Verification of Immune Debts in Children Caused by the COVID-19 Pandemic from an Epidemiological and Clinical Perspective
Abstract
:1. Introduction
2. Epidemiological Changes in Infectious Diseases in Children During and After the COVID-19 Pandemic
2.1. Viral Infections in Children
2.2. Bacterial Infections in Children
3. Were There Any Changes in the Clinical Presentation and Severity of Infectious Diseases After the Pandemic?
3.1. Is There Any Changes in Clinical Manifestation of Mycoplasma Pneumoniae ?
3.2. Deep Neck Abscess-Forming Infection
4. Did the COVID-19 Pandemic Affect the Development of Allergic Diseases?
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Fieten, K.B.; Totté, J.E.E.; Levin, E.; Reyman, M.; Meijer, Y.; Knulst, A.; Schuren, F.; Pasmans, S. Fecal Microbiome and Food Allergy in Pediatric Atopic Dermatitis: A Cross-Sectional Pilot Study. Int. Arch. Allergy Immunol. 2018, 175, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Liu, C.H.; Tzianabos, A.O.; Kasper, D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005, 122, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- Walk, J.; de Bree, L.C.J.; Graumans, W.; Stoter, R.; van Gemert, G.J.; van de Vegte-Bolmer, M.; Teelen, K.; Hermsen, C.C.; Arts, R.J.W.; Behet, M.C.; et al. Outcomes of controlled human malaria infection after BCG vaccination. Nat. Commun. 2019, 10, 874. [Google Scholar] [CrossRef]
- Dong, C. Diversification of T-helper-cell lineages: Finding the family root of IL-17-producing cells. Nat. Reviews. Immunol. 2006, 6, 329–333. [Google Scholar] [CrossRef]
- Laidlaw, B.J.; Craft, J.E.; Kaech, S.M. The multifaceted role of CD4(+) T cells in CD8(+) T cell memory. Nat. Rev. Immunol. 2016, 16, 102–111. [Google Scholar] [CrossRef]
- Yazdanbakhsh, M.; Kremsner, P.G.; van Ree, R. Allergy, parasites, and the hygiene hypothesis. Science 2002, 296, 490–494. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H. The immunology of the allergy epidemic and the hygiene hypothesis. Nat. Immunol. 2017, 18, 1076–1083. [Google Scholar] [CrossRef]
- Cohen, R.; Ashman, M.; Taha, M.K.; Varon, E.; Angoulvant, F.; Levy, C.; Rybak, A.; Ouldali, N.; Guiso, N.; Grimprel, E. Pediatric Infectious Disease Group (GPIP) position paper on the immune debt of the COVID-19 pandemic in childhood, how can we fill the immunity gap? Infect. Dis. Now 2021, 51, 418–423. [Google Scholar] [CrossRef]
- Yang, M.C.; Su, Y.T.; Chen, P.H.; Tsai, C.C.; Lin, T.I.; Wu, J.R. Changing patterns of infectious diseases in children during the COVID-19 pandemic. Front. Cell. Infect. Microbiol. 2023, 13, 1200617. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Autore, G.; Ramundo, G.; Esposito, S. Epidemiology of Respiratory Infections during the COVID-19 Pandemic. Viruses 2023, 15, 1160. [Google Scholar] [CrossRef]
- Takashita, E.; Kawakami, C.; Momoki, T.; Saikusa, M.; Shimizu, K.; Ozawa, H.; Kumazaki, M.; Usuku, S.; Tanaka, N.; Okubo, I.; et al. Increased risk of rhinovirus infection in children during the coronavirus disease-19 pandemic. Influenza Other Respir. Viruses 2021, 15, 488–494. [Google Scholar] [CrossRef]
