Immune Landscape of Intrahepatic Cholangiocarcinoma: Evasion and Therapeutic Insights
Abstract
1. Introduction
2. Current Immunotherapy Landscape in iCCA
3. Immunosuppressive Nature and Immune Cell Profile in iCCA
3.1. Tumor-Associated Macrophages
3.2. Tumor-Infiltrating Lymphocytes
3.2.1. CD8+ Cytotoxic T Lymphocytes
3.2.2. Natural Killer Cells
3.2.3. Regulatory T Cells
3.3. Tumor-Associated Neutrophils
3.4. Myeloid-Derived Suppressor Cells
3.5. Dendritic Cells
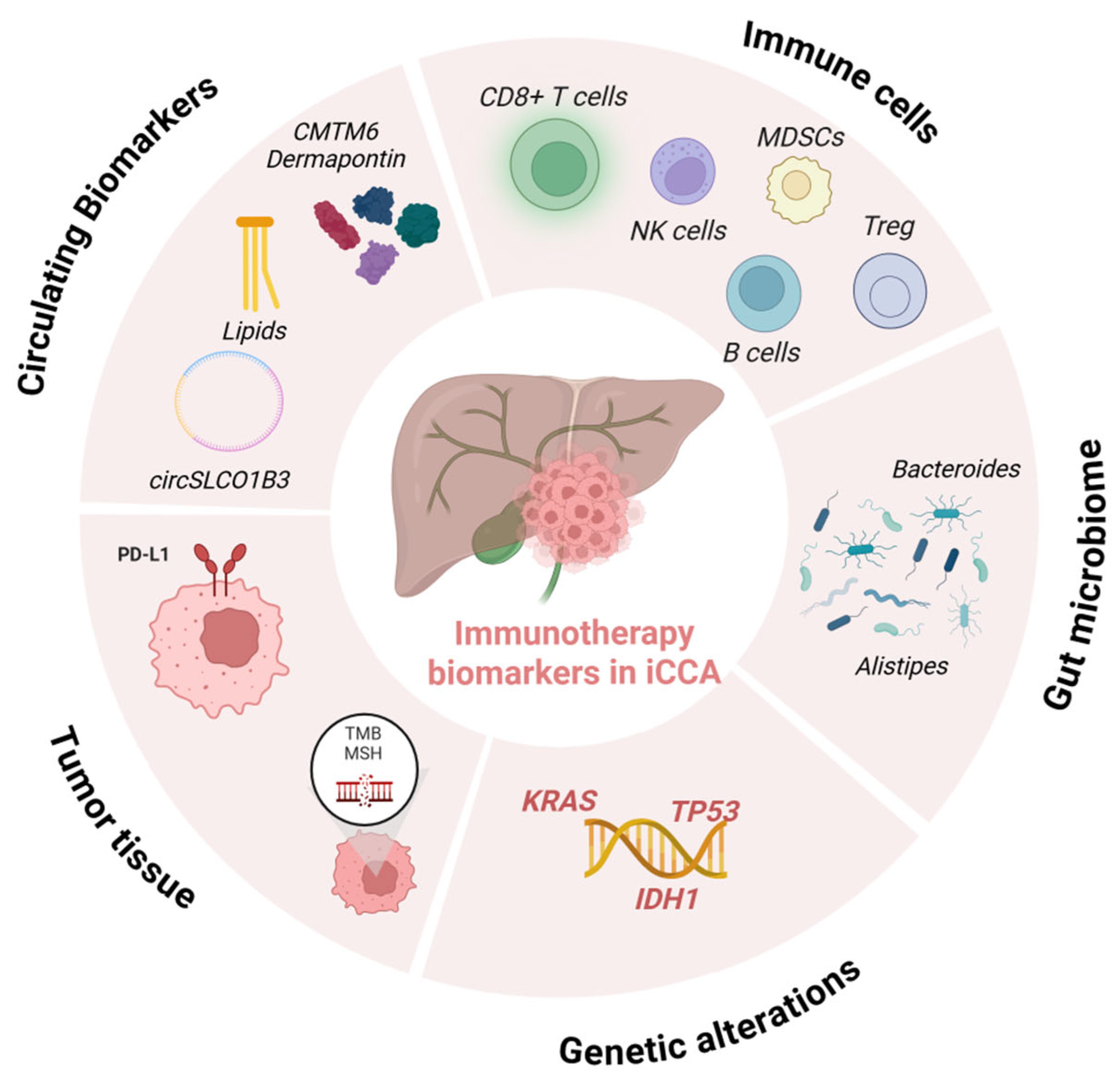
4. Immunotherapy Biomarkers in iCCA
4.1. Current Predictive Biomarkers and Their Limitations
4.2. Emerging Biomarkers for Immunotherapy Response in iCCA
4.2.1. TIME-Based Biomarkers
4.2.2. Mutation-Based Biomarkers
4.2.3. Circulating Biomarkers
4.2.4. Gut Microbiota
5. Novel Immunotherapeutic Approaches
5.1. CAR-T Therapy
5.2. Adoptive TIL Therapy
5.3. Cancer Vaccines
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ADAMTS6 | A Disintegrin and Metalloproteinase with Thrombospondin Motifs 6 |
| ADM | Adrenomedullin |
| ALOX5 | Polyunsaturated fatty acid 5-lipoxygenase |
| APOBEC | Apolipoprotein B mRNA Editing Catalytic Polypeptide-like |
| APOA1 | Apolipoprotein A1 |
| ApoE | Apolipoprotein E |
| B7-H3 | CD276 antigen |
| BCMA | B-cell Maturation Antigen |
| BDCA1 | Blood Dendritic Cell Antigen 2 |
| BTC | Biliary Tract Cancer |
| CAF(s) | Cancer-associated fibroblast(s) |
| CALCRL | Calcitonin receptor-like receptor |
| CAR-NK | Chimeric Antigen Receptor Natural Killer cells |
| CAR-T | Chimeric Antigen Receptor T-cell |
| CCA | Cholangiocarcinoma |
| CCR2/8 | C-C chemokine receptor type 2/8 |
| cDCs | Conventional Dendritic cells |
| CEA | Carcinoembryonic Antigen |
| CMTM6 | CKLF-like MARVEL transmembrane domain-containing protein 6 |
| CSF1R | Macrophage Colony-Stimulating Factor 1 receptor |
| CTL | Cytotoxic T Lymphocyte |
| CTLA-4 | Cytotoxic T-lymphocyte protein 4 |
| CXCL | C-X-C motif chemokine |
| CXCR | C-X-C chemokine receptor type |
| DC(s) | Dendritic Cell(s) |
| DCDC2 | Doublecortin domain-containing 2 |
| DPT | Dermatopontin |
| eMDSCs | Early-stage Myeloid-derived suppressor cells |
| EGFR | Epidermal Growth Factor Receptor |
| EMT | Epithelial-to-mesenchymal transition |
| ENO1 | Enolase 1 |
| FAP | Fibroblast activation protein |
| FasL | Fas ligand |
| FCR | FOXP3+/CD8+ T cell ratio |
| FGFR2 | Fibroblast Growth Factor Receptor 2 |
| FGL1 | Fibrinogen-like protein 1 |
| Flt3L | Fms-related tyrosine kinase 3 ligand |
| FOXP3 | Forkhead Box P3 |
| G-CSF | Granulocyte Colony-Stimulating Factor |
| GemCis | Gemcitabine plus Cisplatin |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| GPC3 | Glypican-3 |
| HCC | Hepatocellular Carcinoma |
| ICB | Immune Checkpoint Blockade |
| iCCA | Intrahepatic cholangiocarcinoma |
| ICI(s) | Immune Checkpoint Inhibitor(s) |
| IDH1/2 | Isocitrate Dehydrogenase 1/2 |
| IDO | Indoleamine-pyrrole 2,3-dioxygenase |
| IFN- α/γ | Interferon alpha/gamma |
| IL | Interleukin |
| KIR | Killer-cell immunoglobulin-like receptor |
| KRAS | Kirsten Rat Sarcoma Viral Oncogene Homolog |
| LAG-3 | Lymphocyte activation gene 3 |
| LGALS1 | Beta-galactoside-binding lectin L-14-I |
| LTB4 | Leukotriene B4 |
| LXR | Liver X nuclear receptor |
| LY6K | Lymphocyte Antigen 6 Complex Locus K |
| M-MDSCs | Monocytic Myeloid-derived suppressor cells |
| MDSC(s) | Myeloid-derived suppressor cell(s) |
| MEOX1 | Mesenchyme homeobox 1 |
| METTL1 | tRNA (guanine-N(7)-)-methyltransferase |
| MHC | Major Histocompatibility Complex |
| MICA/B | MHC class I polypeptide-related sequence A/B |
| MMR-d | Mismatch Repair Deficiency |
| MoDCs | Monocyte-derived Dendritic cells |
| MUC1 | Mucin 1 |
| MSI-H | Microsatellite Instability-High |
| NETs | Neutrophil Extracellular Traps |
| NK | Natural Killer |
| NKG2A/D | NK cell receptor A/D |
| NLR | Neutrophil-to-Lymphocyte Ratio |
| NSCLC | Non-Small Cell Lung Cancer |
| ORR | Objective Response Rate |
| OS | Overall Survival |
| PD-1 | Programmed Cell Death Protein 1 |
| PD-L1 | Programmed Death Ligand 1 |
| pDCs | Plasmacytoid Dendritic cells |
| PFS | Progression-Free Survival |
| PGE2 | Prostaglandin E2 |
| PMN-MDSCs | Polymorphonuclear Myeloid-derived suppressor cells |
| poly(I:C) | Polyinosinic:Polycytidylic acid |
| PORCN | Protein-serine O-palmitoleoyltransferase porcupine |
| PVR | Poliovirus receptor |
| RAMP1 | Receptor activity-modifying protein 1 |
| RFS | Relapse-Free Survival |
| SCFA | Short-Chain Fatty Acid |
| SIRPα | Signal Regulatory Protein alpha |
| SPP1 | Secreted Phosphoprotein 1 |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| TAA | Tumor-Associated Antigen |
| TAM(s) | Tumor-associated macrophage(s) |
| TAN(s) | Tumor-associated neutrophil(s) |
| Tcm | Central Memory T-cell |
| TCR(s) | T-cell receptor(s) |
| TERT | Telomerase Reverse Transcriptase |
| TFF3 | Trefoil Factor 3 |
| TGF-β | Transforming growth factor β |
| TIDE | Tumor Immune Dysfunction and Exclusion |
| TIGIT | T-cell immunoreceptor with Ig and ITIM domains |
| TIL(s) | Tumor-Infiltrating Lymphocyte(s) |
| TIM-3 | T cell Immunoglobulin Domain and Mucin Domain-3 |
| TIME | Tumor Immune Microenvironment |
| TLR4 | Toll-like receptor 4 |
| TLS | Tertiary Lymphoid Structures |
| TMB | Tumor Mutational Burden |
| TMB-H | High Tumor Mutational Burden |
| TNF-α | Tumor necrosis factor alpha |
| TP53 | Tumor protein 53 |
| TRAIL | TNF-related apoptosis-inducing ligand |
| Treg(s) | Regulatory T cell(s) |
| PRKAR1A | cAMP-dependent protein kinase type I-alpha regulatory subunit |
| TRM | Tissue-resident memory |
| TRRAP | Transformation/Transcription Domain-Associated Protein |
| VEGF | Vascular Endothelial Growth Factor |
| WT1 | Wilms Tumor 1 |
References
- Catalano:, M.; Iannone, L.F.; Nesi, G.; Nobili, S.; Mini, E.; Roviello, G. Immunotherapy-related biomarkers: Confirmations and uncertainties. Crit. Rev. Oncol. Hematol. 2023, 192, 104135. [Google Scholar] [CrossRef]
- Sussman, T.A.; Ott, P.A. Adjuvant immunotherapy for melanoma patients: Progress and opportunities. ESMO Open 2024, 9, 102962. [Google Scholar] [CrossRef]
- Reck, M.; Remon, J.; Hellmann, M.D. First-Line Immunotherapy for Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 586–597. [Google Scholar] [CrossRef]
- Voss, M.H.; Motzer, R.J. Adjuvant Immunotherapy for Kidney Cancer—A New Strategy with New Challenges. N. Engl. J. Med. 2024, 390, 1432–1433. [Google Scholar]
- Kim, J.; Maharjan, R.; Park, J. Current Trends and Innovative Approaches in Cancer Immunotherapy. AAPS PharmSciTech 2024, 25, 168. [Google Scholar] [CrossRef]
- Desai, R.; Coxon, A.T.; Dunn, G.P. Therapeutic applications of the cancer immunoediting hypothesis. Semin. Cancer Biol. 2022, 78, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Galassi, C.; Chan, T.A.; Vitale, I.; Galluzzi, L. The hallmarks of cancer immune evasion. Cancer Cell 2024, 42, 1825–1863. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Loeuillard, E.; Conboy, C.B.; Gores, G.J.; Ilyas, S.I. Immunobiology of cholangiocarcinoma. JHEP Rep. 2019, 1, 297–311. [Google Scholar] [CrossRef]
