Experimental Study and Thermodynamic Analysis of Carbon Dioxide Adsorption onto Activated Carbons Prepared from Biowaste Raw Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Activated Carbons from Hazelnut and Walnut Shells
2.2. Adsorptive
2.3. Textural Characteristics of the Carbon Adsorbents
2.4. The Adsorption of Carbon Dioxide onto Carbon Adsorbents Prepared from Nutshells
3. Results and Discussion
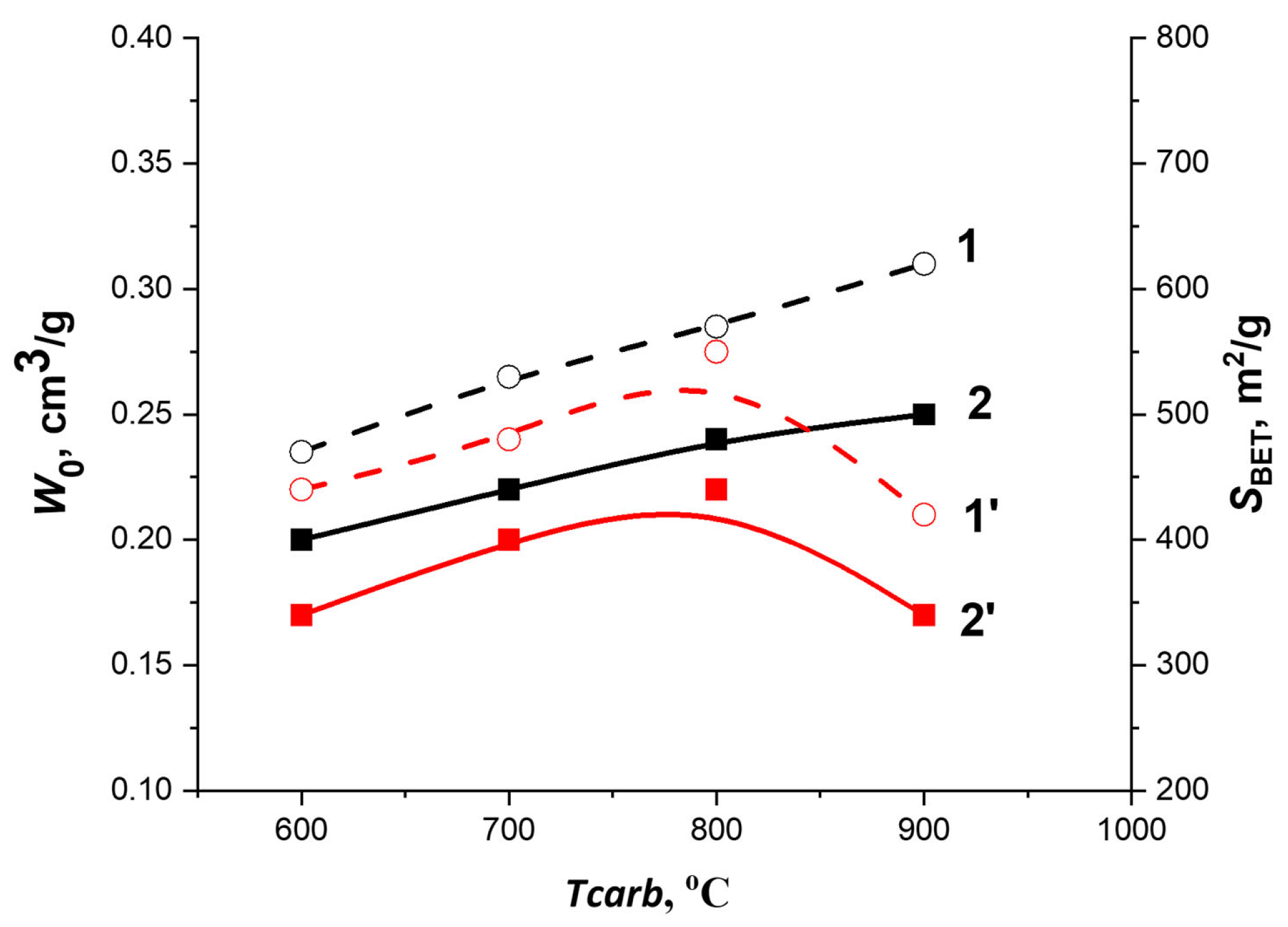
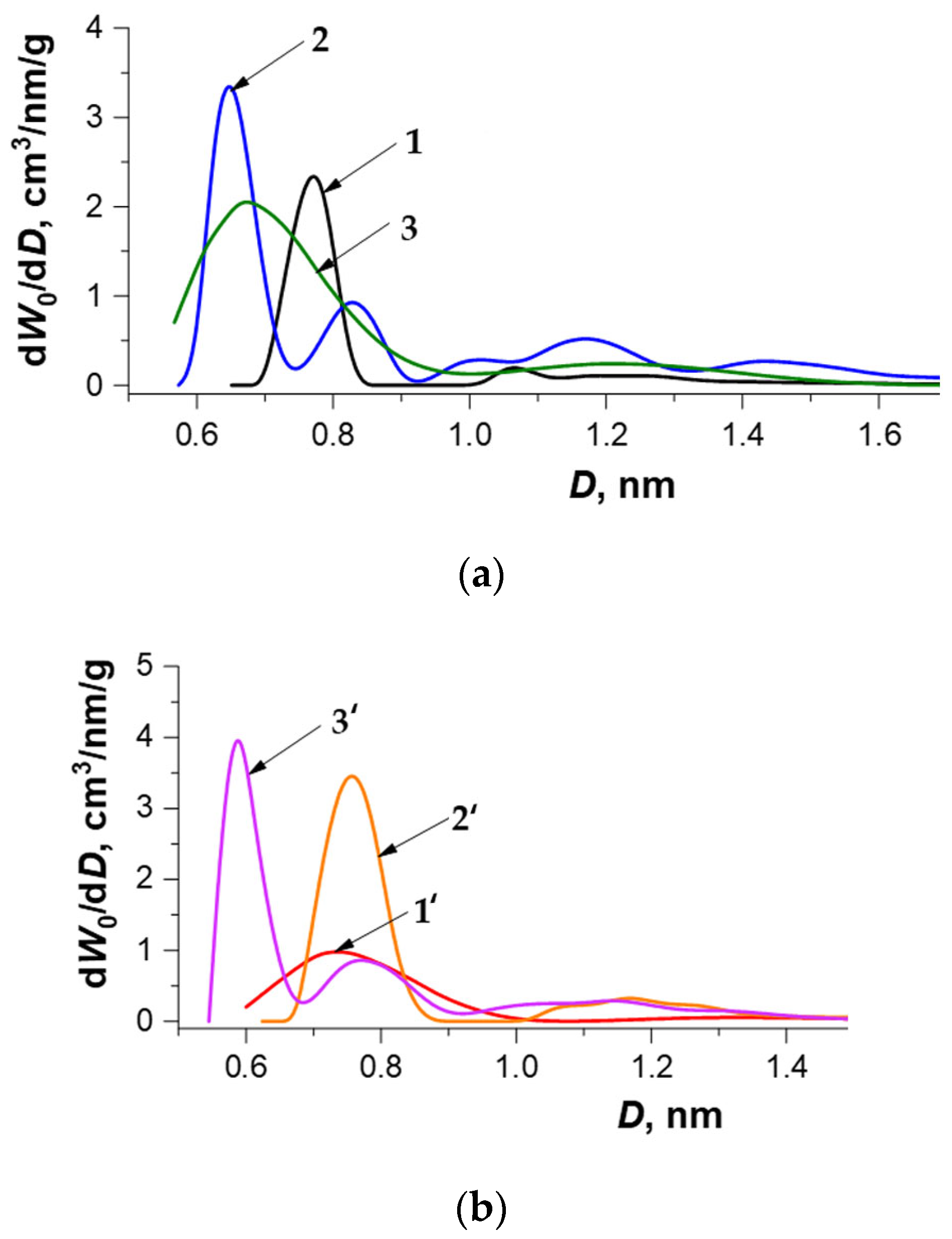
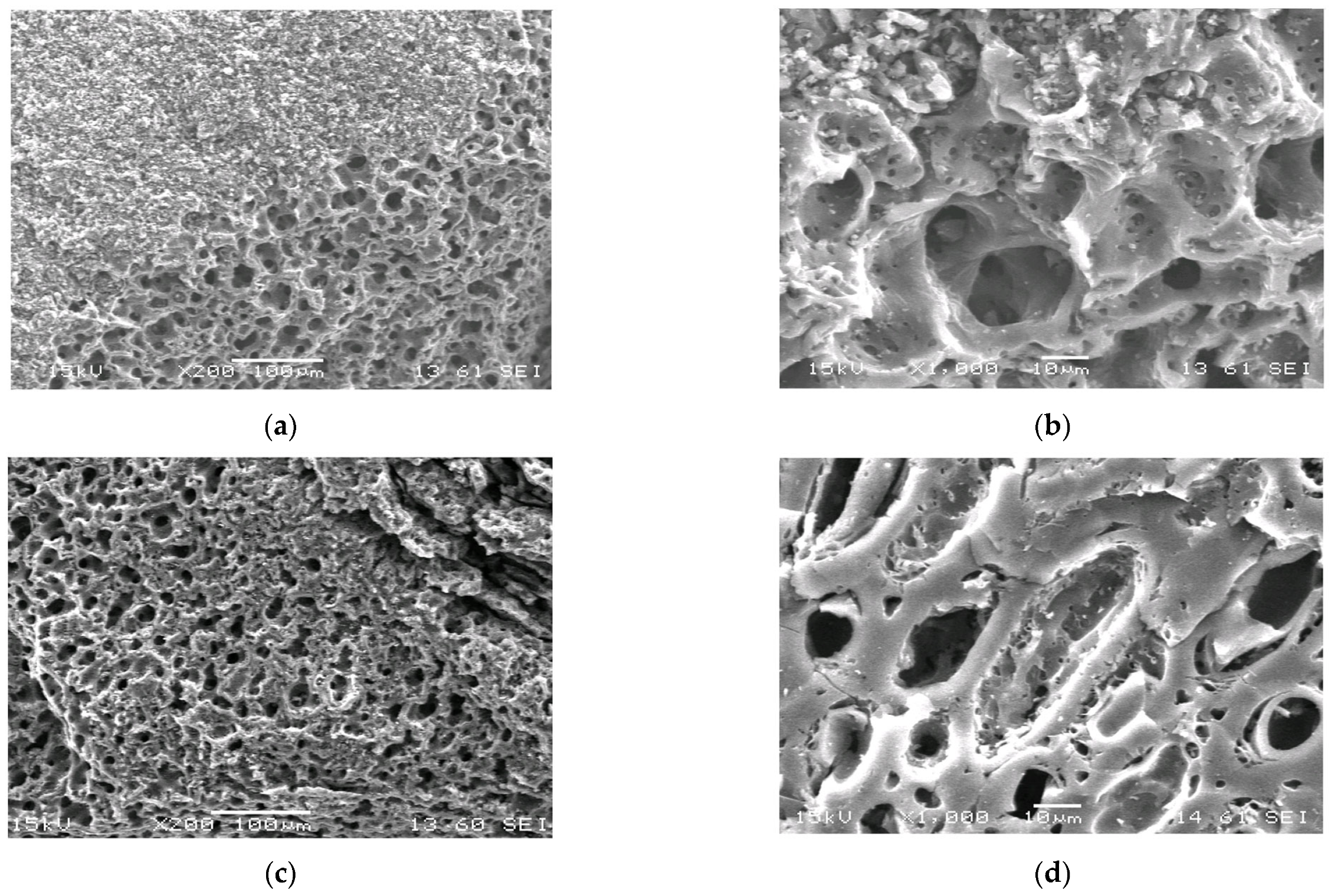
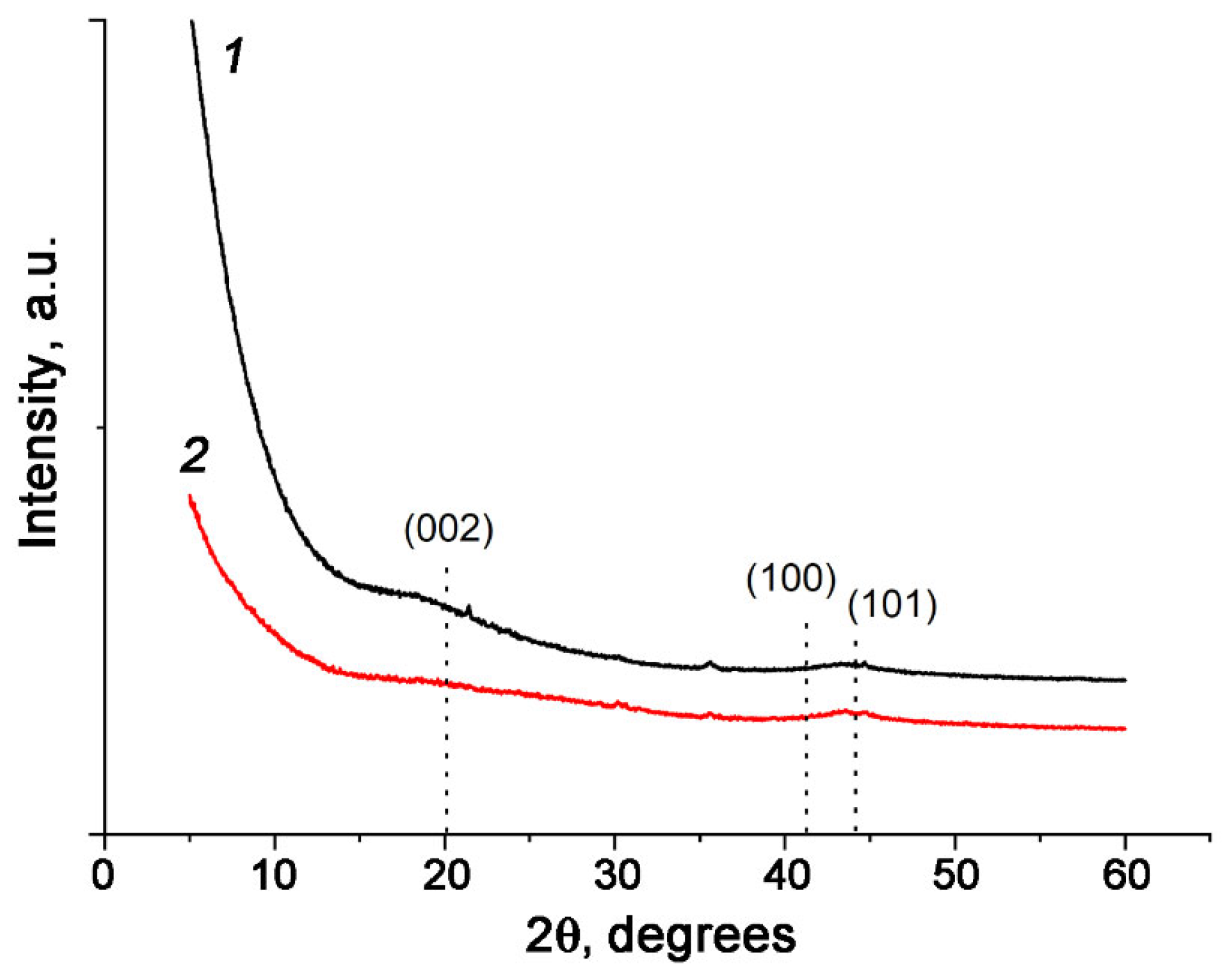
3.1. Optimal Conditions for Synthesis of Carbon Adsorbents and Characterization of Their Textural Properties
- The specific BET surface in the WNS-AC and HNS-AC series increased almost three and two times, respectively;
- The micropore volume in the WNS-AC and HNS-AC series increased by almost 2.6 and 1.9 times, respectively;
- The volume of mesopores in both series increased with increasing content of KOH and achieved a maximum value at the char/KOH ratio = 1:3;
- According to the TVFM-based calculations, the effective width of slit-like micropore increased by a factor of 1.2 for the WNS-AC series and decreased insignificantly for the HNS-AC series;
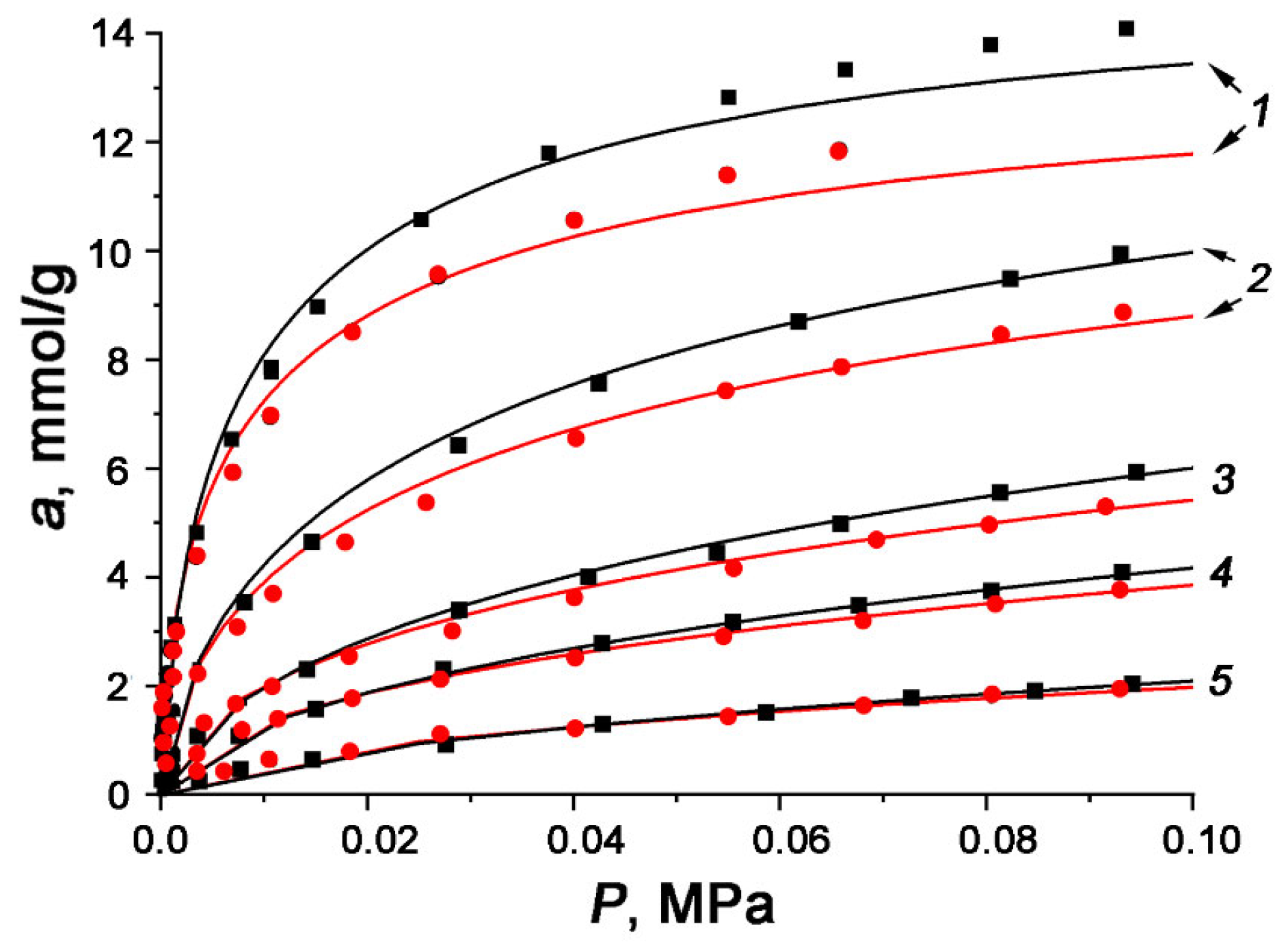
3.2. Carbon Dioxide Adsorption Properties of Nutshell-Derived Carbon Adsorbents
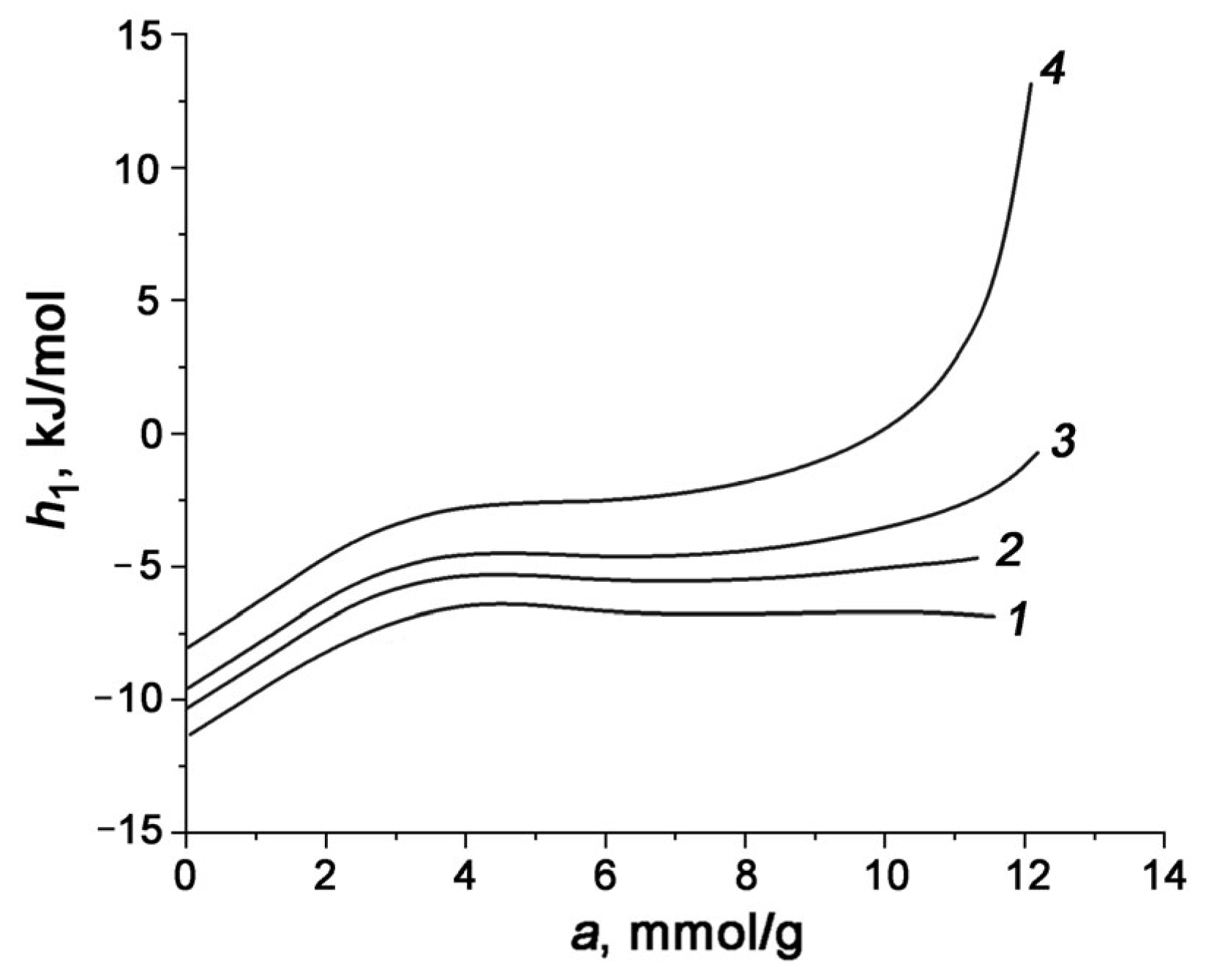
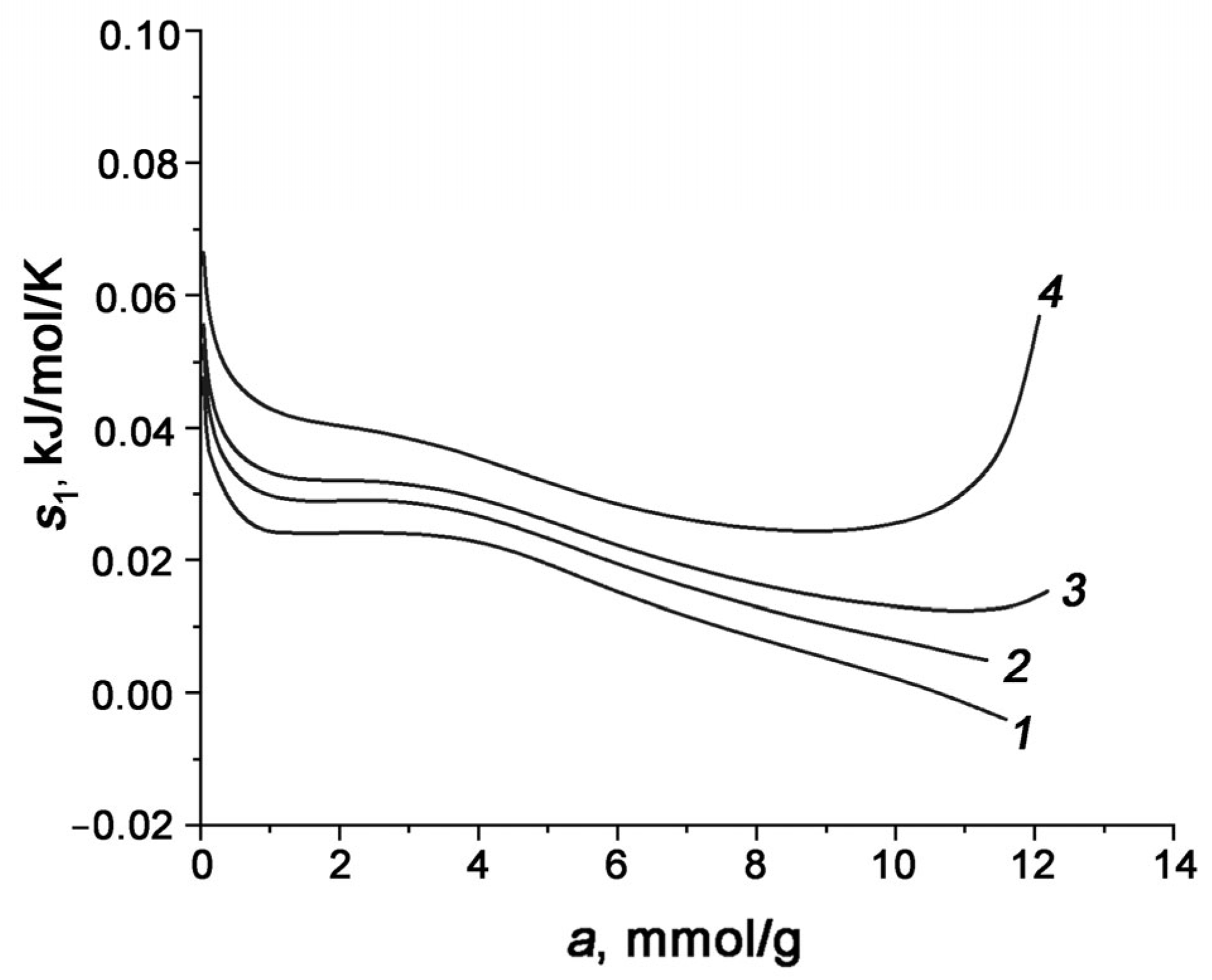
3.3. Differential Molar Isosteric Heat of CO2 Adsorption onto Carbon Adsorbents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Paris Agreement. 2015. Available online: https://www.un.org/ru/climatechange/paris-agreement (accessed on 20 December 2022).
- Cuéllar-Franca, R.M.; Azapagic, A.J. Carbon capture, storage and utilization technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sust. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Nanda, S.; Reddy, S.N.; Mitra, S.K.; Kozinski, J.A. The progressive routes for carbon capture and sequestration. Energy Sci. Eng. 2016, 4, 99–122. [Google Scholar] [CrossRef]
- Aschenbrenner, O.; Styring, P. Comparative study of solvent properties for carbon dioxide absorption. Energy Environ. Sci. 2010, 3, 1106–1113. [Google Scholar] [CrossRef]
- Zhu, X.; Hillesheim, P.C.; Mahurin, S.M.; Wang, C.; Tian, C.; Brown, S.; Luo, H.; Veith, G.M.; Han, K.S.; Hagaman, E.W.; et al. Efficient CO2 capture by porous, nitrogen-doped carbonaceous adsorbents derived from task-specific ionic liquids. Chem. Sus. Chem. 2012, 5, 1912–1917. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Tian, C.; Mahurin, S.M.; Chai, S.-H.; Wang, C.; Brown, S.; Veith, G.M.; Luo, H.; Liu, H.; Dai, S. A superacid-catalyzed synthesis of porous membranes based on triazine frameworks for CO2 separation. J. Am. Chem. Soc. 2012, 134, 10478–10484. [Google Scholar] [CrossRef]
- Tuinier, M.J.; van Sint Annaland, M.; Kramer, G.J.; Kuipers, J.A.M. Cryogenic CO2 capture using dynamically operated packed beds. Chem. Eng. Sci. 2010, 65, 114–119. [Google Scholar] [CrossRef]
- Hinkov, I.; Lamari, F.D.; Langlois, P.; Dicko, M.; Chilev, C.; Pentchev, I. Carbon dioxide capture by adsorption (Review). J. Chem. Technol. Metall. 2016, 51, 609–626. [Google Scholar]
