RIPK2 Inhibition Blocks NOD2-Mediated IL-1β Production by Macrophages In Vitro but Exacerbates Crohn’s Disease-like Ileitis in SHIP–/– Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Macrophage Derivation
2.3. Cell Stimulations
2.4. In Vivo GSK2983559 Treatment
2.5. Cytokine Measurements
2.6. Histological Analyses
2.7. SDS-Page and Western Blotting
2.8. Gene Expression
2.9. Statistical Analyses
3. Results
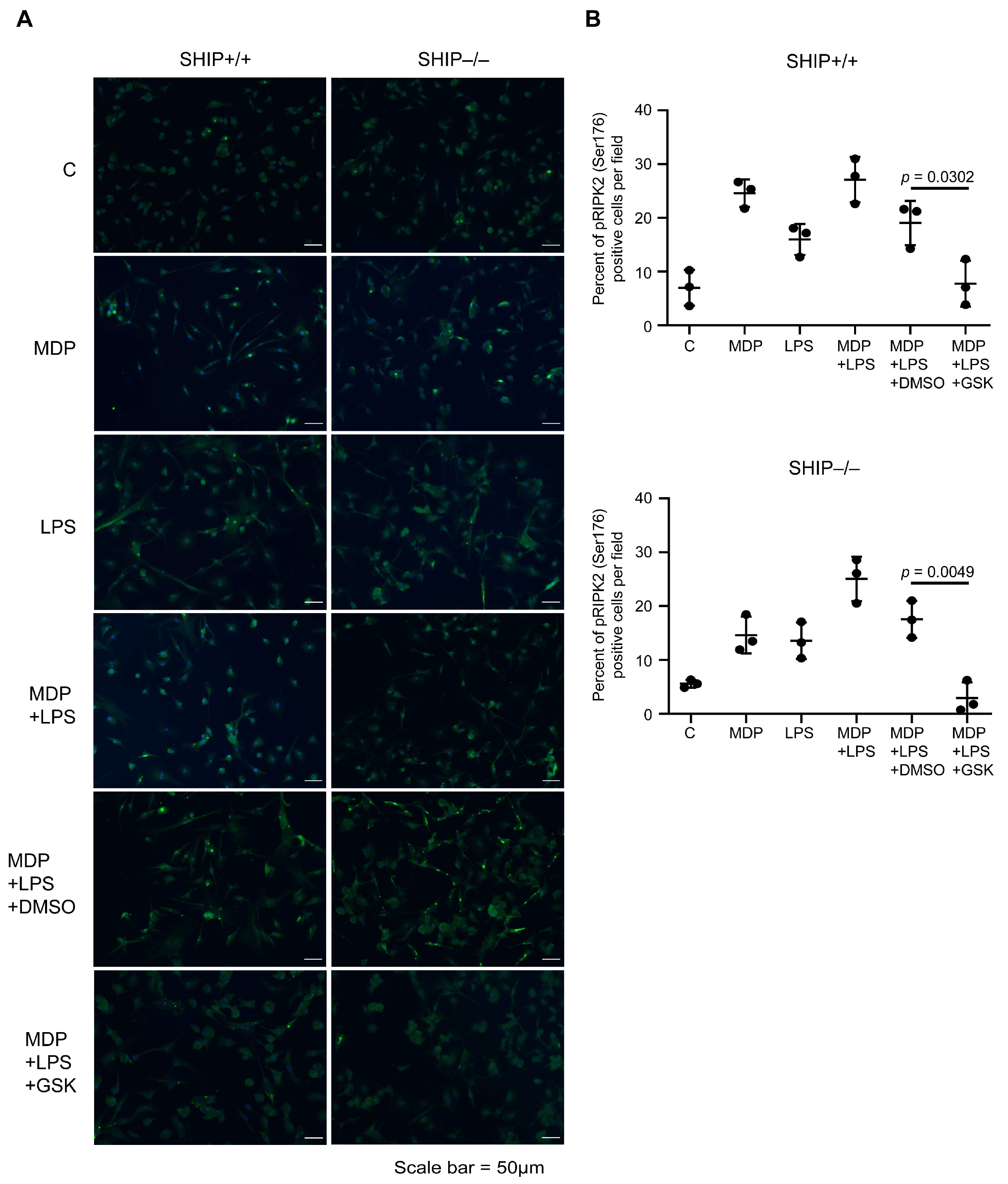
3.1. GSK Blocks RIPK2 Activation in MCSF-Derived BMDMs
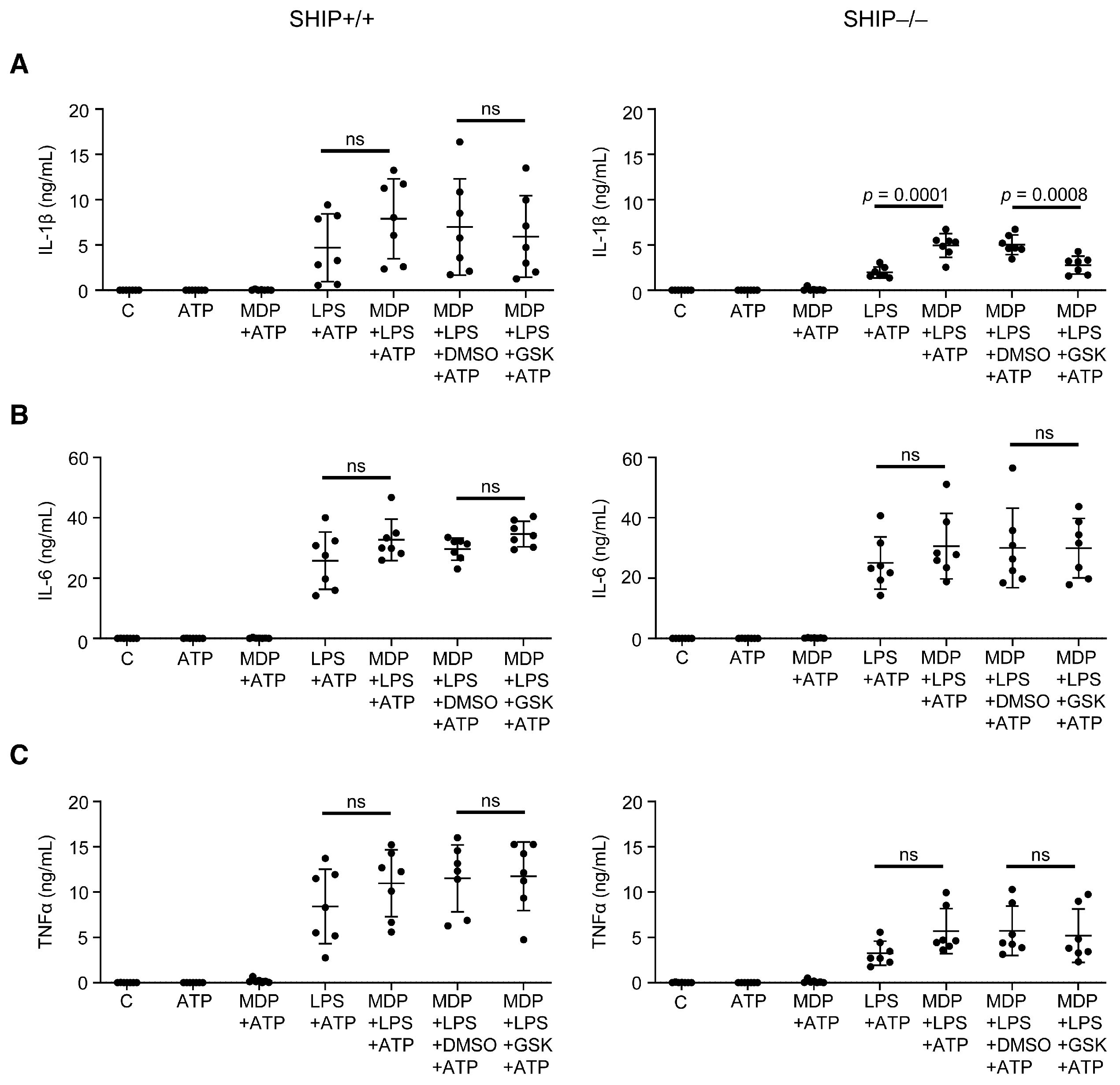
3.2. RIPK2 Inhibition Reduces IL-1β Production by SHIP–/– MCSF-Derived BMDMs Co-Stimulated with MDP and LPS
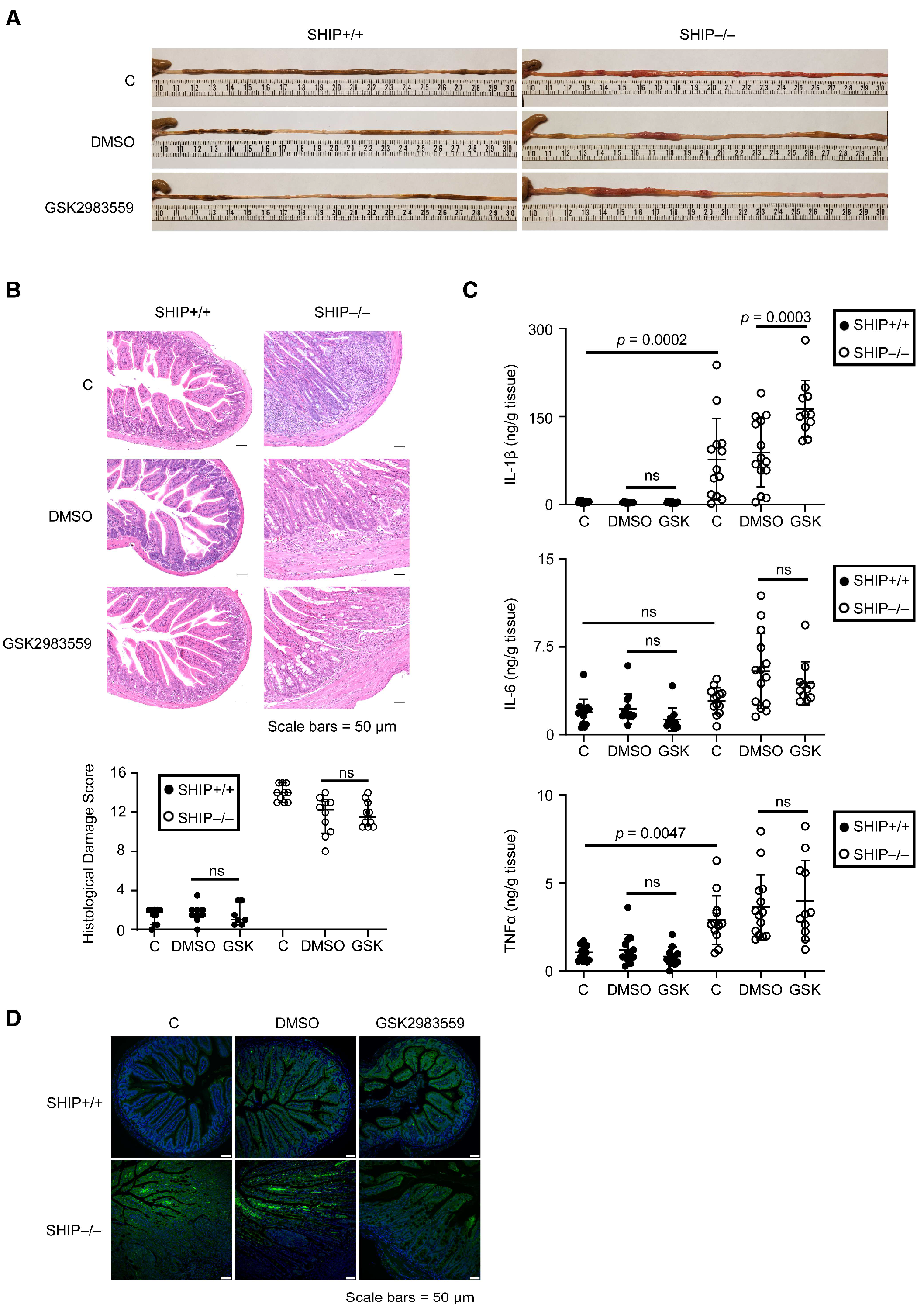
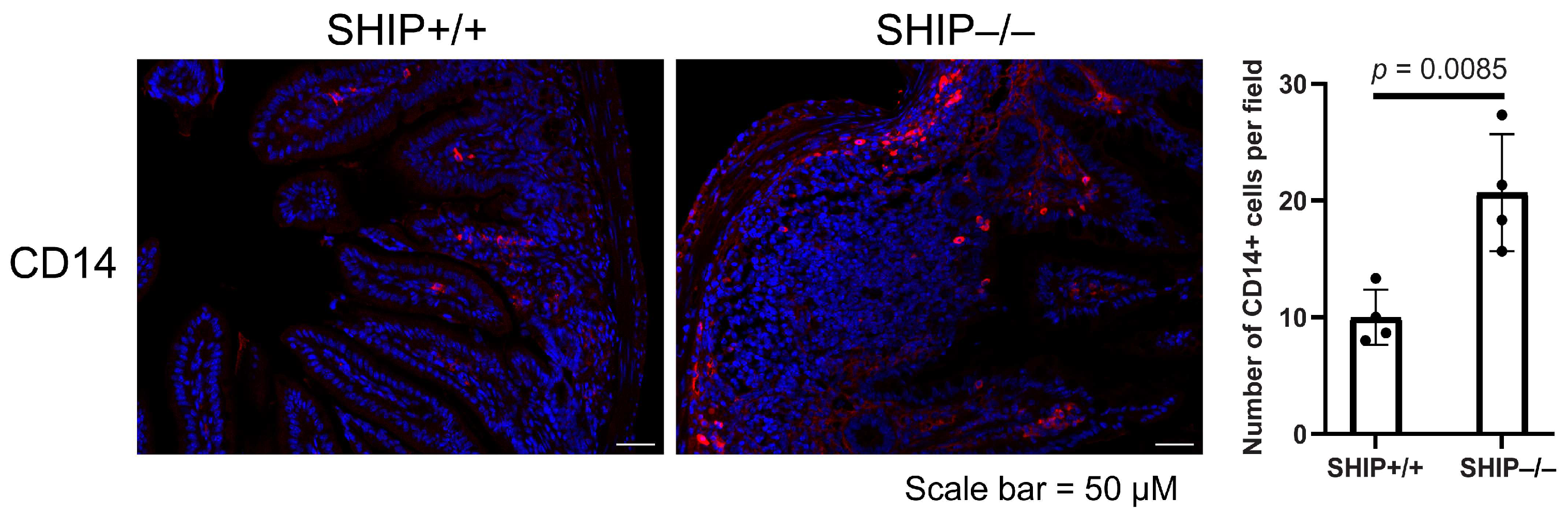
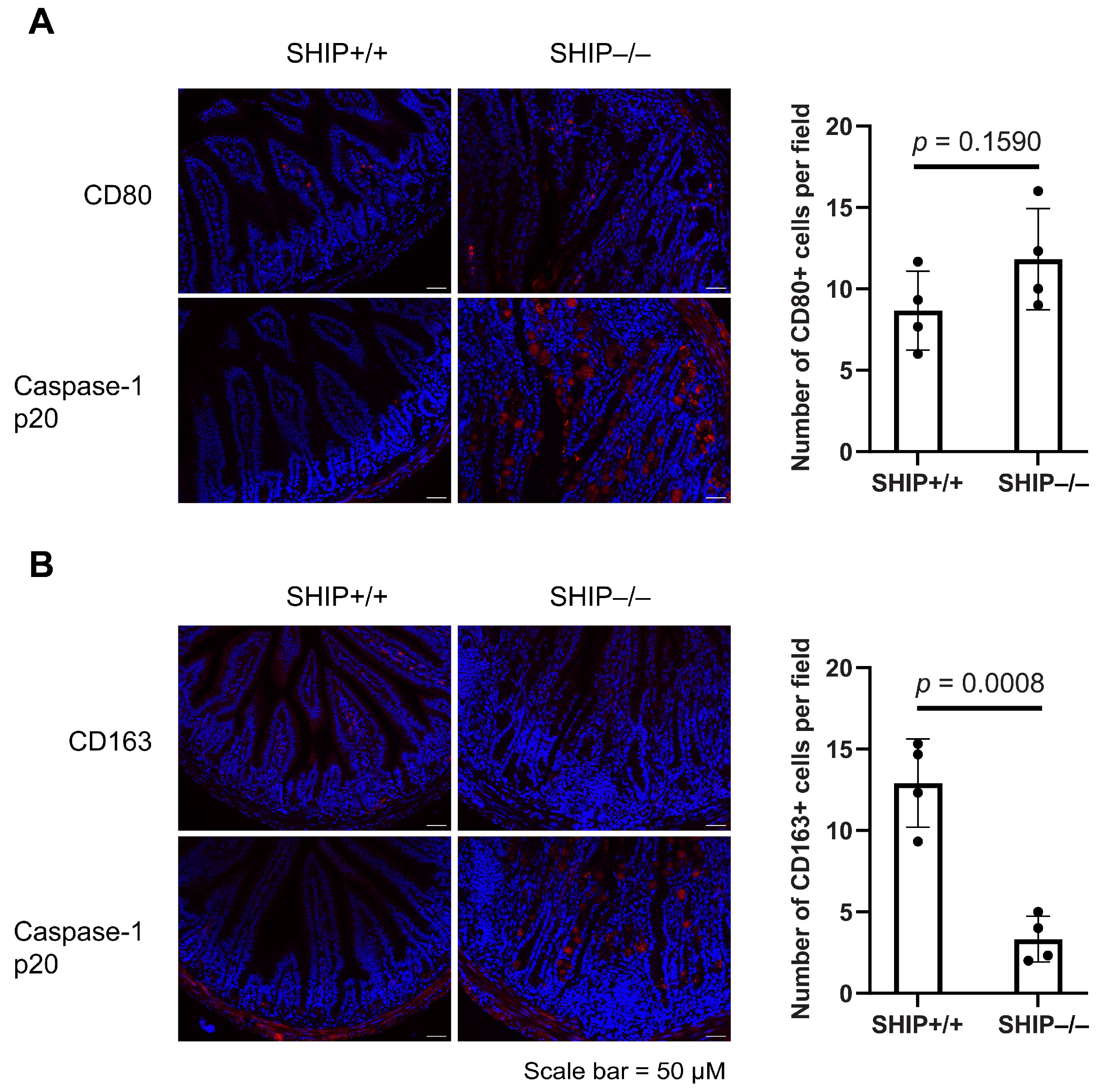
3.3. GSK Treatment Exacerbates Intestinal Inflammation and Increases IL-1β Concentrations in Full-Thickness Ileal Tissue Homogenates from Treated SHIP–/– Mice
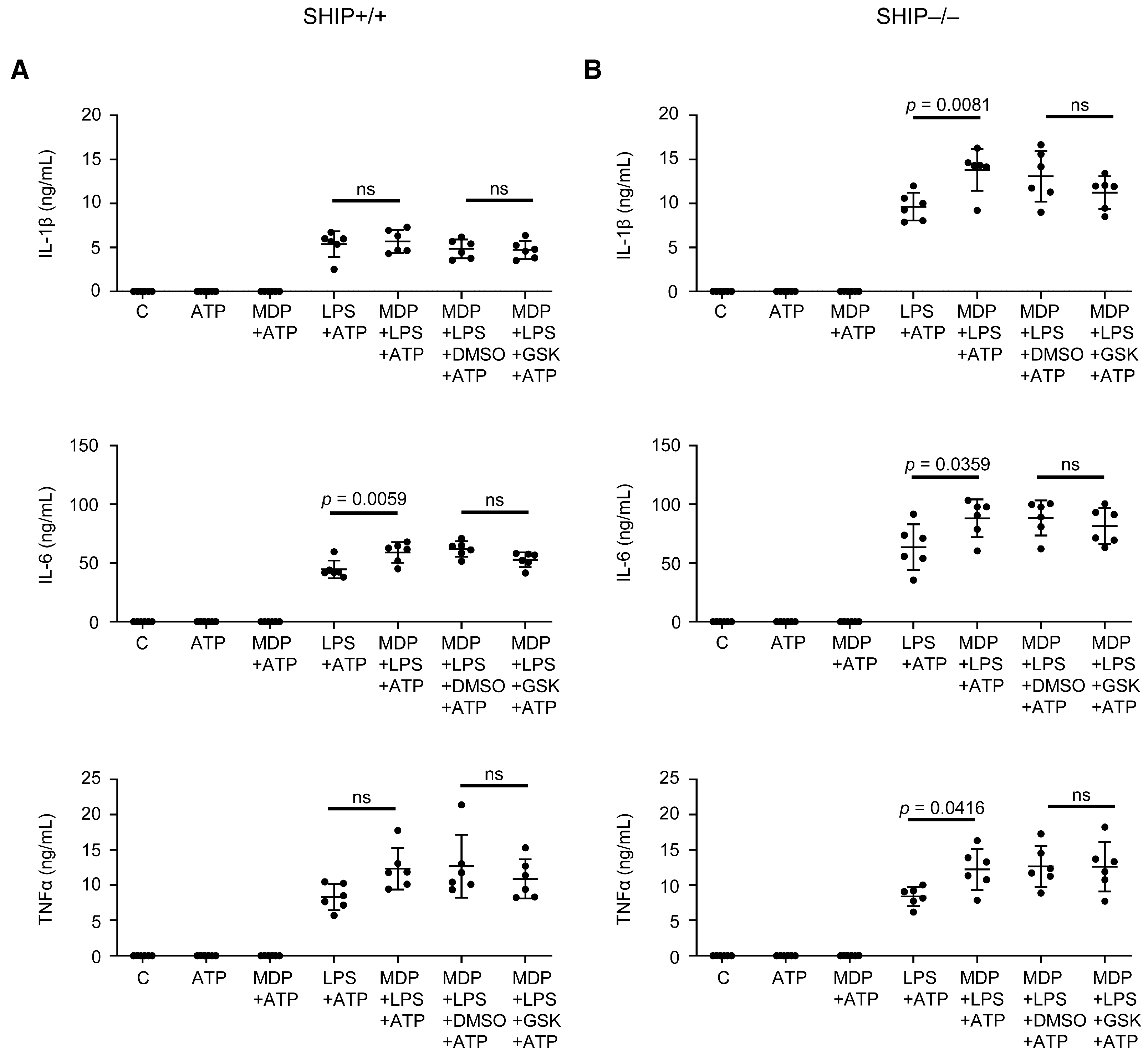
3.4. RIPK2 Inhibition Only Modestly Reduces Pro-Inflammatory Cytokine Production in SHIP–/– Peritoneal Macrophages Compared to BMDMs
3.5. GSK Did Not Reduce Pro-Inflammatory Cytokine Production in Response to Other TLR Ligands
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | adenosine triphosphate |
| BMDMs | bone marrow-derived macrophages |
| CD | Crohn’s disease |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DMSO | dimethyl sulfoxide |
| ELISA | enzyme-linked immunosorbent assay |
| FLA-ST | Salmonella typhimurium flagellin |
| FSL-1 | synthetic diacylated lipoprotein |
| GSK | GSK2983559 |
| IBD | inflammatory bowel disease |
| IL | interleukin |
| LPS | lipopolysaccharide |
| MCSF | macrophage colony-stimulating factor |
| MDP | muramyl dipeptide |
| NFκB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NOD2 | nucleotide-binding oligomerization domain containing 2 |
| ODN1826 | synthetic oligonucleotides containing unmethylated CpG dinucleotides |
| Pam3CSK4 | synthetic triacylated lipopeptide |
| PI3K | phosphatidylinositol 3-kinase |
| PIP3 | phosphatidylinositol (3,4,5)-trisphosphate |
| PRD | proline-rich domain |
| pRIPK2 | phospho-RIPK2 |
| RIPK2 | receptor-interacting serine/threonine-protein kinase 2 |
| SHIP | SH2 domain-containing inositol 5’-phosphatase |