- Woo, S.R.; Corrales, L.; Gajewski, T.F. Innate immune recognition of cancer. Annu. Rev. Immunol. 2015, 33, 445–474. [Google Scholar] [CrossRef]
- Almhanna, K. Immune checkpoint inhibitors in combination with chemotherapy for patients with biliary tract cancer: What did we learn from TOPAZ-1 and KEYNOTE-966. Transl. Cancer Res. 2024, 13, 22–24. [Google Scholar] [CrossRef]
- Havel, J.J.; Chowell, D.; Chan, T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Moris, D.; Palta, M.; Kim, C.; Allen, P.J.; Morse, M.A.; Lidsky, M.E. Advances in the treatment of intrahepatic cholangiocarcinoma: An overview of the current and future therapeutic landscape for clinicians. CA Cancer J. Clin. 2023, 73, 198–222. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Tavolari, S.; Brandi, G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019, 39 (Suppl. S1), 19–31. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL-ILCA Clinical Practice Guidelines on the management of intrahepatic cholangiocarcinoma. J. Hepatol. 2023, 79, 181–208. [Google Scholar] [CrossRef]
- Rodrigues, P.M.; Olaizola, P.; Paiva, N.A.; Olaizola, I.; Agirre-Lizaso, A.; Landa, A.; Bujanda, L.; Perugorria, M.J.; Banales, J.M. Pathogenesis of Cholangiocarcinoma. Annu. Rev. Pathol. 2021, 16, 433–463. [Google Scholar] [CrossRef]
- Elvevi, A.; Laffusa, A.; Scaravaglio, M.; Rossi, R.E.; Longarini, R.; Stagno, A.M.; Cristoferi, L.; Ciaccio, A.; Cortinovis, D.L.; Invernizzi, P.; et al. Clinical treatment of cholangiocarcinoma: An updated comprehensive review. Ann. Hepatol. 2022, 27, 100737. [Google Scholar] [CrossRef]
- Speckart, J.; Rasmusen, V.; Talib, Z.; GnanaDev, D.A.; Rahnemai-Azar, A.A. Emerging Therapies in Management of Cholangiocarcinoma. Cancers 2024, 16, 613. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Hong, B.; Hou, Q.; Chen, W.; Zhang, W.; Zheng, W. PD-1/PD-L1 inhibitors plus chemotherapy versus chemotherapy alone as the first line treatment for advanced biliary tract cancer: A pooled analysis of KEYNOTE-966 and TOPAZ-1 trails. World J. Surg. Oncol. 2025, 23, 228. [Google Scholar] [CrossRef]
- Oh, D.Y.; He, A.R.; Bouattour, M.; Okusaka, T.; Qin, S.; Chen, L.T.; Kitano, M.; Lee, C.K.; Kim, J.W.; Chen, M.H.; et al. Durvalumab or placebo plus gemcitabine and cisplatin in participants with advanced biliary tract cancer (TOPAZ-1): Updated overall survival from a randomised phase 3 study. Lancet Gastroenterol. Hepatol. 2024, 9, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; He, A.R.; Bouattour, M.; Okusaka, T.; Qin, S.; Chen, L.T.; Kitano, M.; Lee, C.K.; Kim, J.W.; Chen, M.H.; et al. Durvalumab plus chemotherapy in advanced biliary tract cancer: 3-year overall survival update from the phase III TOPAZ-1 study. J. Hepatol. 2025, 83, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.; Ueno, M.; Klümpen, H.J.; Kelley, R.K.; Vogel, A.; Furuse, J.; Ren, Z.; Yau, T.; Chan, S.L.; Ozaka, M.; et al. Health-related quality of life in participants with advanced biliary tract cancer from the randomized phase III KEYNOTE-966 study. J. Hepatol. 2025, 83, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Ricci, A.D.; Brandi, G. PD-L1, TMB, MSI, and Other Predictors of Response to Immune Checkpoint Inhibitors in Biliary Tract Cancer. Cancers 2021, 13, 558. [Google Scholar] [CrossRef]
- Lo, J.H.; Agarwal, R.; Goff, L.W.; Heumann, T.R. Immunotherapy in Biliary Tract Cancers: Current Standard-of-Care and Emerging Strategies. Cancers 2023, 15, 3312. [Google Scholar] [CrossRef]
- Vogel, A.; Bathon, M.; Saborowski, A. Immunotherapies in clinical development for biliary tract cancer. Expert. Opin. Investig. Drugs 2021, 30, 351–363. [Google Scholar] [CrossRef]
- Cammarota, A.; Balsano, R.; Pressiani, T.; Bozzarelli, S.; Rimassa, L.; Lleo, A. The Immune-Genomics of Cholangiocarcinoma: A Biological Footprint to Develop Novel Immunotherapies. Cancers 2025, 17, 272. [Google Scholar] [CrossRef]
- Greten, T.F.; Schwabe, R.; Bardeesy, N.; Ma, L.; Goyal, L.; Kelley, R.K.; Wang, X.W. Immunology and immunotherapy of cholangiocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 349–365. [Google Scholar] [CrossRef]
- Fabris, L.; Sato, K.; Alpini, G.; Strazzabosco, M. The Tumor Microenvironment in Cholangiocarcinoma Progression. Hepatology 2021, 73 (Suppl. S1), 75–85. [Google Scholar] [CrossRef]
- Alvisi, G.; Termanini, A.; Soldani, C.; Portale, F.; Carriero, R.; Pilipow, K.; Costa, G.; Polidoro, M.; Franceschini, B.; Malenica, I.; et al. Multimodal single-cell profiling of intrahepatic cholangiocarcinoma defines hyperactivated Tregs as a potential therapeutic target. J. Hepatol. 2022, 77, 1359–1372. [Google Scholar] [CrossRef]
- Jiang, S.; Lu, H.; Pan, Y.; Yang, A.; Aikemu, A.; Li, H.; Hao, R.; Huang, Q.; Qi, X.; Tao, Z.; et al. Characterization of the distinct immune microenvironments between hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Cancer Lett. 2024, 588, 216799. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, J.L.; Valle, J.W.; Ilyas, S.I. Immunobiology of cholangiocarcinoma. J. Hepatol. 2023, 79, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Peng, L.; Dong, L.; Liu, D.; Ma, J.; Lin, J.; Chen, X.; Lin, P.; Song, G.; Zhang, M.; et al. Geospatial Immune Heterogeneity Reflects the Diverse Tumor-Immune Interactions in Intrahepatic Cholangiocarcinoma. Cancer Discov. 2022, 12, 2350–2371. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Peng, L.; Dong, L.; Liu, D.; Ma, J.; Lin, J.; Chen, X.; Lin, P.; Song, G.; Zhang, M.; et al. FAP Promotes Immunosuppression by Cancer-Associated Fibroblasts in the Tumor Microenvironment via STAT3-CCL2 Signaling. Cancer Res. 2016, 76, 4124–4135. [Google Scholar]
- Martin-Serrano, M.A.; Kepecs, B.; Torres-Martin, M.; Bramel, E.R.; Haber, P.K.; Merritt, E.; Rialdi, A.; Param, N.J.; Maeda, M.; Lindblad, K.E.; et al. Novel microenvironment-based classification of intrahepatic cholangiocarcinoma with therapeutic implications. Gut 2023, 72, 736–748. [Google Scholar] [CrossRef]
- Job, S.; Rapoud, D.; Dos Santos, A.; Gonzalez, P.; Desterke, C.; Pascal, G.; Elarouci, N.; Ayadi, M.; Adam, R.; Azoulay, D.; et al. Identification of Four Immune Subtypes Characterized by Distinct Composition and Functions of Tumor Microenvironment in Intrahepatic Cholangiocarcinoma. Hepatology 2020, 72, 965–981. [Google Scholar] [CrossRef]
- Bang, Y.H.; Lee, C.K.; Bang, K.; Kim, H.D.; Kim, K.P.; Jeong, J.H.; Park, I.; Ryoo, B.Y.; Lee, D.K.; Choi, H.J.; et al. Artificial Intelligence-Powered Spatial Analysis of Tumor-Infiltrating Lymphocytes as a Potential Biomarker for Immune Checkpoint Inhibitors in Patients with Biliary Tract Cancer. Clin. Cancer Res. 2024, 30, 4635–4643. [Google Scholar] [CrossRef]
- Dong, Z.R.; Zhang, M.Y.; Qu, L.X.; Zou, J.; Yang, Y.H.; Ma, Y.L.; Yang, C.C.; Cao, X.L.; Wang, L.Y.; Zhang, X.L.; et al. Spatial resolved transcriptomics reveals distinct cross-talk between cancer cells and tumor-associated macrophages in intrahepatic cholangiocarcinoma. Biomark. Res. 2024, 12, 100. [Google Scholar] [CrossRef]
