoo: Foundations for Management and Development; EAZA: Amsterdam, The Netherlands, 2013; Available online: https://www.eaza.net/assets/Uploads/images/Membership-docs-and-images/Zoo-Management-Manual-compressed.pdf (accessed on 15 January 2021).
- Wildfowl & Wetlands Trust. Saving the Madagascar Pochard. Available online: https://www.wwt.org.uk/our-work/projects/saving-the-madagascar-pochard/ (accessed on 15 January 2021).
- Wildfowl & Wetlands Trust. Baer’s Pochard Conservation. Available online: https://www.wwt.org.uk/our-work/projects/baers-pochard-conservation/ (accessed on 15 January 2021).
- Kerr, K.C.R. Zoo animals as “proxy species” for threatened sister taxa: Defining a novel form of species surrogacy. Zoo Biol. 2020. [Google Scholar] [CrossRef]
- Brereton, J.E.; Rose, P.E. Comparing behaviour of wild and captive flamingos: An evaluation of published data on time-activity budgets. Flamingo 2019, e2, 34–49. [Google Scholar]
- Stamp Dawkins, M. Time budgets in red junglefowl as a baseline for the assessment of welfare in domestic fowl. Appl. Anim. Behav. Sci. 1989, 24, 77–80. [Google Scholar] [CrossRef]
- Hosey, G.R. How does the zoo environment affect the behaviour of captive primates? Appl. Anim. Behav. Sci. 2005, 90, 107–129. [Google Scholar] [CrossRef]
- Howell, C.P.; Cheyne, S.M. Complexities of using wild versus captive activity budget comparisons for assessing captive primate welfare. J. Appl. Anim. Welf. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.E.; Croft, D.P. Evaluating the social networks of four flocks of captive flamingos over a five-year period: Temporal, environmental, group and health influences on assortment. Behav. Process. 2020. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.E.; Soole, L. What influences aggression and foraging activity in social birds? Measuring individual, group and environmental characteristics. Ethology 2020. [Google Scholar] [CrossRef]
- Rose, P.E.; Brereton, J.E.; Croft, D.P. Measuring welfare in captive flamingos: Activity patterns and exhibit usage in zoo-housed birds. Appl. Anim. Behav. Sci. 2018, 205, 115–125. [Google Scholar] [CrossRef]
- Carr, N. Ideal animals and animal traits for zoos: General public perspectives. Tour. Manag. 2016, 57, 37–44. [Google Scholar] [CrossRef]
- Carr, N. An analysis of zoo visitors’ favourite and least favourite animals. Tour. Manag. Perspect. 2016, 20, 70–76. [Google Scholar] [CrossRef]
- Albert, C.; Luque, G.M.; Courchamp, F. The twenty most charismatic species. PLoS ONE 2018, 13, e0199149. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.; Bicknell, J.; Sickler, J.; Taylor, A. What information do zoo & aquarium visitors want on animal identification labels? J. Interpret. Res. 2009, 14, 7–18. [Google Scholar] [CrossRef]
- BirdLife International. Phoeniconaias Minor. Available online: http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22697369A129912906.en (accessed on 15 January 2021).
- Apa, A.D.; Wiechman, L.A. Captive-breeding of captive and wild-reared Gunnison sage-grouse. Zoo Biol. 2016, 35, 70–75. [Google Scholar] [CrossRef]
- Earnhardt, J.M.; Thompson, S.D.; Faust, L.J. Extinction risk assessment for the species survival plan (SSP®) population of the Bali mynah (Leucopsar rothschildi). Zoo Biol. 2009, 28, 230–252. [Google Scholar] [CrossRef]
- Earnhardt, J.M.; Vélez-Valentín, J.; Valentin, R.; Long, S.; Lynch, C.; Schowe, K. The Puerto Rican parrot reintroduction program: Sustainable management of the aviary population. Zoo Biol. 2014, 33, 89–98. [Google Scholar] [CrossRef]
- Sankhom, R.; Warrit, N.; Wiwegweaw, A. Screening and application of microsatellite markers for genetic diversity analysis of captive eastern sarus crane Grus antigone sharpii Blanford, 1895 in Thailand. Zoo Biol. 2018, 37, 310–319. [Google Scholar] [CrossRef]
- Ellenberg, U.; Mattern, T.; Seddon, P.J.; Jorquera, G.L. Physiological and reproductive consequences of human disturbance in Humboldt penguins: The need for species-specific visitor management. Biol. Conserv. 2006, 133, 95–106. [Google Scholar] [CrossRef]