- Danino, D.; van der Beek, B.A.; Givon-Lavi, N.; Greenberg, D.; Ben-Shimol, S.; Dagan, R. Unraveling the Impact of Pneumococcal Conjugate Vaccines on Ambulatory Antibiotic Drug Consumption in Young Children: An Interrupted Time-Series Analysis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 73, 1268–1278. [Google Scholar] [CrossRef]
- Rybak, A.; Levy, C.; Angoulvant, F.; Auvrignon, A.; Gembara, P.; Danis, K.; Vaux, S.; Levy-Bruhl, D.; van der Werf, S.; Béchet, S.; et al. Association of Nonpharmaceutical Interventions During the COVID-19 Pandemic With Invasive Pneumococcal Disease, Pneumococcal Carriage, and Respiratory Viral Infections Among Children in France. JAMA Netw. Open 2022, 5, e2218959. [Google Scholar] [CrossRef]
- Delestrain, C.; Danis, K.; Hau, I.; Behillil, S.; Billard, M.N.; Krajten, L.; Cohen, R.; Bont, L.; Epaud, R. Impact of COVID-19 social distancing on viral infection in France: A delayed outbreak of RSV. Pediatr. Pulmonol. 2021, 56, 3669–3673. [Google Scholar] [CrossRef]
- Billard, M.N.; Bont, L.J. Quantifying the RSV immunity debt following COVID-19: A public health matter. Lancet Infect. Dis. 2023, 23, 3–5. [Google Scholar] [CrossRef]
- Weinberger Opek, M.; Yeshayahu, Y.; Glatman-Freedman, A.; Kaufman, Z.; Sorek, N.; Brosh-Nissimov, T. Delayed respiratory syncytial virus epidemic in children after relaxation of COVID-19 physical distancing measures, Ashdod, Israel, 2021. Eurosurveillance 2021, 26, 2100706. [Google Scholar] [CrossRef]
- Nagasawa, M.; Udagawa, T.; Okada, M.; Nakagawa, R.; Yokoyama, H.; Kato, T.; Furuya, M.; Sakaguchi, H. COVID-19 pandemic-altered epidemiology of respiratory syncytial virus and human metapneumovirus infections in young children. GHM Open 2024, 4, 47–49. [Google Scholar] [CrossRef]
- Lee, S.S.; Viboud, C.; Petersen, E. Understanding the rebound of influenza in the post COVID-19 pandemic period holds important clues for epidemiology and control. Int. J. Infect. Dis. 2022, 122, 1002–1004. [Google Scholar] [CrossRef]
- Available online: https://idsc.tmiph.metro.tokyo.lg.jp/weekly/ (accessed on 23 November 2024).
- Belingheri, M.; Paladino, M.E.; Piacenti, S.; Riva, M.A. Effects of COVID-19 lockdown on epidemic diseases of childhood. J. Med. Virol. 2021, 93, 153–154. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.R.; Rybak, A.; Werner, A.; Béchet, S.; Desandes, R.; Hassid, F.; André, J.M.; Gelbert, N.; Thiebault, G.; Kochert, F.; et al. Trends in pediatric ambulatory community acquired infections before and during COVID-19 pandemic: A prospective multicentric surveillance study in France. Lancet Reg. Health Eur. 2022, 22, 100497. [Google Scholar] [CrossRef] [PubMed]
- Bertran, M.; Amin-Chowdhury, Z.; Sheppard, C.L.; Eletu, S.; Zamarreño, D.V.; Ramsay, M.E.; Litt, D.; Fry, N.K.; Ladhani, S.N. Increased Incidence of Invasive Pneumococcal Disease among Children after COVID-19 Pandemic, England. Emerg. Infect. Dis. 2022, 28, 1669–1672. [Google Scholar] [CrossRef]
- Perniciaro, S.; van der Linden, M.; Weinberger, D.M. Reemergence of Invasive Pneumococcal Disease in Germany During the Spring and Summer of 2021. Clin. Infect. Dis. 2022, 75, 1149–1153. [Google Scholar] [CrossRef]
- Otsuka, T.; Chang, B.; Shirai, T.; Iwaya, A.; Wada, A.; Yamanaka, N.; Okazaki, M. Individual risk factors associated with nasopharyngeal colonization with Streptococcus pneumoniae and Haemophilus influenzae: A Japanese birth cohort study. Pediatr. Infect. Dis. J. 2013, 32, 709–714. [Google Scholar] [CrossRef]