- Raggi, C.; Correnti, M.; Sica, A.; Andersen, J.B.; Cardinale, V.; Alvaro, D.; Chiorino, G.; Forti, E.; Glaser, S.; Alpini, G.; et al. Cholangiocarcinoma stem-like subset shapes tumor-initiating niche by educating associated macrophages. J. Hepatol. 2017, 66, 102–115. [Google Scholar] [CrossRef]
- Chen, J.; Tang, Y.; Qin, D.; Yu, X.; Tong, H.; Tang, C.; Tang, Z. ALOX5 acts as a key role in regulating the immune microenvironment in intrahepatic cholangiocarcinoma, recruiting tumor-associated macrophages through PI3K pathway. J. Transl. Med. 2023, 21, 923. [Google Scholar] [CrossRef]
- Jarman, E.J.; Horcas-Lopez, M.; Waddell, S.H.; MacMaster, S.; Gournopanos, K.; Soong, D.Y.H.; Musialik, K.I.; Tsokkou, P.; Ng, M.E.; Cambridge, W.A.; et al. DKK1 drives immune suppressive phenotypes in intrahepatic cholangiocarcinoma and can be targeted with anti-DKK1 therapeutic DKN-01. Liver International. 2023, 43, 208–220. [Google Scholar] [CrossRef]
- Yuan, H.; Lin, Z.; Liu, Y.; Jiang, Y.; Liu, K.; Tu, M.; Yao, N.; Qu, C.; Hong, J. Intrahepatic cholangiocarcinoma induced M2-polarized tumor-associated macrophages facilitate tumor growth and invasiveness. Cancer Cell Int. 2020, 20, 586. [Google Scholar] [CrossRef]
- Guo, Y.; Miao, S.; Jin, Y.; Li, Q.; Wang, Y.; Zhang, X.; Li, J. Tumor-associated macrophages contribute to cholangiocarcinoma progression and chemoresistance through activation of ID1. Ann. Hepatol. 2025, 30, 101773. [Google Scholar] [CrossRef] [PubMed]
- Loeuillard, E.; Yang, J.; Buckarma, E.; Wang, J.; Liu, Y.; Conboy, C.; Pavelko, K.D.; Li, Y.; O’Brien, D.; Wang, C.; et al. Targeting tumor-associated macrophages and granulocytic myeloid-derived suppressor cells augments PD-1 blockade in cholangiocarcinoma. J. Clin. Investig. 2020, 130, 5380–5396. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, P.; Sun, R.; Li, J.; Hu, Z.; Xin, H.; Luo, C.; Zhou, J.; Fan, J.; Zhou, S. Tumor-associated neutrophils and macrophages interaction contributes to intrahepatic cholangiocarcinoma progression by activating STAT3. J. Immunother. Cancer 2021, 9, e001946. [Google Scholar] [CrossRef] [PubMed]
- Zuyin, L.; Zhao, L.; Qian, C.; Changkun, Z.; Delin, M.; Jialing, H.; Zhuomiaoyu, C.; Yuzi, L.; Jiaxi, Z.; Jie, G.; et al. Single-Cell and Spatial Transcriptomics Delineate the Microstructure and Immune Landscape of Intrahepatic Cholangiocarcinoma in the Leading-Edge Area. Adv. Sci. 2025, 12, e2412740. [Google Scholar] [CrossRef]
- Long, F.; Zhong, W.; Zhao, F.; Xu, Y.; Hu, X.; Jia, G.; Huang, L.; Yi, K.; Wang, N.; Si, H.; et al. DAB2 + macrophages support FAP + fibroblasts in shaping tumor barrier and inducing poor clinical outcomes in liver cancer. Theranostics 2024, 14, 4822–4843. [Google Scholar] [CrossRef]
- Fan, G.; Tao, C.; Li, L.; Xie, T.; Tang, L.; Han, X.; Shi, Y. The co-location of MARCO+ tumor-associated macrophages and CTSE+ tumor cells determined the poor prognosis in intrahepatic cholangiocarcinoma. Hepatology 2025, 82, 25–41. [Google Scholar] [CrossRef]
- Yang, H.; Yan, M.; Li, W.; Xu, L. SIRPα and PD1 expression on tumor-associated macrophage predict prognosis of intrahepatic cholangiocarcinoma. J. Transl. Med. 2022, 20, 140. [Google Scholar] [CrossRef]
- Chen, F.; Sheng, J.; Li, X.; Gao, Z.; Hu, L.; Chen, M.; Fei, J.; Song, Z. Tumor-associated macrophages: Orchestrators of cholangiocarcinoma progression. Front. Immunol. 2024, 15, 1451474. [Google Scholar] [CrossRef]
- Yi, M.; Li, T.; Niu, M.; Mei, Q.; Zhao, B.; Chu, Q.; Dai, Z.; Wu, K. Exploiting innate immunity for cancer immunotherapy. Mol. Cancer 2023, 22, 187. [Google Scholar] [CrossRef]
- Cui, T.; Sun, L.; Guo, X.; Cheng, C.; Zhang, N.; Zhou, S.; Chu, Q.; Xing, C.; Liang, S.; Liu, Y.; et al. Tumor-derived CD109 orchestrates reprogramming of tumor-associated macrophages to dampen immune response. J. Hepatol. 2025, 83, 946–958. [Google Scholar]
- Dai, Y.; Dong, C.; Wang, Z.; Zhou, Y.; Wang, Y.; Hao, Y.; Chen, P.; Liang, C.; Li, G. Infiltrating T lymphocytes and tumor microenvironment within cholangiocarcinoma: Immune heterogeneity, intercellular communication, immune checkpoints. Front. Immunol. 2024, 15, 1482291. [Google Scholar] [CrossRef]
- Hua, S.; Gu, X.; Jin, H.; Zhang, X.; Liu, Q.; Yang, J. Tumor-infiltrating T lymphocytes: A promising immunotherapeutic target for preventing immune escape in cholangiocarcinoma. Biomed. Pharmacother. 2024, 177, 117080. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, D.; Qian, H.; Shi, Y.; Tao, Z. CD8+ T cell-based cancer immunotherapy. J. Transl. Med. 2024, 22, 394. [Google Scholar] [CrossRef] [PubMed]
- Vigano, L.; Soldani, C.; Franceschini, B.; Cimino, M.; Lleo, A.; Donadon, M.; Roncalli, M.; Aghemo, A.; Di Tommaso, L.; Torzilli, G. Tumor-Infiltrating Lymphocytes and Macrophages in Intrahepatic Cholangiocellular Carcinoma. Impact on Prognosis after Complete Surgery. J. Gastrointest. Surg. 2019, 23, 2216–2224. [Google Scholar] [CrossRef]
- Lu, J.C.; Wu, L.L.; Sun, Y.N.; Huang, X.Y.; Gao, C.; Guo, X.J.; Zeng, H.Y.; Qu, X.D.; Chen, Y.; Wu, D.; et al. Macro CD5L+ deteriorates CD8+T cells exhaustion and impairs combination of Gemcitabine-Oxaliplatin-Lenvatinib-anti-PD1 therapy in intrahepatic cholangiocarcinoma. Nat. Commun. 2024, 15, 621. [Google Scholar] [CrossRef]
- Wan, W.; Li, Y.; Sun, W.; Cheng, Z.; Ma, F.; Shen, S.; Liu, H.; Zhang, J. The DCDC2/ENO1 axis promotes tumor progression and immune evasion in intrahepatic cholangiocarcinoma via activating FGL1-LAG3 checkpoint. J. Exp. Clin. Cancer Res. 2025, 44, 177. [Google Scholar] [CrossRef]
- Kim, H.D.; Jeong, S.; Park, S.; Lee, Y.J.; Ju, Y.S.; Kim, D.; Song, G.W.; Lee, J.H.; Kim, S.Y.; Shin, J.; et al. Implication of CD69+ CD103+ tissue-resident-like CD8+ T cells as a potential immunotherapeutic target for cholangiocarcinoma. Liver Int. 2021, 41, 764–776. [Google Scholar] [CrossRef]
- Panya, A.; Thepmalee, C.; Sawasdee, N.; Sujjitjoon, J.; Phanthaphol, N.; Junking, M.; Wongkham, S.; Yenchitsomanus, P.T. Cytotoxic activity of effector T cells against cholangiocarcinoma is enhanced by self-differentiated monocyte-derived dendritic cells. Cancer Immunol. Immunother. 2018, 67, 1579–1588. [Google Scholar] [CrossRef]
- Oliveira, G.; Wu, C.J. Dynamics and specificities of T cells in cancer immunotherapy. Nat. Rev. Cancer Nat. Res. 2023, 23, 295–316. [Google Scholar] [CrossRef]
- Oliviero, B.; Varchetta, S.; Mele, D.; Pessino, G.; Maiello, R.; Falleni, M.; Tosi, D.; Donadon, M.; Soldani, C.; Franceschini, B.; et al. MICA/B-targeted antibody promotes NK cell–driven tumor immunity in patients with intrahepatic cholangiocarcinoma. Oncoimmunology 2022, 11, 2035919. [Google Scholar] [CrossRef]
- Li, D.; Andaloori, L.; Crowe, M.; Lin, S.; Hong, J.; Zaidi, N.; Ho, M. Development of CAR-T Therapies and Personalized Vaccines for the Treatment of Cholangiocarcinoma: Current Progress, Mechanisms of Action, and Challenges. Am. J. Pathol. 2025, 195, 453–469. [Google Scholar] [CrossRef]