- Abanades, J.C.; Arias, B.; Lyngfelt, A.; Mattisson, T.; Wiley, D.E.; Li, H.; Ho, M.T.; Mangano, E.; Brandani, S. Emerging CO2 capture systems. Int. J. Greenh. Gas. Control 2015, 40, 126–166. [Google Scholar] [CrossRef]
- Patel, H.A.; Byun, J.; Yavuz, C.T. Carbon Dioxide Capture Adsorbents: Chemistry and Methods. Chem. Sus. Chem. 2017, 10, 1303–1317. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yang, B.; Li, A. Biomass derived porous carbon for efficient capture of carbon dioxide, organic contaminants and volatile iodine with exceptionally high uptake. Chem. Eng. J. 2019, 372, 65–73. [Google Scholar] [CrossRef]
- Quan, C.; Su, R.; Gao, N. Preparation of activated biomass carbon from pine sawdust for supercapacitor and CO2 capture. Int. J. Energy Res. 2020, 14, 4335–4351. [Google Scholar] [CrossRef]
- Serafin, J.; Dziejarski, B.; Cruz Junior, O.F.; Srénscek-Nazzal, J. Design of highly microporous activated carbons based on walnut shell biomass for H2 and CO2 storage. Carbon 2023, 201, 633–647. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Hu, X.; Pastuszyński, P. Analysis of the Influence of Activated Carbons’ Production Conditions on the Porous Structure Formation on the Basis of Carbon Dioxide Adsorption Isotherms. Materials 2022, 15, 7939. [Google Scholar] [CrossRef] [PubMed]
- Siriwardane, R.V.; Shen, M.-S.; Fisher, E.P.; Losch, J. Adsorption of CO2 on zeolites at moderate temperatures. Energy Fuels 2005, 19, 1153–1159. [Google Scholar] [CrossRef]
- Vidoni, A.; Ravikovitch, P.I.; Afeworki, M.; Calabro, D.; Deckman, H.; Ruthven, D. Adsorption of CO2 on high silica MFI and DDR zeolites: Structural defects and differences between adsorbent samples. Microporous Mesoporous Mater. 2020, 294, 109818. [Google Scholar] [CrossRef]
- Younas, M.; Rezakazemi, M.; Daud, M.; Wazir, M.B.; Ahmad, S.; Inamuddin, N.U.; Ramakrishna, S. Recent progress and remaining challenges in postcombustion CO2 capture using metal-organic frameworks (MOFs). Prog. Energy Combust. Sci. 2020, 80, 100849. [Google Scholar] [CrossRef]
- Quan, C.; Wang, H.; Jia, X.; Gao, N.J. Effect of carbonization temperature on CO2 adsorption behavior of activated coal char. Energy Inst. 2021, 97, 92–99. [Google Scholar] [CrossRef]
- Coats, R.; Schefele, J.A.; Kingston, P.W. Adsorption Apparatus. U.S. Patent 6436175, 20 August 2002. [Google Scholar]
- Zhang, S.; Gao, N.; Quan, C.; Wang, F.; Wu, C. Autothermal CaO looping biomass gasification to increase process energy efficiency and reduce ash sintering. Fuel 2020, 277, 118199. [Google Scholar] [CrossRef]
- Ding, S.; Liu, Y. Adsorption of CO2 from flue gas by novel seaweed-based KOH activated porous biochars. Fuel 2020, 260, 116382. [Google Scholar] [CrossRef]
- Li, D.; Ma, T.; Zhang, R.; Tian, Y.; Qiao, Y. Preparation of porous carbons with high low-pressure CO2 uptake by KOH activation of rice husk char. Fuel 2015, 139, 68–70. [Google Scholar] [CrossRef]
- Mukhin, V.M.; Taranchenko, Y.F.; Gimatdinov, T.V. Technology for producing activated carbons based on compacted vegetable raw materials. Theor. Appl. Ecol. 2015, 3, 55–60. [Google Scholar]
- Savelyeva, Y.R.; Kryazhev, A.N.; Bogomolov, M.S.; Ivasenko, V.L.; Novikov, V.T. Production of activated carbon from the shell of pine nuts. Chem. Veg. Raw Mater. 2003, 4, 61–64. [Google Scholar]