- Flamaing, J.; Peetermans, W.E.; Vandeven, J.; Verhaegen, J. Pneumococcal colonization in older persons in a nonoutbreak setting. J. Am. Geriatr. Soc. 2010, 58, 396–398. [Google Scholar] [CrossRef]
- Walter, N.D.; Taylor, T.H.; Shay, D.K.; Thompson, W.W.; Brammer, L.; Dowell, S.F.; Moore, M.R. Influenza circulation and the burden of invasive pneumococcal pneumonia during a non-pandemic period in the United States. Clin. Infect. Dis. 2010, 50, 175–183. [Google Scholar] [CrossRef]
- Fleming-Dutra, K.E.; Taylor, T.; Link-Gelles, R.; Garg, S.; Jhung, M.A.; Finelli, L.; Jain, S.; Shay, D.; Chaves, S.S.; Baumbach, J.; et al. Effect of the 2009 influenza A(H1N1) pandemic on invasive pneumococcal pneumonia. J. Infect. Dis. 2013, 207, 1135–1143. [Google Scholar] [CrossRef]
- Dagan, R.; Danino, D.; Weinberger, D.M. The Pneumococcus-Respiratory Virus Connection-Unexpected Lessons From the COVID-19 Pandemic. JAMA Netw. Open 2022, 5, e2218966. [Google Scholar] [CrossRef]
- Available online: https://www.niid.go.jp/niid/ja/typhi-m/iasr-reference/2606-related-articles/related-articles-515/11766-515r02.html (accessed on 22 November 2024).
- Available online: https://www.niid.go.jp/niid/ja/typhi-m/iasr-reference/2606-related-articles/related-articles-515/11768-515r07.html (accessed on 22 November 2024).
- Nagasawa, M.; Kato, T.; Tanaka, I.; Ono, E. Selective Change in the Bacteria Cultured and Isolated in Respiratory Sputum from Elderly Patients during the SARS-CoV-2 Pandemic. Appl. Microbiol. 2023, 3, 1003–1012. [Google Scholar] [CrossRef]
- Yan, C.; Sun, H.; Zhao, H. Latest Surveillance Data on Mycoplasma pneumoniae Infections in Children, Suggesting a New Epidemic Occurring in Beijing. J. Clin. Microbiol. 2016, 54, 1400–1401. [Google Scholar] [CrossRef] [PubMed]
- Lind, K.; Benzon, M.W.; Jensen, J.S.; Clyde, W.A., Jr. A seroepidemiological study of Mycoplasma pneumoniae infections in Denmark over the 50-year period 1946-1995. Eur. J. Epidemiol. 1997, 13, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, E. Mycoplasma pneumoniae: Now in the focus of clinicians and epidemiologists. Euro Surveill. Bull. Eur. Sur Les Mal. Transm.=Eur. Commun. Dis. Bull. 2012, 17, 20084. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, T.P.; Balish, M.F.; Waites, K.B. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol. Rev. 2008, 32, 956–973. [Google Scholar] [CrossRef]
- Jacobs, E.; Ehrhardt, I.; Dumke, R. New insights in the outbreak pattern of Mycoplasma pneumoniae. Int. J. Med. Microbiol. 2015, 305, 705–708. [Google Scholar] [CrossRef]
- Beeton, M.L.; Zhang, X.S.; Uldum, S.A.; Bébéar, C.; Dumke, R.; Gullsby, K.; Ieven, M.; Loens, K.; Nir-Paz, R.; Pereyre, S.; et al. Mycoplasma pneumoniae infections, 11 countries in Europe and Israel, 2011 to 2016. Euro Surveill. 2020, 25, 1900112. [Google Scholar] [CrossRef]
- Yamazaki, T.; Kenri, T. Epidemiology of Mycoplasma pneumoniae Infections in Japan and Therapeutic Strategies for Macrolide-Resistant M. pneumoniae. Front. Microbiol. 2016, 7, 693. [Google Scholar] [CrossRef]