- Sun, B.Y.; Wang, Z.T.; Chen, K.Z.; Song, Y.; Wu, J.F.; Zhang, D.; Sun, G.Q.; Zhou, J.; Fan, J.; Hu, B.; et al. Mobilization and activation of tumor-infiltrating dendritic cells inhibits lymph node metastasis in intrahepatic cholangiocarcinoma. Cell Death Discov. 2024, 10, 304. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, X.; Ren, X.; Zhang, Y.; Huang, M.; Tan, L.; Dai, Z.; Lai, J.; Xie, W.; Chen, Z.; et al. Targeting tumour-intrinsic N7-methylguanosine tRNA modification inhibits MDSC recruitment and improves anti-PD-1 efficacy. Gut 2023, 72, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Ielpo, S.; Barberini, F.; Dabbagh Moghaddam, F.; Pesce, S.; Cencioni, C.; Spallotta, F.; De Ninno, A.; Businaro, L.; Marcenaro, E.; Bei, R.; et al. Crosstalk and communication of cancer-associated fibroblasts with natural killer and dendritic cells: New frontiers and unveiled opportunities for cancer immunotherapy. Cancer Treat. Rev. 2024, 131, 102843. [Google Scholar] [CrossRef] [PubMed]
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural killer cells: Development, maturation, and clinical utilization. Front. Immunol. 2018, 9, 1869. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Sharabi, A. Targeting myeloid-derived suppressor cells to enhance natural killer cell-based immunotherapy. Pharmacol. Ther. 2022, 235, 108114. [Google Scholar] [CrossRef]
- Rimassa, L.; Personeni, N.; Aghemo, A.; Lleo, A. The immune milieu of cholangiocarcinoma: From molecular pathogenesis to precision medicine. J. Autoimmun. 2019, 100, 17–26. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, Q.; Greten, T.F. MDSCs in liver cancer: A critical tumor-promoting player and a potential therapeutic target. Cell Immunol. 2021, 361, 104295. [Google Scholar] [CrossRef]
- Han, J.; Wang, H. Cytokine-overexpressing dendritic cells for cancer immunotherapy. Exp. Mol. Med. 2024, 56, 2559–2568. [Google Scholar] [CrossRef] [PubMed]
- Tay, C.; Tanaka, A.; Sakaguchi, S. Tumor-infiltrating regulatory T cells as targets of cancer immunotherapy. Cancer Cell 2023, 41, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Imianowski, C.J.; Chen, Q.; Workman, C.J.; Vignali, D.A.A. Regulatory T cells in the tumour microenvironment. Nat. Rev. Cancer 2025, 25, 703–722. [Google Scholar] [CrossRef] [PubMed]
- Konishi, D.; Umeda, Y.; Yoshida, K.; Shigeyasu, K.; Yano, S.; Toji, T.; Takeda, S.; Yoshida, R.; Yasui, K.; Fuji, T.; et al. Regulatory T cells induce a suppressive immune milieu and promote lymph node metastasis in intrahepatic cholangiocarcinoma. Br. J. Cancer 2022, 127, 757–765. [Google Scholar] [CrossRef]
- Nie, J.; Zhang, S.; Guo, Y.; Liu, C.; Shi, J.; Wu, H.; Na, R.; Liang, Y.; Yu, S.; Quan, F.; et al. Mapping of the T-cell Landscape of Biliary Tract Cancer Unravels Anatomic Subtype-Specific Heterogeneity. Cancer Res. 2025, 85, 704–722. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, H.; Wan, L.; Wang, Z.; Wang, H.; Ge, C.; Liu, Y.; Hao, Y.; Zhang, D.; Shi, G.; et al. Single-cell transcriptomic architecture and intercellular crosstalk of human intrahepatic cholangiocarcinoma. J. Hepatol. 2020, 73, 1118–1130. [Google Scholar] [CrossRef]
- Gao, X.; Xu, M.; Xiao, H.; Han, Z.; Wang, Z.; Sun, G.; Zhang, D.; Shuangjian, Q.; Ren, N.; Zhou, C.; et al. Tumor-associated neutrophils: A complex role in cancer. Clin. Immunol. 2025, 280, 110558. [Google Scholar] [CrossRef]
- Zhou, Y.; Shen, G.; Zhou, X.; Li, J. Therapeutic potential of tumor-associated neutrophils: Dual role and phenotypic plasticity. Signal Transduct. Target. Ther. 2025, 10, 178. [Google Scholar] [CrossRef]
- Sugahara, O.; Koga, D.; Oka, T.; Sugiyama, S.; Wada, R.; Higa, T.; Nakayama, K.I. Antitumor Activity of Tumor-Infiltrating Neutrophils Revealed by a Syngeneic Mouse Model of Cholangiocarcinoma. Cancer Sci. 2025, 116, 2457–2470. [Google Scholar] [CrossRef]
- Mao, Z.Y.; Zhu, G.Q.; Xiong, M.; Ren, L.; Bai, L. Prognostic value of neutrophil distribution in cholangiocarcinoma. World J. Gastroenterol. 2015, 21, 4961–4968. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Heij, L.R.; Czigany, Z.; Dahl, E.; Dulk, M.D.; Lang, S.A.; Ulmer, T.F.; Neumann, U.P.; Bednarsch, J. The prognostic value of neutrophil-to-lymphocyte ratio in cholangiocarcinoma: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 12691. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct. Target. Ther. 2021, 6, 362. [Google Scholar] [CrossRef] [PubMed]
- Corzo, C.A.; Condamine, T.; Lu, L.; Cotter, M.J.; Youn, J.I.; Cheng, P.; Cho, H.I.; Celis, E.; Quiceno, D.G.; Padhya, T.; et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. 2010, 207, 2439–2453. [Google Scholar] [CrossRef]
- Kwak, T.; Wang, F.; Deng, H.; Condamine, T.; Kumar, V.; Perego, M.; Kossenkov, A.; Montaner, L.J.; Xu, X.; Xu, W.; et al. Distinct Populations of Immune-Suppressive Macrophages Differentiate from Monocytic Myeloid-Derived Suppressor Cells in Cancer. Cell Rep. 2020, 33, 108571. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, C.; Duan, Y.; Heinrich, B.; Rosato, U.; Diggs, L.P.; Ma, L.; Roy, S.; Fu, Q.; Brown, Z.J.; et al. Gut Microbiome Directs Hepatocytes to Recruit MDSCs and Promote Cholangiocarcinoma. Cancer Discov. 2021, 11, 1248–1267. [Google Scholar] [CrossRef]
- Takagi, S.; Miyagawa, S.; Ichikawa, E.; Soeda, J.; Miwa, S.; Miyagawa, Y.; Iijima, S.; Noike, T.; Kobayashi, A.; Kawasaki, S. Dendritic cells, T-cell infiltration, and grp94 expression in cholangiocellular carcinoma. Hum. Pathol. 2004, 35, 881–886. [Google Scholar] [CrossRef]
- Mazzoccoli, L.; Liu, B. Dendritic Cells in Shaping Anti-Tumor T Cell Response. Cancers 2024, 16, 2211. [Google Scholar] [CrossRef]
- Mentucci, F.M.; Ferrara, M.G.; Ercole, A.; Rumie Vittar, N.B.; Lamberti, M.J. Interplay between cancer-associated fibroblasts and dendritic cells: Implications for tumor immunity. Front. Immunol. 2025, 16, 1515390. [Google Scholar] [CrossRef]
- Chen, J.; Duan, Y.; Che, J.; Zhu, J. Dysfunction of dendritic cells in tumor microenvironment and immunotherapy. Cancer Commun. 2024, 44, 1047–1070. [Google Scholar] [CrossRef]
- Hansen, F.J.; David, P.; Weber, G.F. The Multifaceted Functionality of Plasmacytoid Dendritic Cells in Gastrointestinal Cancers: A Potential Therapeutic Target? Cancers 2024, 16, 2216. [Google Scholar] [CrossRef]
- Hu, Z.Q.; Zhou, Z.J.; Luo, C.B.; Xin, H.Y.; Li, J.; Yu, S.Y.; Zhou, S.L. Peritumoral plasmacytoid dendritic cells predict a poor prognosis for intrahepatic cholangiocarcinoma after curative resection. Cancer Cell Int. 2020, 20, 582. [Google Scholar] [CrossRef]
- Kendall, T.; Verheij, J.; Gaudio, E.; Evert, M.; Guido, M.; Goeppert, B.; Carpino, G. Anatomical, histomorphological and molecular classification of cholangiocarcinoma. Liver Int. 2019, 39, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, D.; Zhao, B.; Ren, L.; Huang, R.; Feng, B.; Chen, H. The predictive value of PD-L1 expression in patients with advanced hepatocellular carcinoma treated with PD-1/PD-L1 inhibitors: A systematic review and meta-analysis. Cancer Med. 2023, 12, 9282–9292. [Google Scholar] [CrossRef]