- Bogaev, A.V.; Lebedev, I.A.; Karchevsky, D.F.; Berestennikov, D.A.; Vtorushina, O.O. Production of active coals from the shells of pine nuts. Polzunovsky Bull. 2013, 1, 282–284. [Google Scholar]
- Al Mesfer, M.K. Synthesis and characterization of high-performance activated carbon from walnut shell biomass for CO2 capture. Environ. Sci. Pollut. Res. 2020, 27, 15020–15028. [Google Scholar] [CrossRef]
- Liu, S.; Ma, R.; Hu, X.; Wang, L.; Wang, X.; Radosz, M.; Fan, M. CO2 adsorption on hazelnut-shell-derived nitrogen-doped porous carbons synthesized by single-step sodium amide activation. Ind. Eng. Chem. Res. 2020, 59, 7046–7053. [Google Scholar] [CrossRef]
- Laikam, K.E.; Vorob’eva, N.A.; Vysotskaya, N.A.; Demina, L.V.; Epikhina, A.V.; Zinchenko, A.P.; Kiselev, S.V.; Lennik, A.V.; Nesterov, V.N.; Novokshchenova, E.I. (Eds.) The Main Results of the All-Russian Agricultural Census of 2016; Federal State Statistics Service; Statistika Rossii: Moscow, Russia, 2016; Volume 1. Book 1. (in Russian) [Google Scholar]
- Kapica, J.; Pełech, R.; Przepiórski, J.; Morawski, A.W. Kinetics of the Adsorption of copper and lead ions from aqueous solution on to WD-ekstra activated carbon. Adsorpt. Sci. Technol. 2002, 20, 441–452. [Google Scholar] [CrossRef]
- Davidi, S.; Lashanizadegan, A.; Sharififard, H. Walnut shell activated carbon: Optimization of synthesis process, characterization and application for Zn (II) removal in batch and continuous process. Mater. Res. Express 2019, 6, 085621. [Google Scholar] [CrossRef]
- Mukhin, V.M.; Tarasov, A.V.; Klushin, V.N. Active Coals of Russia; Metallurgy: Moscow, Russia, 2000. [Google Scholar]
- Kambarova, G.B.; Sarymsakov, S. Obtaining activated carbon from walnut shells. Chem. Solid Fuels 2008, 3, 42–46. [Google Scholar]
- Deng, S.; Wei, H.; Chen, T.; Wang, B.; Huang, J.; Yu, G. Superior CO2 adsorption on pine nut shell-derived activated carbons and the effective micropores at different temperatures. Chem. Eng. J. 2014, 253, 46–54. [Google Scholar] [CrossRef]
- Nouralishahi, A.; Bahaeddini, M.; Rashidi, A.; Mahinnezhad, S.; Fazeli, A. Activated nanoporous carbon from walnut shell as a promising adsorbent for methane storage in adsorbed natural gas technology. Sci. Iran. 2019, 26, 3447–3455. [Google Scholar]
- Chomiak, K.; Gryglewicz, S.; Kierzek, K.; Machnikowski, J.J. Optimizing the properties of granular walnut-shell based KOH activated carbons for carbon dioxide adsorption. J. CO2 Util. 2017, 21, 436–443. [Google Scholar] [CrossRef]
- Mohammad-Khah, A.; Ansari, R. Activated Charcoal: Preparation, characterization and Applications: A review article. Int. J. Chem. Tech. Res. 2009, 1, 859–864. [Google Scholar]
- Bedia, J.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodriguez, J.J.; Belver, C. Review on Activated Carbons by Chemical Activation with FeCl3. J. Carbon Res. 2020, 6, 21. [Google Scholar] [CrossRef]
- Wei, H.; Deng, S.; Hu, B.; Chen, Z.; Wang, B.; Huang, J.; Yu, G. Granular bamboo-Derived activated carbon for high CO2 adsorption: The dominant role of narrow micropores. Chem. Sus. Chem. 2012, 5, 2354–2360. [Google Scholar] [CrossRef] [PubMed]