- Cheng, Y.; Cheng, Y.; Dai, S.; Hou, D.; Ge, M.; Zhang, Y.; Fan, L.; Pei, Y.; Yu, L.; Xue, G.; et al. The Prevalence of Mycoplasma Pneumoniae Among Children in Beijing Before and During the COVID-19 Pandemic. Front. Cell. Infect. Microbiol. 2022, 12, 854505. [Google Scholar] [CrossRef]
- Meyer Sauteur, P.M.; Beeton, M.L. Mycoplasma pneumoniae: Delayed re-emergence after COVID-19 pandemic restrictions. Lancet Microbe 2024, 5, e100–e101. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, C.; Sun, P.; Zeng, F.; Wang, Q.; Wu, J.; Fang, C.; Zhang, C.; Wang, J.; Gu, Y.; et al. Epidemic features and megagenomic analysis of childhood Mycoplasma pneumoniae post COVID-19 pandemic: A 6-year study in southern China. Emerg. Microbes Infect. 2024, 13, 2353298. [Google Scholar] [CrossRef]
- Edouard, S.; Boughammoura, H.; Colson, P.; La Scola, B.; Fournier, P.E.; Fenollar, F. Large-Scale Outbreak of Mycoplasma pneumoniae Infection, Marseille, France, 2023–2024. Emerg. Infect. Dis. 2024, 30, 1481–1484. [Google Scholar] [CrossRef] [PubMed]
- Edens, C.; Clopper, B.R.; DeVies, J.; Benitez, A.; McKeever, E.R.; Johns, D.; Wolff, B.; Selvarangan, R.; Schuster, J.E.; Weinberg, G.A.; et al. Notes from the Field: Reemergence of Mycoplasma pneumoniae Infections in Children and Adolescents After the COVID-19 Pandemic, United States, 2018–2024. MMWR. Morb. Mortal. Wkly. Rep. 2024, 73, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.B.; He, W.; Gui, Y.H.; Lu, Q.; Yin, Y.; Zhang, J.H.; Dong, X.Y.; Wang, Y.W.; Ye, Y.Z.; Xu, H.; et al. Current Mycoplasma pneumoniae epidemic among children in Shanghai: Unusual pneumonia caused by usual pathogen. World J. Pediatr. 2024, 20, 5–10. [Google Scholar] [CrossRef]
- Wu, Q.; Pan, X.; Han, D.; Ma, Z.; Zhang, H. New Insights into the Epidemiological Characteristics of Mycoplasma pneumoniae Infection before and after the COVID-19 Pandemic. Microorganisms 2024, 12, 2019. [Google Scholar] [CrossRef]
- Shaw, D.; Abad, R.; Amin-Chowdhury, Z.; Bautista, A.; Bennett, D.; Broughton, K.; Cao, B.; Casanova, C.; Choi, E.H.; Chu, Y.W.; et al. Trends in invasive bacterial diseases during the first 2 years of the COVID-19 pandemic: Analyses of prospective surveillance data from 30 countries and territories in the IRIS Consortium. Lancet Digit. Health 2023, 5, e582–e593. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Nagaoka, K.; Kawasuji, H.; Kawagishi, T.; Fuchigami, T.; Ikeda, K.; Kanatani, J.I.; Doi, T.; Oishi, K.; Yamamoto, Y. COVID-19 complicated with severe M1(UK)-lineage Streptococcus pyogenes infection in elderly patients: A report of two cases. Int. J. Infect. Dis. 2024, 148, 107246. [Google Scholar] [CrossRef]
- Peetermans, M.; Matheeussen, V.; Moerman, C.; De Rydt, F.; Thieren, S.; Pollet, E.; Casaer, M.; De Backer, B.; De Paep, R.; Debaveye, Y.; et al. Clinical and molecular epidemiological features of critically ill patients with invasive group A Streptococcus infections: A Belgian multicenter case-series. Ann. Intensive Care 2024, 14, 19. [Google Scholar] [CrossRef]
- Vieira, A.; Wan, Y.; Ryan, Y.; Li, H.K.; Guy, R.L.; Papangeli, M.; Huse, K.K.; Reeves, L.C.; Soo, V.W.C.; Daniel, R.; et al. Rapid expansion and international spread of M1(UK) in the post-pandemic UK upsurge of Streptococcus pyogenes. Nat. Commun. 2024, 15, 3916. [Google Scholar] [CrossRef]