| ssRNA40 | single-stranded RNA 40 |
| TLR | Toll-like receptor |
| TNFα | tumor necrosis factor alpha |
| UC | ulcerative colitis |
| XIAP | X-linked inhibitor of apoptosis protein |
References
- Coward, S.; Clement, F.; Benchimol, E.I.; Bernstein, C.N.; Avina-Zubieta, J.A.; Bitton, A.; Carroll, M.W.; Hazlewood, G.; Jacobson, K.; Jelinski, S.; et al. Past and Future Burden of Inflammatory Bowel Diseases Based on Modeling of Population-Based Data. Gastroenterology 2019, 156, 1345–1353. [Google Scholar] [CrossRef]
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The Global, Regional, and National Burden of Inflammatory Bowel Disease in 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- Coward, S.; Benchimol, E.I.; Kuenzig, M.E.; Windsor, J.W.; Bernstein, C.N.; Bitton, A.; Jones, J.L.; Lee, K.; Murthy, S.K.; Targownik, L.E.; et al. The 2023 Impact of Inflammatory Bowel Disease in Canada: Epidemiology of IBD. J. Can. Assoc. Gastroenterol. 2023, 6, 9–15. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwon, J.E.; Cho, M. Immunological Pathogenesis of Inflammatory Bowel Disease. Intest. Res. 2018, 16, 26–42. [Google Scholar] [CrossRef]
- Wallace, K.L.; Zheng, L.; Kanazawa, Y.; Shih, D.Q. Immunopathology of Inflammatory Bowel Disease. World J. Gastroenterol. 2014, 20, 6–21. [Google Scholar] [CrossRef]
- Murthy, S.K.; Weizman, A.V.; Kuenzig, M.E.; Windsor, J.W.; Kaplan, G.G.; Benchimol, E.I.; Bernstein, C.N.; Bitton, A.; Coward, S.; Jones, J.L.; et al. The 2023 Impact of Inflammatory Bowel Disease in Canada: Treatment Landscape. J. Can. Assoc. Gastroenterol. 2023, 6, 97–110. [Google Scholar] [CrossRef]
- Ben-Horin, S.; Kopylov, U.; Chowers, Y. Optimizing Anti-TNF Treatments in Inflammatory Bowel Disease. Autoimmun. Rev. 2014, 13, 24–30. [Google Scholar] [CrossRef]
- Engelman, J.A.; Luo, J.; Cantley, L.C. The Evolution of Phosphatidylinositol 3-Kinases as Regulators of Growth and Metabolism. Nat. Rev. Genet. 2006, 7, 606–619. [Google Scholar] [CrossRef]
- Katso, R.; Okkenhaug, K.; Ahmadi, K.; White, S.; Timms, J.; Waterfield, M.D. Cellular Function of Phosphoinositide 3-Kinases: Implications for Development, Immunity, Homeostasis, and Cancer. Annu. Rev. Cell Dev. Biol. 2001, 17, 615–675. [Google Scholar] [CrossRef]