- Nor, N.M.; Chung, L.L.; Teong, L.K.; Mohamed, A.R. Synthesis of activated carbon from lignocellulosic biomass and its applications in air pollution control—A review. J. Chem. Eng. 2013, 1, 658–666. [Google Scholar]
- Reddy, M.S.B.; Ponnamma, D.; Sadasivuni, K.K.; Kumar, B.; Abdullah, A.M. Carbon dioxide adsorption based on porous materials. RSC Adv. 2021, 11, 12658–12681. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, M.A.W.; Houshmand, A.; Shamiri, A. A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Khosrowshahi, M.S.; Abdol, M.A.; Mashhadimoslem, H.; Khakpour, E.; Emrooz, H.B.M.; Sadegh Sadeghzadeh, S.; Ghaemi, A. The role of surface chemistry on CO2 adsorption in biomass-derived porous carbons by experimental results and molecular dynamics simulations. Sci. Rep. 2022, 12, 8917. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Policicchio, A.; Seredych, M.; Bandosz, T.J. Evaluation of CO2 interactions with S-doped nanoporous carbon and its composites with a reduced GO: Effect of surface features on an apparent physical adsorption mechanism. Carbon 2016, 98, 250–258. [Google Scholar] [CrossRef]
- Seema, H.; Kemp, K.C.; Le, N.H.; Park, S.-W.; Chandra, V.; Lee, J.W.; Kim, K.S. Highly selective CO2 capture by S-doped microporous carbon materials. Carbon 2014, 66, 320–326. [Google Scholar] [CrossRef]
- Ma, X.; Yang, Y.; Wu, Q.; Liu, B.; Li, D.; Chen, R.; Wang, C.; Li, H.; Zeng, Z.; Li, L. Underlying mechanism of CO2 uptake onto biomass-based porous carbons: Do adsorbents capture CO2 chiefly through narrow micropores? Fuel 2020, 282, 118727. [Google Scholar] [CrossRef]
- Presser, V.; McDonough, J.; Yeon, S.-H.; Gogotsi, Y. Effect of pore size on carbon dioxide sorption by carbide derived carbon. Energy Environ. Sci 2011, 4, 3059–3066. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, J.; Xing, W.; Xue, Q.; Yan, Z.; Zhuo, S.; Qiao, S.Z. Critical role of small micropores in high CO2 uptake. Phys. Chem. Chem. Phys. 2013, 15, 2523–2529. [Google Scholar] [CrossRef]
- Casco, M.E.; Martinez-Escandell, M.; Silvestre-Albero, J.; Rodriguez-Reinoso, F. Effect of the porous structure in carbon materials for CO2 capture at atmospheric and high-pressure. Carbon 2014, 67, 230–235. [Google Scholar] [CrossRef]
- Yakovlev, V.Y.; Shkolin, A.V.; Fomkin, A.A.; Gorelikov, V.N.; Men’shchikov, I.E. Adsorption of Carbon Dioxide onto Model Carbon Structures with Slit-like Micropores. Prot. Met. Phys. Chem. Surf. 2021, 57, 1105–1114. [Google Scholar] [CrossRef]
- Lee, J.H.; Kwack, K.; Lee, H.J.; Lim, S.Y.; Jung, D.S.; Jung, Y.; Choi, J.W. Optimal activation of porous carbon for high performance CO2 capture. Chem. Nano Mat. 2016, 2, 528–533. [Google Scholar]
- Dubinin, M.M. Physical Adsorption of Gases and Vapors in Micropores. In Progress Surface Membrane Science, 1st ed.; Cadenhead, D.A., Danielli, J.F., Rosenberg, M.D., Eds.; Academic Press: New York, NY, USA, 1975; Volume 9, pp. 1–70. [Google Scholar]
- Dubinin, M.M. Fundamentals of the theory of adsorption in micropores of carbon adsorbents: Characteristics of their adsorption properties and microporous structures. Carbon 1989, 27, 457–467. [Google Scholar] [CrossRef]
- Hill, T.L. Theory of physical adsorption. Adv Catal. 1952, 4, 211–258. [Google Scholar]
- Myers, A.L. Thermodynamics of Adsorption. AIChE J. 2002, 48, 145–160. [Google Scholar] [CrossRef]