- Ngo, P.; Cooper, W.A.; Wade, S.; Fong, K.M.; Canfell, K.; Karikios, D.; Weber, M. Why PD-L1 expression varies between studies of lung cancer: Results from a Bayesian meta-analysis. Sci. Rep. 2025, 15, 4166. [Google Scholar] [CrossRef] [PubMed]
- Kaunitz, G.J.; Cottrell, T.R.; Lilo, M.; Muthappan, V.; Esandrio, J.; Berry, S.; Xu, H.; Ogurtsova, A.; Anders, R.A.; Fischer, A.H.; et al. Melanoma subtypes demonstrate distinct PD-L1 expression profiles. Lab. Investig. 2017, 97, 1063–1071. [Google Scholar] [CrossRef]
- Frega, G.; Cossio, F.P.; Banales, J.M.; Cardinale, V.; Macias, R.I.R.; Braconi, C.; Lamarca, A. Lacking Immunotherapy Biomarkers for Biliary Tract Cancer: A Comprehensive Systematic Literature Review and Meta-Analysis. Cells 2023, 12, 2098. [Google Scholar] [CrossRef] [PubMed]
- Kubecek, O.; Trojanova, P.; Molnarova, V.; Kopecky, J. Microsatellite instability as a predictive factor for immunotherapy in malignant melanoma. Med. Hypotheses 2016, 93, 74–76. [Google Scholar] [CrossRef]
- Akagi, K.; Oki, E.; Taniguchi, H.; Nakatani, K.; Aoki, D.; Kuwata, T.; Yoshino, T. Real-world data on microsatellite instability status in various unresectable or metastatic solid tumors. Cancer Sci. 2021, 112, 1105–1113. [Google Scholar] [CrossRef]
- Tian, J.; Wang, H.; Lu, C.; Liu, L.; Zhang, X.; Xie, Y.; Li, R.; Lv, X.; Fu, D.; Zhang, L.; et al. Genomic characteristics and prognosis of lung cancer patients with MSI-H: A cohort study. Lung Cancer 2023, 181, 107255. [Google Scholar] [CrossRef]
- Shao, C.; Li, G.; Huang, L.; Pruitt, S.; Castellanos, E.; Frampton, G.; Carson, K.R.; Snow, T.; Singal, G.; Fabrizio, D.; et al. Prevalence of High Tumor Mutational Burden and Association with Survival in Patients with Less Common Solid Tumors. JAMA Netw Open 2020, 3, e2025109. [Google Scholar] [CrossRef]
- Gabbia, D.; De Martin, S. Tumor Mutational Burden for Predicting Prognosis and Therapy Outcome of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2023, 24, 3441. [Google Scholar] [CrossRef]
- Jung, J.; Heo, Y.J.; Park, S. High tumor mutational burden predicts favorable response to anti-PD-(L)1 therapy in patients with solid tumor: A real-world pan-tumor analysis. J. Immunother. Cancer 2023, 11, e006454. [Google Scholar] [CrossRef] [PubMed]
- Doi, N.; Ino, Y.; Fuse, M.; Esaki, M.; Shimada, K.; Hiraoka, N. Correlation of Vein-Rich Tumor Microenvironment of Intrahepatic Cholangiocarcinoma with Tertiary Lymphoid Structures and Patient Outcome. Mod. Pathol. 2024, 37, 100401. [Google Scholar] [CrossRef] [PubMed]
- Karapetyan, L.; Li, A.; Vargas De Stefano, D.; Abushukair, H.M.; Al-Bzour, A.N.; Knight, A.; Layding, C.; Wang, H.; Xu, J.; Yao, J.; et al. Differences in the pathological, transcriptomic, and prognostic implications of lymphoid structures between primary and metastatic cutaneous melanomas. J. Immunother. Cancer 2024, 12, e009231. [Google Scholar] [CrossRef] [PubMed]
- Elfving, H.; Yu, H.; Fessehatsion, K.K.; Brunnström, H.; Botling, J.; Gulyas, M.; Backman, M.; Lindberg, A.; Strell, C.; Micke, P. Spatial distribution of tertiary lymphoid structures in the molecular and clinical context of non-small cell lung cancer. Cell. Oncol. 2025, 48, 801–813. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Fu, H.; Li, J.; Xu, L.; Wang, G.; Wu, H. Peritumoral Tertiary Lymphoid Structures Correlate With Protective Immunity and Improved Prognosis in Patients with Hepatocellular Carcinoma. Front. Immunol. 2021, 12, 648812. [Google Scholar] [CrossRef]
- Dong, L.; Lu, D.; Chen, R.; Lin, Y.; Zhu, H.; Zhang, Z.; Cai, S.; Cui, P.; Song, G.; Rao, D.; et al. Proteogenomic characterization identifies clinically relevant subgroups of intrahepatic cholangiocarcinoma. Cancer Cell. 2022, 40, 70–87.e15. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, D.H.; Park, W.Y.; Shin, N.; Kim, A.; Lee, H.J.; Kim, Y.K.; Choi, K.U.; Kim, J.Y.; Yang, Y.I.; et al. IDH1 R132C mutation is detected in clear cell hepatocellular carcinoma by pyrosequencing. World J. Surg. Oncol. 2017, 15, 82. [Google Scholar] [CrossRef]
- Shibata, T.; Kokubu, A.; Miyamoto, M.; Sasajima, Y.; Yamazaki, N. Mutant IDH1 confers an in vivo growth in a melanoma cell line with BRAF mutation. Am. J. Pathol. 2011, 178, 1395–1402. [Google Scholar] [CrossRef]
- Vogel, A.; Segatto, O.; Stenzinger, A.; Saborowski, A. FGFR2 Inhibition in Cholangiocarcinoma. Annu. Rev. Med. 2023, 74, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Bold, N.; Buyanbat, K.; Enkhtuya, A.; Myagmar, N.; Batbayar, G.; Sandag, Z.; Damdinbazar, D.; Oyunbat, N.; Boldbaatar, T.; Enkhbaatar, A.; et al. High-Frequency Mutations in TP53, AXIN1, CTNNB1, and KRAS, and Polymorphisms in JAK1 Genes Among Mongolian HCC Patients. Cancer Rep. 2025, 8, e70227. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.K.H.; Skoulidis, F.; Kerr, K.M.; Ahn, M.J.; Kapp, J.R.; Soares, F.A.; Yatabe, Y. KRAS G12C in advanced NSCLC: Prevalence, co-mutations, and testing. Lung Cancer 2023, 184, 107293. [Google Scholar] [CrossRef]
- Rabbie, R.; Ferguson, P.; Wong, K.; Couturier, D.L.; Moran, U.; Turner, C.; Emanuel, P.; Haas, K.; Saunus, J.M.; Davidson, M.R.; et al. The mutational landscape of melanoma brain metastases presenting as the first visceral site of recurrence. Br. J. Cancer. 2021, 124, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Bousou, T.E.; Sarantis, P.; Anastasiou, I.A.; Trifylli, E.M.; Liapopoulos, D.; Korakaki, D.; Koustas, E.; Katsimpoulas, M.; Karamouzis, M.V. Biomarkers for the Evaluation of Immunotherapy in Patients with Cholangiocarcinoma. Cancers 2025, 17, 555. [Google Scholar] [CrossRef]
- Weinberg, B.A.; Xiu, J.; Lindberg, M.R.; Shields, A.F.; Hwang, J.J.; Poorman, K.; Salem, M.E.; Pishvaian, M.J.; Holcombe, R.F.; Marshall, J.L.; et al. Molecular profiling of biliary cancers reveals distinct molecular alterations and potential therapeutic targets. J. Gastrointest. Oncol. 2019, 10, 652–662. [Google Scholar] [CrossRef]
- Kim, R.; Park, J.K.; Kwon, M.; An, M.; Hong, J.Y.; Park, J.O.; Lim, S.H.; Kim, S.T. Comprehensive molecular characterization to predict immunotherapy response in advanced biliary tract cancer: A phase II trial of pembrolizumab. Oncol. Res. 2025, 33, 57–65. [Google Scholar]
- Chan, T.A.; Yarchoan, M.; Jaffee, E.; Swanton, C.; Quezada, S.A.; Stenzinger, A.; Peters, S. Development of tumor mutation burden as an immunotherapy biomarker: Utility for the oncology clinic. Ann. Oncol. 2019, 30, 44–56. [Google Scholar] [CrossRef]
- Jardim, D.L.; Goodman, A.; de Melo Gagliato, D.; Kurzrock, R. The Challenges of Tumor Mutational Burden as an Immunotherapy Biomarker. Cancer Cell. 2021, 39, 154–173. [Google Scholar] [CrossRef]
- Zhang, Y.; Gou, M. Combined Chemotherapy-Immunotherapy for Advanced Biliary Tract Cancer (BTC): A Clinical, Genomic, and Biomarker Analysis. J. Gastrointest. Cancer. 2025, 56, 90. [Google Scholar] [CrossRef]