- Dobranowski, P.; Sly, L.M. SHIP Negatively Regulates Type II Immune Responses in Mast Cells and Macrophages. J. Leukoc. Biol. 2018, 103, 1053–1064. [Google Scholar] [CrossRef]
- Condé, C.; Rambout, X.; Lebrun, M.; Lecat, A.; Di Valentin, E.; Dequiedt, F.; Piette, J.; Gloire, G.; Legrand, S. The Inositol Phosphatase SHIP-1 Inhibits NOD2-Induced NF-κB Activation by Disturbing the Interaction of XIAP with RIP. PLoS ONE 2012, 7, e41005. [Google Scholar] [CrossRef]
- Fernandes, S.; Srivastava, N.; Sudan, R.; Middleton, F.A.; Shergill, A.K.; Ryan, J.C.; Kerr, W.G. SHIP1 Deficiency in Inflammatory Bowel Disease Is Associated With Severe Crohn’s Disease and Peripheral T Cell Reduction. Front. Immunol. 2018, 9, 1100. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Bonen, D.K.; Inohara, N.; Nicolae, D.L.; Chen, F.F.; Ramos, R.; Britton, H.; Moran, T.; Karaliuskas, R.; Duerr, R.H.; et al. A Frameshift Mutation in NOD2 Associated with Susceptibility to Crohn’s Disease. Nature 2001, 411, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Girardin, S.E.; Boneca, I.G.; Viala, J.; Chamaillard, M.; Labigne, A.; Thomas, G.; Philpott, D.J.; Sansonetti, P.J. Nod2 Is a General Sensor of Peptidoglycan through Muramyl Dipeptide (MDP) Detection. J. Biol. Chem. 2003, 278, 8869–8872. [Google Scholar] [CrossRef]
- Hugot, J.-P.; Chamaillard, M.; Zouali, H.; Lesage, S.; Cézard, J.-P.; Belaiche, J.; Almer, S.; Tysk, C.; O’Morain, C.A.; Gassull, M.; et al. Association of NOD2 Leucine-Rich Repeat Variants with Susceptibility to Crohn’s Disease. Nature 2001, 411, 599–603. [Google Scholar] [CrossRef]
- Lesage, S.; Zouali, H.; Cézard, J.-P.; the EPWG-IBD group; Colombel, J.-F.; the EPIMAD group; Belaiche, J.; the GETAID group; Almer, S.; Tysk, C.; et al. CARD15/NOD2 Mutational Analysis and Genotype-Phenotype Correlation in 612 Patients with Inflammatory Bowel Disease. Am. J. Hum. Genet. 2002, 70, 845–857. [Google Scholar] [CrossRef]
- Ranson, N.; Veldhuis, M.; Mitchell, B.; Fanning, S.; Cook, A.L.; Kunde, D.; Eri, R. NLRP3-Dependent and -Independent Processing of Interleukin (IL)-1β in Active Ulcerative Colitis. Int. J. Mol. Sci. 2019, 20, 57. [Google Scholar] [CrossRef]