- Du, X.; Wu, T.; Hou, Z.; Liu, Z.; Huo, L.; Hao, Y.; Zhao, Y. Adsorption equilibrium and thermodynamic analysis of CO2 and CH4 on Quinshui Basin Anthracite. Geofluids 2019, 2019, 8268050. [Google Scholar] [CrossRef]
- Singh, V.K.; Kumar, E.A. Estimation of Thermodynamic Properties of CO2 Adsorption on Activated Carbon, Isotherm and Thermodynamic Analysis of Carbon Dioxide on Activated Carbon. ICAER 2017, Conference Paper. Available online: https://www.researchgate.net/publication/321824663_Estimation_of_Thermodynamic_Properties_of_CO2_Adsorption_on_Activated_Carbon/citations#fullTextFileContent (accessed on 23 April 2023).
- Singh, V.K.; Kumar, E.A. Experimental investigation and thermodynamic analysis of CO2 adsorption on activated carbons for cooling system. J. CO2 Util. 2017, 17, 290–304. [Google Scholar] [CrossRef]
- Calbry-Muzyka, A.S.; Edwards, C.F. Thermodynamic benchmarking of CO2 capture systems: Exergy analysis methodology for adsorption processes. Energy Procedia 2014, 63, 1–17. [Google Scholar] [CrossRef]
- Men’shchikov, I.E.; Fomkin, A.A.; Romanov, Y.A.; Kiselev, M.R.; Pulin, A.L.; Chugaev, S.S.; Shkolin, A.V. Carbon Nanoporous Adsorbents Prepared from Walnut Shell for Liquefied Natural Gas Vapor Recovery in Cryogenic Storage Systems. Prot. Met. Phys. Chem. Surf. 2020, 56, 1122–1133. [Google Scholar] [CrossRef]
- Bell, I.H.; Wronski, J.; Quoilin, S.; Lemort, V. Pure and pseudo-pure fluid thermophysical property evaluation and the open-source thermophysical property library CoolProp. Ind. Eng. Chem. Res. 2014, 53, 2498–2508. [Google Scholar] [CrossRef]
- Poling, B.E.; Prausnitz, J.M.; O’Connell, J.P. The Properties of Gases and Liquids; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Rouquerol, J.; Llewellyn, P.; Rouquerol, F. Is the BET equation applicable to microporous adsorbents? Stud. Surf. Sci. Catal. 2007, 160, 49–56. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Oliver, J.P.; Rodrigues-Reinoso, F.; Rouquerol, J.; Sing, K. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity; Academic Press: London, UK; New York, NY, USA, 1982. [Google Scholar]
- Neimark, A.V.; Lin, Y.; Ravikovitch, P.I.; Thommes, M. Quenched solid density functional theory and pore size analysis of micro-mesoporous carbons. Carbon 2009, 47, 1617–1628. [Google Scholar] [CrossRef]
- Shkolin, A.V.; Fomkin, A.A. Measurement of Carbon-Nanotube Adsorption of Energy-Carrier Gases for Alternative Energy Systems. Meas. Tech. 2018, 61, 395–401. [Google Scholar] [CrossRef]
- Shkolin, A.V.; Fomkin, A.A.; Men’shchikov, I.E.; Kharitonov, V.M.; Pulin, A.L. Bench for Measuring the Adsorption of Gases and Vapors by the Gravimetric Method and the Method of Its Operational Use. R.U. Patent No. 2732199, 14 September 2020. [Google Scholar]
- Nikolaev, K.M.; Dubinin, M.M. Concerning adsorption properties of carbon adsorbents 3. A study of adsorption isotherms of gases and vapors on active carbons over a wide interval of temperatures, including the critical region. Russ. Chem. Bull. 1958, 7, 1124–1133. [Google Scholar] [CrossRef]