- Nakamura, H.; Arai, Y.; Totoki, Y.; Shirota, T.; Elzawahry, A.; Kato, M.; Hama, N.; Hosoda, F.; Urushidate, T.; Ohashi, S.; et al. Genomic spectra of biliary tract cancer. Nat. Genet. 2015, 47, 1003–1010. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Ricci, A.D.; Rizzo, A.; Brandi, G. Immunotherapy in Biliary Tract Cancer: Worthy of a Second Look. Cancer Control. 2020, 27, 1073274820948047. [Google Scholar] [CrossRef]
- Lin, G.; Liu, Y.; Li, S.; Mao, Y.; Wang, J.; Shuang, Z.; Chen, J.; Li, S. Elevated neutrophil-to-lymphocyte ratio is an independent poor prognostic factor in patients with intrahepatic cholangiocarcinoma. Oncotarget 2016, 7, 50963–50971. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xie, Y.; Cai, Y.; Hu, H.; He, M.; Liu, L.; Liao, C.; Wang, Y.; Wang, J.; Ren, X.; et al. Multiomic Analysis Reveals Comprehensive Tumor Heterogeneity and Distinct Immune Subtypes in Multifocal Intrahepatic Cholangiocarcinoma. Clin. Cancer Res. 2022, 28, 1896–1910. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, Y.; Tan, Z.; Song, Y.; Chen, K.; Liu, S.; Peng, C.; Chen, X. Machine Learning-based Macrophage Signature for Predicting Prognosis and Immunotherapy Benefits in Cholangiocarcinoma. Curr. Med. Chem. 2024, 32, 8945–8958. [Google Scholar] [CrossRef] [PubMed]
- Kida, A.; Mizukoshi, E.; Tamai, T.; Terashima, T.; Kitahara, M.; Arai, K.; Yamashita, T.; Fushimi, K.; Honda, M.; Kaneko, S. Immune responses against tumour-associated antigen-derived cytotoxic T lymphocyte epitopes in cholangiocarcinoma patients. Liver Int. 2018, 38, 2040–2050. [Google Scholar]
- Shang, T.; Jiang, T.; Lu, T.; Wang, H.; Cui, X.; Pan, Y.; Xu, M.; Pei, M.; Ding, Z.; Feng, X.; et al. Tertiary lymphoid structures predict the prognosis and immunotherapy response of cholangiocarcinoma. Front. Immunol. 2023, 14, 1166497. [Google Scholar] [CrossRef]
- Chen, X.; Wang, D.; Liu, J.; Qiu, J.; Zhou, J.; Ying, J.; Shi, Y.; Wang, Z.; Lou, H.; Cui, J.; et al. Genomic alterations in biliary tract cancer predict prognosis and immunotherapy outcomes. J. Immunother. Cancer 2021, 9, e003214. [Google Scholar] [CrossRef]
- Wu, M.J.; Shi, L.; Dubrot, J.; Merritt, J.; Vijay, V.; Wei, T.Y.; Kessler, E.; Olander, K.E.; Adil, R.; Pankaj, A.; et al. Mutant IDH Inhibits IFNγ-TET2 Signaling to Promote Immunoevasion and Tumor Maintenance in Cholangiocarcinoma. Cancer Discov. 2022, 12, 812–835. [Google Scholar]
- Aguado-Fraile, E.; Tassinari, A.; Ishii, Y.; Sigel, C.; Lowery, M.A.; Goyal, L.; Gliser, C.; Jiang, L.; Pandya, S.S.; Wu, B.; et al. Molecular and morphological changes induced by ivosidenib correlate with efficacy in mutant-IDH1 cholangiocarcinoma. Future Oncol. 2021, 17, 2057–2074. [Google Scholar] [CrossRef]
- Uson Junior, P.L.S.; Borad, M.J. Clinical Utility of Ivosidenib in the Treatment of IDH1-Mutant Cholangiocarcinoma: Evidence To Date. Cancer Manag. Res. 2023, 15, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, G.; Wu, Z.; Dong, Y.; Shi, Y.; Yang, F.; Chen, X.; Wang, J.; Du, S.; Xu, H.; et al. Exosomal circ-PTPN22 and circ-ADAMTS6 mark T cell exhaustion and neutrophil extracellular traps in Asian intrahepatic cholangiocarcinoma. Mol. Ther. Nucleic Acids 2023, 31, 151–163. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, M.; Pu, H.; Guo, S.; Zhang, S.; Wang, Y. Prognostic Implications of Pan-Cancer CMTM6 Expression and Its Relationship with the Immune Microenvironment. Front. Oncol. 2020, 10, 585961. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Li, S.; Liu, K.; Fan, R.; Liu, F.; Zhang, H.; Liu, D.; Shen, D. Downregulation of dermatopontin in cholangiocarcinoma cells suppresses CCL19 secretion of macrophages and immune infiltration. J. Cancer Res. Clin. Oncol. 2024, 150, 66. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, D.; Sima, X.; Fu, Y.; Zeng, H.; Hu, Z.; Hou, J.; Pan, Y.; Zhang, Y.; Zhou, Z.; et al. Levels of pretreatment serum lipids predict responses to PD-1 inhibitor treatment in advanced intrahepatic cholangiocarcinoma. Int. Immunopharmacol. 2023, 115, 109687. [Google Scholar] [CrossRef] [PubMed]
- Park, E.M.; Chelvanambi, M.; Bhutiani, N.; Kroemer, G.; Zitvogel, L.; Wargo, J.A. Targeting the gut and tumor microbiota in cancer. Nat. Med. 2022, 28, 690–703. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Sepich-Poore, G.D.; Zitvogel, L.; Straussman, R.; Hasty, J.; Wargo, J.A.; Knight, R. The microbiome and human cancer. Science 2021, 371, eabc4552. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Xia, Q. Role of gut microbiome in cancer immunotherapy: From predictive biomarker to therapeutic target. Exp. Hematol. Oncol. 2023, 12, 84. [Google Scholar] [CrossRef]
- Xin, H.Y.; Zou, J.X.; Sun, R.Q.; Hu, Z.Q.; Chen, Z.; Luo, C.B.; Zhou, Z.J.; Wang, P.C.; Li, J.; Yu, S.Y.; et al. Characterization of tumor microbiome and associations with prognosis in intrahepatic cholangiocarcinoma. J. Gastroenterol. 2024, 59, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wang, Y.; Zhu, R.; Wang, S.; Xue, J.; Zhang, D.; Lan, Z.; Zhang, C.; Liang, Y.; Zhang, N.; et al. Gut microbiota and metabolites signatures of clinical response in anti-PD-1/PD-L1 based immunotherapy of biliary tract cancer. Biomark. Res. 2024, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Roussel, E.; Brasse-Lagnel, C.; Tuech, J.J.; Montialoux, H.; Papet, E.; Tortajada, P.; Bekri, S.; Schwarz, L. Influence of Probiotics Administration Before Liver Resection in Patients with Liver Disease: A Randomized Controlled Trial. World J. Surg. 2022, 46, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ke, Y.; Liu, Q.; Yang, J.; Liu, F.; Xu, R.; Zhou, H.; Chen, A.; Xiao, J.; Meng, F.; et al. Engineered Lactococcus lactis secreting Flt3L and OX40 ligand for in situ vaccination-based cancer immunotherapy. Nat. Commun. 2022, 13, 7466. [Google Scholar] [CrossRef]
- Singh, A.; Alexander, S.G.; Martin, S. Gut microbiome homeostasis and the future of probiotics in cancer immunotherapy. Front. Immunol. 2023, 14, 1114499. [Google Scholar] [CrossRef]
- Spencer, C.N.; McQuade, J.L.; Gopalakrishnan, V.; McCulloch, J.A.; Vetizou, M.; Cogdill, A.P.; Khan, M.A.W.; Zhang, X.; White, M.G.; Peterson, C.B.; et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science 2021, 374, 1632–1640. [Google Scholar] [CrossRef]
- Kang, X.; Liu, C.; Ding, Y.; Ni, Y.; Ji, F.; Lau, H.C.H.; Jiang, L.; Sung, J.J.; Wong, S.H.; Yu, J.; et al. Roseburia intestinalis generated butyrate boosts anti-PD-1 efficacy in colorectal cancer by activating cytotoxic CD8+ T cells. Gut 2023, 72, 2112–2122. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, M.; Liu, X.; Chai, M.; Diao, L.; Ma, L.; Nie, S.; Xu, M.; Wang, Y.; Mo, F.; et al. Probiotics formulation and cancer nanovaccines show synergistic effect in immunotherapy and prevention of colon cancer. iScience 2023, 26, 107167. [Google Scholar] [CrossRef]