- McAlindon, M.; Hawkey, C.; Mahida, Y. Expression of Interleukin 1β and Interleukin 1β Converting Enzyme by Intestinal Macrophages in Health and Inflammatory Bowel Disease. Gut 1998, 42, 214–219. [Google Scholar] [CrossRef]
- Reinecker, H.C.; Steffen, M.; Witthoeft, T.; Pflueger, I.; Schreiber, S.; MacDermott, R.P.; Raedler, A. Enhanced Secretion of Tumour Necrosis Factor-Alpha, IL-6, and IL-1 Beta by Isolated Lamina Propria Mononuclear Cells from Patients with Ulcerative Colitis and Crohn’s Disease. Clin. Exp. Immunol. 1993, 94, 174–181. [Google Scholar] [CrossRef]
- McLarren, K.W.; Cole, A.E.; Weisser, S.B.; Voglmaier, N.S.; Conlin, V.S.; Jacobson, K.; Popescu, O.; Boucher, J.-L.; Sly, L.M. SHIP-Deficient Mice Develop Spontaneous Intestinal Inflammation and Arginase-Dependent Fibrosis. Am. J. Pathol. 2011, 179, 180–188. [Google Scholar] [CrossRef]
- Kerr, W.G.; Park, M.-Y.; Maubert, M.; Engelman, R.W. SHIP Deficiency Causes Crohn’s Disease-like Ileitis. Gut 2011, 60, 177–188. [Google Scholar] [CrossRef]
- Caprilli, R. Why Does Crohn’s Disease Usually Occur in Terminal Ileum? J. Crohns Colitis 2008, 2, 352–356. [Google Scholar] [CrossRef]
- Ngoh, E.N.; Weisser, S.B.; Lo, Y.; Kozicky, L.K.; Jen, R.; Brugger, H.K.; Menzies, S.C.; McLarren, K.W.; Nackiewicz, D.; van Rooijen, N.; et al. Activity of SHIP, Which Prevents Expression of Interleukin 1β, Is Reduced in Patients With Crohn’s Disease. Gastroenterology 2016, 150, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Dobranowski, P.A.; Tang, C.; Sauvé, J.P.; Menzies, S.C.; Sly, L.M. Compositional Changes to the Ileal Microbiome Precede the Onset of Spontaneous Ileitis in SHIP Deficient Mice. Gut Microbes 2019, 10, 578–598. [Google Scholar] [CrossRef]
- Lauro, M.L.; D’Ambrosio, E.A.; Bahnson, B.J.; Grimes, C.L. The Molecular Recognition of Muramyl Dipeptide Occurs in the Leucine-Rich Repeat Domain of Nod. ACS Infect. Dis. 2017, 3, 264–270. [Google Scholar] [CrossRef]
- Ogura, Y.; Inohara, N.; Benito, A.; Chen, F.F.; Yamaoka, S.; Núñez, G. Nod2, a Nod1/Apaf-1 Family Member That Is Restricted to Monocytes and Activates NF-κB. J. Biol. Chem. 2001, 276, 4812–4818. [Google Scholar] [CrossRef]
- Dorsch, M.; Wang, A.; Cheng, H.; Lu, C.; Bielecki, A.; Charron, K.; Clauser, K.; Ren, H.; Polakiewicz, R.D.; Parsons, T.; et al. Identification of a Regulatory Autophosphorylation Site in the Serine–Threonine Kinase RIP. Cell Signal. 2006, 18, 2223–2229. [Google Scholar] [CrossRef]