- Men’shchikov, I.E.; Fomkin, A.A.; Arabei, A.B.; Shkolin, A.V.; Strizhenov, E.M. Description of methane adsorption on microporous carbon adsorbents on the range of supercritical temperatures on the basis of the Dubinin–Astakhov equation. Prot. Met. Phys. Chem. Surf. 2016, 52, 575–580. [Google Scholar] [CrossRef]
- Sugden, S. The Parachor and Valency; G. Routledge: London, UK, 1930. [Google Scholar]
- Antreich, S.J.; Huss, J.C.; Xiao, N.X.; Singh, A.; Gierlinger, N. The walnut shell network: 3D visualisation of symplastic and apoplastic transport routes in sclerenchyma tissue. Planta 2022, 256, 49. [Google Scholar] [CrossRef]
- Kaya, N.; Yildiz, Z.; Ceylan, S. Preparation and Characterisation of Biochar from Hazelnut Shell and Its Adsorption Properties for Methylene Blue Dye. J. Polytech. 2018, 21, 765–776. [Google Scholar] [CrossRef]
- Gomez-Delgado, E.; Nunell, G.; Cukierman, A.L.; Bonelli, P. Tailoring activated carbons from Pinus canariensis cones for post-combustion CO2 capture. Environ. Sci. Pollut. Res. 2020, 27, 13915–13929. [Google Scholar] [CrossRef]
- Ayala, J.; Fernández, B. Removal of zinc, cadmium and nickel from mining waste leachate using walnut shells. Environ. Prot. Eng. 2019, 45, 141–158. [Google Scholar] [CrossRef]
- Khodadadi, B. Hazelnut shell as a valuable bio-waste support for green synthesis of Ag NPs using Origanum vulgare leaf extract: Catalytic activity for reduction of methyl orange and Congo red. Iran J. Catal. 2017, 7, 111–119. [Google Scholar]
- Lillo-Ródenas, M.A.; Cazorla-Amorós, D.; Linares-Solano, A. Understanding chemical reactions between carbons and NaOH and KOH: An insight into the chemical activation mechanism. Carbon 2003, 41, 267–275. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-based supercapacitors produced by activation of graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Fomkin, A.A. Adsorption of gases, vapors and liquids by microporous adsorbents. Adsorption 2005, 11, 425–436. [Google Scholar] [CrossRef]
- Vargas, D.P.; Giraldo, L.; Erto, A.; Moreno-Pirajan, J.C. Chemical modification of activated carbon monoliths for CO2 adsorption. J. Therm. Anal. Calorim. 2013, 114, 1039–1047. [Google Scholar] [CrossRef]
- Nelson, K.M.; Mahurin, S.M.; Mayes, R.T.; Williamson, B.; Teague, C.M.; Binder, A.J.; Baggetto, L.; Veith, G.M.; Dai, S. Preparation and CO2 adsorption properties of soft-templated mesoporous carbons derived from chestnut tannin precursors. Microporous Mesoporous Mater. 2016, 222, 94–103. [Google Scholar] [CrossRef]
- Li, M.; Xiao, R. Preparation of a dual Pore Structure Activated Carbon from Rice Husk Char as an Adsorbent for CO2 Capture. Fuel Process Technol. 2019, 186, 35–39. [Google Scholar] [CrossRef]
- Goskula, S.; Siliveri, S.; Gujjula, S.R.; Chirra, S.; Adepu, A.K.; Narayanan, V. Sustainable Micro Porous Activated Carbons Synthesis from Gum Arabic Tree Seed Shell Using KOH as an Activation Agent and Their CO2 Capture. Available online: https://ssrn.com/abstract=4231053 (accessed on 20 May 2023).
- Hong, S.M.; Jang, E.; Dysart, A.D.; Pol, V.G.; Lee, K.B. CO2 Capture in the sustainable wheat-derived activated microporous carbon compartments. Sci. Rep. 2016, 6, 34590. [Google Scholar] [CrossRef]
- Alabadi, A.; Razzaque, S.; Yang, Y.; Chen, S.; Tan, B. Highly porous activated carbon materials from carbonized biomass with high CO2 capturing capacity. Chem. Eng. J. 2015, 281, 606–612. [Google Scholar] [CrossRef]