- Boucher, E.; Plazy, C.; Richard, M.L.; Suau, A.; Mangin, I.; Cornet, M.; Aldebert, D.; Toussaint, B.; Hannani, D. Inulin prebiotic reinforces host cancer immunosurveillance via ɣδ T cell activation. Front. Immunol. 2023, 14, 1104224. [Google Scholar] [CrossRef]
- Huang, J.; Gong, C.; Zhou, A. Modulation of gut microbiota: A novel approach to enhancing the effects of immune checkpoint inhibitors. Ther. Adv. Med. Oncol. 2023, 15, 17588359231204854. [Google Scholar] [CrossRef]
- Dadgar, N.; Arunachalam, A.K.; Hong, H.; Phoon, Y.P.; Arpi-Palacios, J.E.; Uysal, M.; Wehrle, C.J.; Aucejo, F.; Ma, W.W.; Melenhorst, J.J.; et al. Advancing Cholangiocarcinoma Care: Insights and Innovations in T Cell Therapy. Cancers 2024, 16, 3232. [Google Scholar] [CrossRef]
- Castellarin, M.; Watanabe, K.; June, C.H.; Kloss, C.C.; Posey, A.D. Driving cars to the clinic for solid tumors. Gene Ther. 2018, 25, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Li, X.; Wang, X.; Cheng, L.; Li, Z.; Zhang, C.; Ye, Z.; Qian, Q. Current Progress in CAR-T Cell Therapy for Solid Tumors. Int. J. Biol. Sci. 2019, 15, 2548–2560. [Google Scholar] [CrossRef] [PubMed]
- Amorós-Pérez, B.; Rivas-Pardo, B.; Gómez del Moral, M.; Subiza, J.L.; Martínez-Naves, E. State of the Art in CAR-T Cell Therapy for Solid Tumors: Is There a Sweeter Future? Cells 2024, 13, 725. [Google Scholar] [CrossRef] [PubMed]
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- Uslu, U.; June, C.H. Beyond the blood: Expanding CAR T cell therapy to solid tumors. Nat. Biotechnol. 2025, 43, 506–515. [Google Scholar] [CrossRef]
- Supimon, K.; Sangsuwannukul, T.; Sujjitjoon, J.; Phanthaphol, N.; Chieochansin, T.; Poungvarin, N.; Wongkham, S.; Junking, M.; Yenchitsomanus, P.T. Anti-mucin 1 chimeric antigen receptor T cells for adoptive T cell therapy of cholangiocarcinoma. Sci. Rep. 2021, 11, 6276. [Google Scholar] [CrossRef]
- Mao, L.; Su, S.; Li, J.; Yu, S.; Gong, Y.; Chen, C.; Hu, Z.; Huang, X. Development of Engineered CAR T Cells Targeting Tumor-Associated Glycoforms of MUC1 for the Treatment of Intrahepatic Cholangiocarcinoma. J. Immunother. 2023, 46, 89–95. [Google Scholar] [CrossRef]
- Gomes, R.V.; Rodrigues, M.Â.; Rodrigues, J.B.S.R.; Vidigal, P.T.; Damasceno, K.A.; Lima, H.A.; Gomes, D.A.; Machado, C.J.; Resende, V. Expression of epidermal growth factor receptor (EGFR) in cholangiocarcinomas: Predictive factors and survival. Rev. Col. Bras. Cir. 2018, 45, e1826. [Google Scholar] [CrossRef]
- Guo, Y.; Feng, K.; Liu, Y.; Wu, Z.; Dai, H.; Yang, Q.; Wang, Y.; Jia, H.; Han, W. Phase I Study of Chimeric Antigen Receptor-Modified T Cells in Patients with EGFR-Positive Advanced Biliary Tract Cancers. Clin. Cancer Res. 2018, 24, 1277–1286. [Google Scholar] [CrossRef]
- Feng, K.C.; Guo, Y.L.; Liu, Y.; Dai, H.R.; Wang, Y.; Lv, H.Y.; Huang, J.H.; Yang, Q.M.; Han, W.D. Cocktail treatment with EGFR-specific and CD133-specific chimeric antigen receptor-modified T cells in a patient with advanced cholangiocarcinoma. J. Hematol. Oncol. 2017, 10, 4. [Google Scholar] [CrossRef]
- Paijens, S.T.; Vledder, A.; de Bruyn, M.; Nijman, H.W. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell. Mol. Immunol. 2021, 18, 842–859. [Google Scholar] [CrossRef]
- Kumar, A.; Watkins, R.; Vilgelm, A.E. Cell Therapy with TILs: Training and Taming T Cells to Fight Cancer. Front. Immunol. 2021, 12, 690499. [Google Scholar] [CrossRef]
- Higuchi, R.; Yamamoto, M.; Hatori, T.; Shimizu, K.; Imai, K.; Takasaki, K. Intrahepatic cholangiocarcinoma with lymph node metastasis successfully treated by immunotherapy with CD3-activated T cells and dendritic cells after surgery: Report of a case. Surg. Today 2006, 36, 559–562. [Google Scholar] [CrossRef]
- Tran, E.; Turcotte, S.; Gros, A.; Robbins, P.F.; Lu, Y.C.; Dudley, M.E.; Wunderlich, J.R.; Somerville, R.P.; Hogan, K.; Hinrichs, C.S.; et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014, 344, 641–645. [Google Scholar] [CrossRef]
- Amhis, N.; Carignan, J.; Tai, L.H. Transforming pancreaticobiliary cancer treatment: Exploring the frontiers of adoptive cell therapy and cancer vaccines. Mol. Ther. Oncol. 2024, 32, 200825. [Google Scholar] [CrossRef]
- Ding, G.Y.; Ma, J.Q.; Yun, J.P.; Chen, X.; Ling, Y.; Zhang, S.; Shi, J.Y.; Chang, Y.Q.; Ji, Y.; Wang, X.Y.; et al. Distribution and density of tertiary lymphoid structures predict clinical outcome in intrahepatic cholangiocarcinoma. J. Hepatol. 2022, 76, 608–618. [Google Scholar] [CrossRef]
- Fridman, W.H.; Petitprez, F.; Meylan, M.; Chen, T.W.; Sun, C.M.; Roumenina, L.T.; Sautès-Fridman, C. B cells and cancer: To B or not to B? J. Exp. Med. 2021, 218, e20200851. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Meylan, M.; Petitprez, F.; Sun, C.M.; Italiano, A.; Sautès-Fridman, C. B cells and tertiary lymphoid structures as determinants of tumour immune contexture and clinical outcome. Nat. Rev. Clin. Oncol. 2022, 19, 441–457. [Google Scholar] [CrossRef] [PubMed]
- Vonderheide, R.H.; Glennie, M.J. Agonistic CD40 antibodies and cancer therapy. Clin. Cancer Res. 2013, 19, 1035–1043. [Google Scholar] [CrossRef]
- Casolino, R.; Braconi, C. CD40-agonist: A new avenue for immunotherapy combinations in cholangiocarcinoma. J. Hepatol. 2021, 74, 1021–1024. [Google Scholar] [CrossRef] [PubMed]
- Kverneland, A.H.; Chamberlain, C.A.; Borch, T.H.; Nielsen, M.; Mørk, S.K.; Kjeldsen, J.W.; Lorentzen, C.L.; Jørgensen, L.P.; Riis, L.B.; Yde, C.W.; et al. Adoptive cell therapy with tumor-infiltrating lymphocytes supported by checkpoint inhibition across multiple solid cancer types. J. Immunother. Cancer 2021, 9, e003499. [Google Scholar] [CrossRef] [PubMed]
- Vitale, L.A.; Thomas, L.J.; He, L.Z.; O’Neill, T.; Widger, J.; Crocker, A.; Sundarapandiyan, K.; Storey, J.R.; Forsberg, E.M.; Weidlick, J.; et al. Development of CDX-1140, an agonist CD40 antibody for cancer immunotherapy. Cancer Immunol. Immunother. 2019, 68, 233–245. [Google Scholar] [PubMed]
- Hochnadel, I.; Kossatz-Boehlert, U.; Jedicke, N.; Lenzen, H.; Manns, M.P.; Yevsa, T. Cancer vaccines and immunotherapeutic approaches in hepatobiliary and pancreatic cancers. Hum. Vaccin. Immunother. 2017, 13, 2931–2952. [Google Scholar] [CrossRef][Green Version]
- Zhao, L.M.; Shi, A.D.; Yang, Y.; Liu, Z.L.; Hu, X.Q.; Shu, L.Z.; Tang, Y.C.; Zhang, Z.L. Advances in molecular and cell therapy for immunotherapy of cholangiocarcinoma. Front. Oncol. 2023, 13, 1140103. [Google Scholar] [CrossRef]
- Igarashi, Y.; Sasada, T. Cancer Vaccines: Toward the Next Breakthrough in Cancer Immunotherapy. J. Immunol. Res. 2020, 2020, 5825401. [Google Scholar] [CrossRef]
- Kamigaki, T.; Takimoto, R.; Okada, S.; Ibe, H.; Oguma, E.; Goto, S. Personalized Dendritic-cell-based Vaccines Targeting Cancer Neoantigens. Anticancer Res. 2024, 44, 3713–3724. [Google Scholar] [CrossRef]
- Popovic, A.; Jaffee, E.M.; Zaidi, N. Emerging strategies for combination checkpoint modulators in cancer immunotherapy. J. Clin. Investig. 2018, 128, 3209–3218. [Google Scholar] [CrossRef]