- Haile, P.A.; Casillas, L.N.; Votta, B.J.; Wang, G.Z.; Charnley, A.K.; Dong, X.; Bury, M.J.; Romano, J.J.; Mehlmann, J.F.; King, B.W.; et al. Discovery of a First-in-Class Receptor Interacting Protein 2 (RIP2) Kinase Specific Clinical Candidate, 2-((4-(Benzo[d]Thiazol-5-Ylamino)-6-(Tert-Butylsulfonyl)Quinazolin-7-Yl)Oxy)Ethyl Dihydrogen Phosphate, for the Treatment of Inflammatory Diseases. J. Med. Chem. 2019, 62, 6482–6494. [Google Scholar] [CrossRef]
- Yang, S.; Tamai, R.; Akashi, S.; Takeuchi, O.; Akira, S.; Sugawara, S.; Takada, H. Synergistic Effect of Muramyldipeptide with Lipopolysaccharide or Lipoteichoic Acid To Induce Inflammatory Cytokines in Human Monocytic Cells in Culture. Infect. Immun. 2001, 69, 2045–2053. [Google Scholar] [CrossRef]
- Wolfert, M.A.; Murray, T.F.; Boons, G.-J.; Moore, J.N. The Origin of the Synergistic Effect of Muramyl Dipeptide with Endotoxin and Peptidoglycan. J. Biol. Chem. 2002, 277, 39179–39186. [Google Scholar] [CrossRef]
- Grimm, M.; Pullman, W.; Bennett, G.; Sullivan, P.; Pavli, P.; Doe, W. Direct Evidence of Monocyte Recruitment to Inflammatory Bowel Disease Mucosa. J. Gastroenterol. Hepatol. 1995, 10, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Kamada, N.; Hisamatsu, T.; Okamoto, S.; Chinen, H.; Kobayashi, T.; Sato, T.; Sakuraba, A.; Kitazume, M.T.; Sugita, A.; Koganei, K.; et al. Unique CD14+ Intestinal Macrophages Contribute to the Pathogenesis of Crohn Disease via IL-23/IFN-γ Axis. J. Clin. Investig. 2008, 118, 2269–2280. [Google Scholar] [CrossRef]
- Thiesen, S.; Janciauskiene, S.; Uronen-Hansson, H.; Agace, W.; Högerkorp, C.-M.; Spee, P.; Håkansson, K.; Grip, O. CD14(Hi)HLA-DR(Dim) Macrophages, with a Resemblance to Classical Blood Monocytes, Dominate Inflamed Mucosa in Crohn’s Disease. J. Leukoc. Biol. 2014, 95, 531–541. [Google Scholar] [CrossRef]
- Lampinen, M.; Waddell, A.; Ahrens, R.; Carlson, M.; Hogan, S.P. CD14+CD33+ Myeloid Cell-CCL11-Eosinophil Signature in Ulcerative Colitis. J. Leukoc. Biol. 2013, 94, 1061–1070. [Google Scholar] [CrossRef]
- Zajd, C.M.; Ziemba, A.M.; Miralles, G.M.; Nguyen, T.; Feustel, P.J.; Dunn, S.M.; Gilbert, R.J.; Lennartz, M.R. Bone Marrow-Derived and Elicited Peritoneal Macrophages Are Not Created Equal: The Questions Asked Dictate the Cell Type Used. Front. Immunol. 2020, 11, 269. [Google Scholar] [CrossRef]