- Lewicka, K.; Polish, J. Activated carbons prepared from hazelnut shells, walnut shells and peanut shells for high CO2 adsorption. Chem. Tech. 2017, 19, 38–43. [Google Scholar] [CrossRef]
- Serafin, J.; Kiełbasa, K.; Michalkiewicz, B. The new tailored nanoporous carbons from the common polypody (Polypodium vulgare): The role of textural properties for enhanced CO2 adsorption. Chem. Eng. J. 2022, 429, 131751. [Google Scholar] [CrossRef]
- Xing, W.; Liu, C.; Zhou, Z.; Zhang, L.; Zhou, J.; Zhuo, S.; Yan, Z.; Gao, H.; Wang, G.; Qiao, S.Z. Superior CO2 uptake of N-doped activated carbon through hydrogenbonding interaction. Energy Environ. Sci. 2012, 5, 7323–7327. [Google Scholar] [CrossRef]
- Pramanik, P.; Patel, H.; Charola, S.; Neogi, S.; Maiti, S. High surface area porous carbon from cotton stalk agro-residue for CO2 adsorption and study of techno-economic viability of commercial production. J. CO2 Util. 2021, 45, 101450. [Google Scholar] [CrossRef]
- Cruz, O.F., Jr.; ·Campello-Gómez, I.; Casco, M.E.; Serafin, J.; Silvestre-Albero, J.; Martínez-Escandell, M.; Hotza, D.; Rambo, C.R. Enhanced CO2 capture by cupuassu shell-derived activated carbon with high microporous volume. Carbon Lett. 2022, 33, 727–735. [Google Scholar] [CrossRef]
- de Souza, L.K.C.; Gonçalves, A.A.S.; Queiroz, L.S.; Chaar, J.S.; da Rocha Filho, G.N.; da Costa, C.E.F. Utilization of acai stone biomass for the sustainable production of nanoporous carbon for CO2 capture. Sustain. Mater. Technol. 2020, 25, e00168. [Google Scholar] [CrossRef]
- Deng, S.; Hu, B.; Chen, T.; Wang, B.; Huang, J.; Wang, Y.; Yu, G. Activated carbons prepared from peanut shell and sunfower seed shell for high CO2 adsorption. Adsorption 2015, 21, 125–133. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Hu, G.; Hu, X.; Li, Z.; Shen, S.; Radosz, M.; Fan, M. Enhanced CO2 capture capacity of nitrogen-doped biomass-derived porous carbons. ACS Sustain. Chem. Eng. 2016, 4, 1439–1445. [Google Scholar] [CrossRef]
- Boujibar, O.; Souikny, A.; Ghamouss, F.; Achak, O.; Dahbi, M.; Chafik, T. CO2 capture using N-containing nanoporous activated carbon obtained from argan fruit shells. J. Environ. Chem. Eng. 2018, 6, 1995–2002. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, G.; Chen, W.; Chen, Q.; Jiao, H.; Liu, L.; Wang, X.; Deng, X. Molten Salt Template Synthesis of Hierarchical Porous Nitrogen-Containing Activated Carbon Derived from Chitosan for CO2 Capture. ACS Omega 2020, 36, 23460–23467. [Google Scholar] [CrossRef]
- Serafin, J.; Narkiewicz, U.; Morawski, A.W.; Wróbel, R.J.; Michalkiewicz, B. Highly microporous activated carbons from biomass for CO2 capture and effective micropores at different conditions. J. CO2 Util. 2017, 18, 73–79. [Google Scholar] [CrossRef]
- Gross, K.J.; Carrington, K.R.; Barcelo, S.; Karkamkar, A.; Purewal, J.; Ma, S.; Zhou, H.-C.; Dantzer, P.; Ott, K.; Burrell, T.; et al. Recommended Best Practices for the Characterization of Storage Properties of Hydrogen Storage Materials, US Department of Energy Hydrogen Program. 2012. Available online: http://www1.eere.energy.gov/hydrogenandfuelcells/pdfs/best_practices_hydrogen_storage.pdf (accessed on 30 November 2021).