- Kotera, Y.; Kougen, Y.; Aruga, A.; Yamamoto, M. Dendritic cell vaccine for intrahepatic cholangio cellular carcinoma--a study of relationship between immuno-reaction and clinical outcome. Gan To Kagaku Ryoho 2009, 36, 1964–1966. [Google Scholar]
- Shimizu, K.; Kotera, Y.; Aruga, A.; Takeshita, N.; Takasaki, K.; Yamamoto, M. Clinical utilization of postoperative dendritic cell vaccine plus activated T-cell transfer in patients with intrahepatic cholangiocarcinoma. J. Hepatobiliary Pancreat. Sci. 2012, 19, 171–178. [Google Scholar] [CrossRef]
- Kobayashi, M.; Sakabe, T.; Abe, H.; Tanii, M.; Takahashi, H.; Chiba, A.; Yanagida, E.; Shibamoto, Y.; Ogasawara, M.; Tsujitani, S.; et al. Dendritic cell-based immunotherapy targeting synthesized peptides for advanced biliary tract cancer. J. Gastrointest. Surg. 2013, 17, 1609–1617. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ueno, T.; Kawaoka, T.; Hazama, S.; Fukui, M.; Suehiro, Y.; Hamanaka, Y.; Ikematsu, Y.; Imai, K.; Oka, M.; et al. MUC1 peptide vaccination in patients with advanced pancreas or biliary tract cancer. Anticancer Res. 2005, 25, 3575–3579. [Google Scholar]
- Aruga, A.; Takeshita, N.; Kotera, Y.; Okuyama, R.; Matsushita, N.; Ohta, T.; Takeda, K.; Yamamoto, M. Long-term Vaccination with Multiple Peptides Derived from Cancer-Testis Antigens Can Maintain a Specific T-cell Response and Achieve Disease Stability in Advanced Biliary Tract Cancer. Clin. Cancer Res. 2013, 19, 2224–2231. [Google Scholar] [CrossRef]
- Tang, T.Y.; Huang, X.; Zhang, G.; Lu, M.H.; Liang, T.B. mRNA vaccine development for cholangiocarcinoma: A precise pipeline. Mil. Med. Res. 2022, 9, 40. [Google Scholar] [CrossRef]
- Huang, X.; Tang, T.; Zhang, G.; Liang, T. Identification of tumor antigens and immune subtypes of cholangiocarcinoma for mRNA vaccine development. Mol. Cancer 2021, 20, 50. [Google Scholar] [CrossRef]

| Immune Cell Type | Functional Role in iCCA | Therapeutic Strategies Under Investigation |
|---|---|---|
| TAMs | Promote cancer progression, therapy resistance, and immunosuppression | Dual inhibition of TAMs (anti–CSF1R) and G-MDSCs (anti–Ly6G or GW3965) + anti–PD-1; dual checkpoint blockade (CD47–SIRPα and PD-1); dual inhibition of CSF1R and ALOX5; Dual blockade of CD109 and PD-L1 |
| CD8+ T cells | Central antitumor effectors, but frequently excluded or functionally exhausted within the TIME | Restoration of activity with ICIs + myeloid targeting, CXCR2 blockade or β-catenin inhibition, DC-based vaccines, adoptive transfer (CAR-T, TILs), GOLP therapy (gemcitabine, oxaliplatin, lenvatinib, and anti–PD1) |
| NK cells | Mediate tumor clearance and immune modulation; possibly dysfunctional in iCCA, role insufficiently defined | Restoration of function (anti-7C6); ICIs targeting NK-specific receptors (TIGIT, KIR or NKG2A); CAR-NK or allogeneic NK therapies; cytokine stimulation (IL-15, IL-2, or IFN-α) |
| Tregs | Mediate potent immunosuppression by suppressing effector T cells and facilitating immune escape, contributing to therapy resistance | Selective depletion/reprogramming (galectin-1 or MEOX1 inhibition, CD25 or CCR8 targeting); disruption of immune checkpoints (TIGIT–PVR axis); combination with ICIs |
| TANs | Innate immune effectors with dual N1 (antitumor) or N2 (protumor) phenotypes; in iCCA, their role is ambiguous and context dependent | Enhancement of maturation (recombinant G-CSF + gemcitabine/cisplatin); dual blockade of TAMs and G-MDSCs/TANs + anti–PD-1; triple combination of METTL1 depletion, CXCR2 inhibition, and anti–PD-1 |
| MDSCs | Promote cancer progression, pre-metastatic niche formation, and immune evasion | Blockade of recruitment/signaling (CXCR2 antagonists, anti-Ly6G or combined blockade of METTL1 and the CXCL8-CXCR2 axis) + ICIs |
| DCs | Coordinate T cell responses and cancer immunosurveillance; in iCCA, impaired function contributes to immune escape | Expansion of DCs (Flt3L + poly(I:C)); restoration of recruitment (β-catenin inhibition); vaccination strategies (engineered or tumor-lysate DCs with T cell transfer); indirect modulation (CAF or PORCN targeting) |
| Biomarker/Mutation | iCCA (%) | HCC (%) | NSCLC (%) | Melanoma (%) | Reference |
|---|---|---|---|---|---|
| PD-L1 positivity | ~27% | ~34.5% | ~58% | ~19–92% | [24,94,95,96] |
| MSI-H | ~2.5% | ~0% to 2.9% | Low | Low | [97,98,99,100] |
| High TMB | ~4.6% | ~4–5.4% | ~20–40% | ~71% | [97,101,102,103] |
| TLS density (high intratumoral) | Low/ Intermediate | Variable | High | High | [104,105,106,107] |
| IDH1 mutations | 10–15% | ~2.5% | Rare | 3–5% | [108,109,110] |
| FGFR2 fusions | 15% | Rare | 4% | 10% | [111] |
| KRAS mutations | 9–24% | <10% | 25–30% | 2–4% | [112,113,114] |
| Therapy | Target/Approach | Development Stage | Clinical Trial ID | Key Notes |
|---|---|---|---|---|
| CAR-T | MUC1, EGFR, CEA, CD133 | Preclinical and clinical | NCT03633773, NCT01869166, NCT06043466, NCT02541370 | Tumor killing shown in xenograft models; persistence and off-tumor toxicity remain challenges |
| Tumor-Infiltrating Lymphocytes | Autologous tumor-infiltrating lymphocytes | Early clinical | NCT01174121, NCT03801083, NCT05088190, NCT03820310 | Case reports with long-term remission; trials ongoing with IL-2 and PD-1+ TILs |
| B cell/CD40 agonists | B cell–mediated antigen presentation, CD40–CD40L axis | Preclinical and clinical | NCT05849480 (CDX-1140) | Enhance T cell cytotoxicity; combination with PD-1 and chemotherapy effective in mice |
| DC vaccines | Antigen-loaded DCs (MUC1, WT1, tumor lysate) | Clinical | NCT02829941 | Safe, induce CTLs, synergistic with chemotherapy; modest responses |
| Peptide/Protein vaccines | MUC1, LY6K, DEPDC1, IMP3, TTK | Early clinical | N/A * | Immunogenic, safe, associated with improved PFS/OS in small cohorts |
| mRNA Vaccines | CD247, FCGR1A, TRRAP | Preclinical | N/A* | Proposed based on transcriptomic profiling; IS2 immune desert subtype most promising |
| Microbiome modulation | Probiotics, microbial metabolites | Preclinical and clinical | NCT05032014 | May enhance ICI response; still exploratory in BTC/iCCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porro, N.; Spínola-Lasso, E.; Marra, F.; Gentilini, A. Immune Landscape of Intrahepatic Cholangiocarcinoma: Evasion and Therapeutic Insights. Immuno 2025, 5, 40. https://doi.org/10.3390/immuno5030040
Porro N, Spínola-Lasso E, Marra F, Gentilini A. Immune Landscape of Intrahepatic Cholangiocarcinoma: Evasion and Therapeutic Insights. Immuno. 2025; 5(3):40. https://doi.org/10.3390/immuno5030040
Chicago/Turabian StylePorro, Nunzia, Elena Spínola-Lasso, Fabio Marra, and Alessandra Gentilini. 2025. "Immune Landscape of Intrahepatic Cholangiocarcinoma: Evasion and Therapeutic Insights" Immuno 5, no. 3: 40. https://doi.org/10.3390/immuno5030040
APA StylePorro, N., Spínola-Lasso, E., Marra, F., & Gentilini, A. (2025). Immune Landscape of Intrahepatic Cholangiocarcinoma: Evasion and Therapeutic Insights. Immuno, 5(3), 40. https://doi.org/10.3390/immuno5030040