- Wang, C.; Yu, X.; Cao, Q.; Wang, Y.; Zheng, G.; Tan, T.K.; Zhao, H.; Zhao, Y.; Wang, Y.; Harris, D.C. Characterization of Murine Macrophages from Bone Marrow, Spleen and Peritoneum. BMC Immunol. 2013, 14, 6. [Google Scholar] [CrossRef]
- Lu, C.; Wang, A.; Dorsch, M.; Tian, J.; Nagashima, K.; Coyle, A.J.; Jaffee, B.; Ocain, T.D.; Xu, Y. Participation of Rip2 in Lipopolysaccharide Signaling Is Independent of Its Kinase Activity. J. Biol. Chem. 2005, 280, 16278–16283. [Google Scholar] [CrossRef]
- Moreira, L.O.; El Kasmi, K.C.; Smith, A.M.; Finkelstein, D.; Fillon, S.; Kim, Y.-G.; Núñez, G.; Tuomanen, E.; Murray, P.J. The TLR2-MyD88-NOD2-RIPK2 Signalling Axis Regulates a Balanced pro-Inflammatory and IL-10-Mediated Anti-Inflammatory Cytokine Response to Gram-Positive Cell Walls. Cell Microbiol. 2008, 10, 2067–2077. [Google Scholar] [CrossRef]
- Hasegawa, M.; Fujimoto, Y.; Lucas, P.C.; Nakano, H.; Fukase, K.; Núñez, G.; Inohara, N. A Critical Role of RICK/RIP2 Polyubiquitination in Nod-Induced NF-κB Activation. EMBO J. 2007, 27, 373–383. [Google Scholar] [CrossRef]
- Magalhaes, J.G.; Lee, J.; Geddes, K.; Rubino, S.; Philpott, D.J.; Girardin, S.E. Essential Role of Rip2 in the Modulation of Innate and Adaptive Immunity Triggered by Nod1 and Nod2 Ligands. Eur. J. Immunol. 2011, 41, 1445–1455. [Google Scholar] [CrossRef]
- Eickhoff, J.; Hanke, M.; Stein-Gerlach, M.; Kiang, T.P.; Herzberger, K.; Habenberger, P.; Müller, S.; Klebl, B.; Marschall, M.; Stamminger, T.; et al. RICK Activates a NF-κB-Dependent Anti-Human Cytomegalovirus Response. J. Biol. Chem. 2004, 279, 9642–9652. [Google Scholar] [CrossRef]
- Windheim, M.; Lang, C.; Peggie, M.; Plater, L.A.; Cohen, P. Molecular Mechanisms Involved in the Regulation of Cytokine Production by Muramyl Dipeptide. Biochem. J. 2007, 404, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Nembrini, C.; Kisielow, J.; Shamshiev, A.T.; Tortola, L.; Coyle, A.J.; Kopf, M.; Marsland, B.J. The Kinase Activity of Rip2 Determines Its Stability and Consequently Nod1- and Nod2-Mediated Immune Responses. J. Biol. Chem. 2009, 284, 19183–19188. [Google Scholar] [CrossRef] [PubMed]