- Sutherland, W.J.; Armstrong, D.; Butchart, S.H.M.; Earnhardt, J.M.; Ewen, J.G.; Jamieson, I.; Jones, C.G.; Lee, R.; Newbery, P.; Nichols, J.D.; et al. Standards for documenting and monitoring bird reintroduction projects. Conserv. Lett. 2010, 3, 229–235. [Google Scholar] [CrossRef]
- Collins, M.S.; Smith, T.B.; Seibels, R.E.; Putra, I.M.W.A. Approaches to the reintroduction of the Bali mynah. Zoo Biol. 1998, 17, 267–284. [Google Scholar] [CrossRef]
- Wirtz, S.; Böhm, C.; Fritz, J.; Kotrschal, K.; Veith, M.; Hochkirch, A. Optimizing the genetic management of reintroduction projects: Genetic population structure of the captive Northern Bald Ibis population. Conserv. Genet. 2018, 19, 853–864. [Google Scholar] [CrossRef]
- Burgess, M.D.; Woolcock, D.; Hales, R.B.; Waite, R.; Hales, A.J. Captive husbandry and socialization of the red-billed chough (Pyrrhocorax pyrrhocorax). Zoo Biol. 2012, 31, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Yaacob, M.N. Captive-breeding and reintroduction project for the milky stork Mycteriu cinerea: At Zoo Negara, Malaysia. Int. Zoo Yearb. 1994, 33, 39–48. [Google Scholar] [CrossRef]
- Lieberman, A.; Rodriguez, J.V.; Paez, J.M.; Wiley, J. The reintroduction of the Andean condor into Colombia, South America: 1989–1991. Oryx 1993, 27, 83–90. [Google Scholar] [CrossRef]
- Rose, P.E.; O’Brien, M. Welfare assessment for captive Anseriformes: A guide for practitioners and animal keepers. Animals 2020, 10, 1132. [Google Scholar] [CrossRef] [PubMed]
- Melfi, V.A. There are big gaps in our knowledge, and thus approach, to zoo animal welfare: A case for evidence-based zoo animal management. Zoo Biol. 2009, 28, 574–588. [Google Scholar] [CrossRef]
- Rose, P.E.; Brereton, J.E.; Gardner, L. Developing flamingo husbandry practices through workshop communication. J. Anim. Ecol. 2016, 4, 115–121. [Google Scholar]
- Trask, A.; Canessa, S.; Moehrenschlager, A.; Newland, S.; Medina, S.; Ewen, J.G. Extinct-in-the-wild species’ last stand. Science 2020, 369, 516. [Google Scholar]
- Smithsonian’s National Zoo & Conservation Biology Institute. Guam Kingfisher. Available online: https://nationalzoo.si.edu/animals/guam-kingfisher (accessed on 15 January 2021).
- Haig, S.M.; Ballou, J.D.; Casna, N.J. Genetic identification of kin in Micronesian kingfishers. J. Hered. 1995, 86, 423–431. [Google Scholar] [CrossRef]
- U.S. Fish & Wildlife Service. Final Revised Recovery Plan for the Sihek or Guam Micronesian Kingfisher (Halcyon Cinnamomina Cinnamomina); U.S. Fish & Wildlife Service: Albuquerque, NM, USA, 2008; p. 117. Available online: https://www.fws.gov/pacific/ecoservices/documents/Sihek_Revised_RP.pdf (accessed on 15 January 2021).
- Conservation Nation. Setting the Stage for Rewilding the Guam Kingfisher. Available online: https://conservationnation.org/setting-the-stage-for-rewilding-the-guam-kingfisher/ (accessed on 15 January 2021).
- Asa, C.S.; Traylor-Holzer, K.; Lacy, R.C. Can conservation-breeding programmes be improved by incorporating mate choice? Int. Zoo Yearb. 2011, 45, 203–212. [Google Scholar] [CrossRef]
- Birdlife International. Hypotaenidia Owstoni. Available online: https://dx.doi.org/10.2305/IUCN.UK.2019-3.RLTS.T22692441A156506469.en (accessed on 15 January 2021).
- Bridge, D. The UK common crane reintroduction 2010–2014. In Proceedings of the VIII European Crane Conference, Gallocanta, Spain, 10–14 November 2014; pp. 10–14. [Google Scholar]
- Mason, G.J.; Clubb, R.; Latham, N.; Vickery, S. Why and how should we use environmental enrichment to tackle stereotypic behaviour? Appl. Anim. Behav. Sci. 2007, 102, 163–188. [Google Scholar] [CrossRef]
- Rose, P.E.; Nash, S.M.; Riley, L.M. To pace or not to pace? A review of what Abnormal Repetitive Behavior tells us about zoo animal management. J. Vet. Behav. 2017, 20, 11–21. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).