- Tsivadze AYu Aksyutin, O.E.; Ishkov, A.G.; Marina KKnyazeva, M.K.; Solovtsova, O.V.; Men’shchikov, I.E.; Fomkin, A.A.; Shkolin, A.A.; Khozina, E.V.; Grachev, V.A. Metal-organic framework structures: Adsorbents for natural gas storage. Russ. Chem. Rev. 2019, 88, 925–978. [Google Scholar] [CrossRef]
- Madden, D.G.; O’Nolan, D.; Rampal, N.; Babu, R.; Çamur, C.; Al Shakhs, A.N.; Zhang, S.Y.; Rance, G.A.; Perez, J.; Maria Casati, N.P.; et al. Densified HKUST-1 Monoliths as a Route to High Volumetric and Gravimetric Hydrogen Storage Capacity. J. Am. Chem. Soc. 2022, 144, 13729–13739. [Google Scholar] [CrossRef]
- Bakaev, V.A. One possible formulation of the thermodynamics of sorption equilibrium. Bull. Acad. Sci. USSR Div. Chem. Sci. 1971, 20, 2516–2520. [Google Scholar] [CrossRef]
- Bakaev, V.A. Molecular Theory of Physical Adsorption. Ph.D. Thesis, Moscow State University, Moscow, Russia, 1990. (In Russian). [Google Scholar]
- Shkolin, A.V.; Men’shchikov, I.E.; Khozina, E.V.; Yakovlev, V.Y.; Fomkin, A.A. Isotropic and anisotropic properties of adsorption-induced deformation of porous carbon materials. Adsorption 2022, 1–17. [Google Scholar] [CrossRef]
- Men’shchikov, I.E.; Shkolin, A.V.; Fomkin, A.A.; Khozina, E.V. Thermodynamics of methane adsorption on carbon adsorbent prepared from mineral coal. Adsorption 2021, 27, 1095–1107. [Google Scholar] [CrossRef]




| M | Tb [K] | Tcr [K] | Tλ [K] | ρcr [kg/m3] | Pcr [MPa] | dkin [nm] |
|---|---|---|---|---|---|---|
| 44.01 | 194.65 | 304.19 | 216.55 | 468 | 7.382 | 0.33 |
| Sample | Char/KOH Ratio | W0 [cm3/g] | E0 [kJ/mol] | X0 [nm] | SBET [m2/g] | Ws [cm3/g] | Wme [cm3/g] |
|---|---|---|---|---|---|---|---|
| WNS-AC series | |||||||
| WNS-AC-1 | 1:1 | 0.24 | 24.5 | 0.49 | 590 | 0.27 | 0.03 |
| WNS-AC-2 | 1:2 | 0.60 | 24.9 | 0.48 | 1510 | 0.65 | 0.05 |
| WNS-AC-3 | 1:3 | 0.62 | 23.4 | 0.61 | 1550 | 0.71 | 0.09 |
| HNS-AC series | |||||||
| HNS-AC-1 | 1:1 | 0.29 | 24.2 | 0.50 | 670 | 0.32 | 0.03 |
| HNS-AC-2 | 1:2 | 0.44 | 25.9 | 0.46 | 1030 | 0.47 | 0.03 |
| HNS-AC-3 | 1:3 | 0.54 | 24.6 | 0.49 | 1300 | 0.59 | 0.05 |
| Sample | C | O | K | Metals (Cr, Fe) |
|---|---|---|---|---|
| WNS-AC-2 | 92.83 | 6.08 | 1.09 | - |
| HNS-AC-3 | 83.69 | 9.53 | 4.59 | 2.19 |
| Carbon Source | Activation | W0 [cm3/g] | SBET [m2/g] | Adsorption Capacity [mmol/g] at P = 0.1 MPa | Ref. | |
|---|---|---|---|---|---|---|
| 273 K | 298 K | |||||
| Palm stone | Chemical (H3PO4) activation + NH3 chemical modification | 0.24 | 587 | 3.1 | n/a | [81] |
| Chestnut | Chemical (NH3) activation | ~0.22 | 561 | 3.4 | 2.3 | [82] |
| Rice husk | CO2 activation + leaching with K2CO3 | 0.34 | 1097 | n/a | 3.1 | [83] |
| Gum Arabic tree seed shell | KOH activation | 0.58 | 1472 | n/a | 3.4 | [84] |
| Wheat | KOH activation | 0.58 | 1438 | 5.7 | 3.5 | [85] |
| Starch | Dry chemical activation | n/a | 1957 | 7.5 | 3.8 | [86] |
| Hazelnut shell | KOH activation | 0.49 | 1270 | 5.6 | 3.9 (293 K) | this work |
| Hazelnut shell | KOH activation | 0.64 | 1963 | 5.0 | 4.0 | [87] |
| Fern leaves | KOH activation | 0.33 | 1593 | 4.5 | 4.1 | [88] |
| Peanut shell | KOH activation | 0.68 | 1962 | 5.4 | 4.1 | [87] |
| Bean dreg | KOH activation + N-doping | 0.43 | 1060 | n/a | 4.2 | [89] |
| Cotton stalk | KOH activation | n/a | 2695 | 6.9 | 4.2 | [90] |
| Carrot peels | KOH activation | 0.40 | 1379 | 5.6 | 4.2 | [88] |
| Walnut shells | KOH activation | 0.52 | 1468 | 4.3 | 4.3 | [87] |
| Cupuassu shell | KOH activation | 0.43 | 2221 | 7.8 | 4.4 | [91] |
| Walnut shells | KOH activation | 0.55 | 1420 | 6.2 | 4.4 (293 K) | this work |
| Bamboo | KOH activation | ~ 0.36 | ~1846 | 7.0 | 4.5 | [39] |
| Assai stone | KOH(CO2) activation | 0.86 | 3250 | 6.1 | 4.5 | [92] |
| Peanut shell | KOH activation | ~ 0.3 | 956 | 7.1 | 4.6 | [93] |
| Pine nutshell | KOH activation | n/a | n/a | 7.7 | 5.0 | [34] |
| Coconut shell | KOH + N-doping | 0.73 | 1535 | ~7.0 | 5.0 | [94] |
| Walnut shell | KOH activation | 0.94 | 1868 | 9.6 | 5.2 | [14] |
| Argan fruit shell | KOH(NaOH) activation | 1.04 | 2251 | n/a | 5.6 | [95] |
| Chitosan | Molten salt template synthesis | 0.56 | 2025 | 7.9 | 5.6 | [96] |
| Common Polypody | KOH activation | 0.92 | 1994 | 9.1 | 5.7 | [97] |
| Adsorbent | Total Capacity, [m3(NTP)/m3] | Deliverable Capacity, [m3(NTP)/m3] |
|---|---|---|
| WNS-AC-2 | 270 | 115 |
| HN-AC-3 | 240 | 110 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solovtsova, O.V.; Men’shchikov, I.E.; Shkolin, A.V.; Grinchenko, A.E.; Khozina, E.V.; Fomkin, A.A. Experimental Study and Thermodynamic Analysis of Carbon Dioxide Adsorption onto Activated Carbons Prepared from Biowaste Raw Materials. Gases 2023, 3, 112-135. https://doi.org/10.3390/gases3030008
Solovtsova OV, Men’shchikov IE, Shkolin AV, Grinchenko AE, Khozina EV, Fomkin AA. Experimental Study and Thermodynamic Analysis of Carbon Dioxide Adsorption onto Activated Carbons Prepared from Biowaste Raw Materials. Gases. 2023; 3(3):112-135. https://doi.org/10.3390/gases3030008
Chicago/Turabian StyleSolovtsova, Olga V., Ilya E. Men’shchikov, Andrey V. Shkolin, Alexander E. Grinchenko, Elena V. Khozina, and Anatoly A. Fomkin. 2023. "Experimental Study and Thermodynamic Analysis of Carbon Dioxide Adsorption onto Activated Carbons Prepared from Biowaste Raw Materials" Gases 3, no. 3: 112-135. https://doi.org/10.3390/gases3030008
APA StyleSolovtsova, O. V., Men’shchikov, I. E., Shkolin, A. V., Grinchenko, A. E., Khozina, E. V., & Fomkin, A. A. (2023). Experimental Study and Thermodynamic Analysis of Carbon Dioxide Adsorption onto Activated Carbons Prepared from Biowaste Raw Materials. Gases, 3(3), 112-135. https://doi.org/10.3390/gases3030008