- Hrdinka, M.; Schlicher, L.; Dai, B.; Pinkas, D.M.; Bufton, J.C.; Picaud, S.; Ward, J.A.; Rogers, C.; Suebsuwong, C.; Nikhar, S.; et al. Small Molecule Inhibitors Reveal an Indispensable Scaffolding Role of RIPK2 in NOD2 Signaling. EMBO J. 2018, 37, e99372. [Google Scholar] [CrossRef]
- Pham, A.-T.; Ghilardi, A.F.; Sun, L. Recent Advances in the Development of RIPK2 Modulators for the Treatment of Inflammatory Diseases. Front. Pharmacol. 2023, 14, 1127722. [Google Scholar] [CrossRef]
- Nachbur, U.; Stafford, C.A.; Bankovacki, A.; Zhan, Y.; Lindqvist, L.M.; Fiil, B.K.; Khakham, Y.; Ko, H.-J.; Sandow, J.J.; Falk, H.; et al. A RIPK2 Inhibitor Delays NOD Signalling Events yet Prevents Inflammatory Cytokine Production. Nat. Commun. 2015, 6, 6442. [Google Scholar] [CrossRef]
- Mazzini, E.; Massimiliano, L.; Penna, G.; Rescigno, M. Oral Tolerance Can Be Established via Gap Junction Transfer of Fed Antigens from CX3CR1+ Macrophages to CD103+ Dendritic Cells. Immunity 2014, 40, 248–261. [Google Scholar] [CrossRef]
- Hadis, U.; Wahl, B.; Schulz, O.; Hardtke-Wolenski, M.; Schippers, A.; Wagner, N.; Müller, W.; Sparwasser, T.; Förster, R.; Pabst, O. Intestinal Tolerance Requires Gut Homing and Expansion of FoxP3+ Regulatory T Cells in the Lamina Propria. Immunity 2011, 34, 237–246. [Google Scholar] [CrossRef]
- Murai, M.; Turovskaya, O.; Kim, G.; Madan, R.; Karp, C.L.; Cheroutre, H.; Kronenberg, M. Interleukin 10 Acts on Regulatory T Cells to Maintain Expression of the Transcription Factor Foxp3 and Suppressive Function in Mice with Colitis. Nat. Immunol. 2009, 10, 1178–1184. [Google Scholar] [CrossRef]
- Kim, M.; Galan, C.; Hill, A.A.; Wu, W.-J.; Fehlner-Peach, H.; Song, H.W.; Schady, D.; Bettini, M.L.; Simpson, K.W.; Longman, R.S.; et al. Critical Role for the Microbiota in CX3CR1+ Intestinal Mononuclear Phagocyte Regulation of Intestinal T Cell Responses. Immunity 2018, 49, 151–163. [Google Scholar] [CrossRef]
- Rugtveit, J.; Haraldsen, G.; Høgåsen, A.K.; Bakka, A.; Brandtzaeg, P.; Scott, H. Respiratory Burst of Intestinal Macrophages in Inflammatory Bowel Disease Is Mainly Caused by CD14+L1+ Monocyte Derived Cells. Gut 1995, 37, 367–373. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, L.; Yu, C.; Yang, X.-F.; Wang, H. Monocyte and Macrophage Differentiation: Circulation Inflammatory Monocyte as Biomarker for Inflammatory Diseases. Biomark. Res. 2014, 2, 1. [Google Scholar] [CrossRef]
- Li, Y.; Lee, P.Y.; Sobel, E.S.; Narain, S.; Satoh, M.; Segal, M.S.; Reeves, W.H.; Richards, H.B. Increased Expression of FcγRI/CD64 on Circulating Monocytes Parallels Ongoing Inflammation and Nephritis in Lupus. Arthritis Res. Ther. 2009, 11, R6. [Google Scholar] [CrossRef] [PubMed]
- Theeuwes, W.F.; Di Ceglie, I.; Dorst, D.N.; Blom, A.B.; Bos, D.L.; Vogl, T.; Tas, S.W.; Jimenez-Royo, P.; Bergstrom, M.; Cleveland, M.; et al. CD64 as Novel Molecular Imaging Marker for the Characterization of Synovitis in Rheumatoid Arthritis. Arthritis Res. Ther. 2023, 25, 158. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.L.; Sly, L.M.; Krystal, G.; Finlay, B.B. The Inositol Phosphatase SHIP Controls Salmonella Enterica Serovar Typhimurium Infection In Vivo. Infect. Immun. 2008, 76, 2913–2922. [Google Scholar] [CrossRef] [PubMed]


| Macrophage | DMSO, MDP+LPS+ATP (ng/mL) | GSK, MDP+LPS+ATP (ng/mL) | % Reduction |
|---|---|---|---|
| MCSF BMDMs | 5.03 | 2.78 | 44.73 |
| Peritoneal macrophages | 13.21 | 11.44 | 13.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, Y.C.F.; Ma, W.J.; Menzies, S.C.; Sly, L.M. RIPK2 Inhibition Blocks NOD2-Mediated IL-1β Production by Macrophages In Vitro but Exacerbates Crohn’s Disease-like Ileitis in SHIP–/– Mice. Immuno 2025, 5, 37. https://doi.org/10.3390/immuno5030037
Pang YCF, Ma WJ, Menzies SC, Sly LM. RIPK2 Inhibition Blocks NOD2-Mediated IL-1β Production by Macrophages In Vitro but Exacerbates Crohn’s Disease-like Ileitis in SHIP–/– Mice. Immuno. 2025; 5(3):37. https://doi.org/10.3390/immuno5030037
Chicago/Turabian StylePang, Yvonne C. F., Wei Jen Ma, Susan C. Menzies, and Laura M. Sly. 2025. "RIPK2 Inhibition Blocks NOD2-Mediated IL-1β Production by Macrophages In Vitro but Exacerbates Crohn’s Disease-like Ileitis in SHIP–/– Mice" Immuno 5, no. 3: 37. https://doi.org/10.3390/immuno5030037
APA StylePang, Y. C. F., Ma, W. J., Menzies, S. C., & Sly, L. M. (2025). RIPK2 Inhibition Blocks NOD2-Mediated IL-1β Production by Macrophages In Vitro but Exacerbates Crohn’s Disease-like Ileitis in SHIP–/– Mice. Immuno, 5(3), 37. https://doi.org/10.3390/immuno5030037