- Lynskey, N.N.; Jauneikaite, E.; Li, H.K.; Zhi, X.; Turner, C.E.; Mosavie, M.; Pearson, M.; Asai, M.; Lobkowicz, L.; Chow, J.Y.; et al. Emergence of dominant toxigenic M1T1 Streptococcus pyogenes clone during increased scarlet fever activity in England: A population-based molecular epidemiological study. Lancet Infect. Dis. 2019, 19, 1209–1218. [Google Scholar] [CrossRef]
- Takahashi, S.; Kishino, A.; Miyai, K.; Takishima, S.; Omori, T.; Furuno, H.; Iemura, R.; Ono, M.; Ogasawara, K.; Sutani, A.; et al. Impact of the COVID-19 Pandemic on Epidemiological Trends in Pediatric Cervical Abscess-Forming Infections. Microorganisms 2025, 13, 190. [Google Scholar] [CrossRef]
- Oh, J.; Lee, M.; Kim, M.; Kim, H.J.; Lee, S.W.; Rhee, S.Y.; Koyanagi, A.; Smith, L.; Kim, M.S.; Lee, H.; et al. Incident allergic diseases in post-COVID-19 condition: Multinational cohort studies from South Korea, Japan and the UK. Nat. Commun. 2024, 15, 2830. [Google Scholar] [CrossRef] [PubMed]
- da Silva Alves, C.; Baptista Pestana, R.; Morais-Almeida, M. Recent insights into the impacts of COVID-19 on pediatric asthma. Expert Rev. Clin. Immunol. 2024, 20, 1347–1366. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, S.; Khatami, S.; Mesdaghi, M. The Effect of COVID-19 Pandemic on the Infants’ Microbiota and the Probability of Development of Allergic and Autoimmune Diseases. Int. Arch. Allergy Immunol. 2022, 183, 435–442. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 237ra265. [Google Scholar] [CrossRef]
- Meropol, S.B.; Edwards, A. Development of the infant intestinal microbiome: A bird’s eye view of a complex process. Birth Defects Research. Part C Embryo Today Rev. 2015, 105, 228–239. [Google Scholar] [CrossRef]
- Yee, A.L.; Gilbert, J.A. MICROBIOME. Is triclosan harming your microbiome? Science 2016, 353, 348–349. [Google Scholar] [CrossRef]
- Tun, H.M.; Konya, T.; Takaro, T.K.; Brook, J.R.; Chari, R.; Field, C.J.; Guttman, D.S.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; et al. Exposure to household furry pets influences the gut microbiota of infant at 3-4 months following various birth scenarios. Microbiome 2017, 5, 40. [Google Scholar] [CrossRef]
- Lavorini, F. The challenge of delivering therapeutic aerosols to asthma patients. ISRN Allergy 2013, 2013, 102418. [Google Scholar] [CrossRef]
- Giaxi, P.; Maniatelli, E.; Vivilaki, V.G. Evaluation of mode of delivery in pregnant women infected with COVID-19. Eur. J. Midwifery 2020, 4, 28. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Z.; Zhang, J.; Zhu, F.; Tang, Y.; Shen, X. A Case of 2019 Novel Coronavirus in a Pregnant Woman With Preterm Delivery. Clin. Infect. Dis. 2020, 71, 844–846. [Google Scholar] [CrossRef]
- Hantoushzadeh, S.; Shamshirsaz, A.A.; Aleyasin, A.; Seferovic, M.D.; Aski, S.K.; Arian, S.E.; Pooransari, P.; Ghotbizadeh, F.; Aalipour, S.; Soleimani, Z.; et al. Maternal death due to COVID-19. Am. J. Obstet. Gynecol. 2020, 223, 109.e1–109.e16. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Liccardi, G.; Bilò, M.B.; Milanese, M.; Martini, M.; Pane, G.; De Maio, A.; Rogliani, P. COVID-19 lockdown, personal protective equipment, hyper-hygiene and allergy. Eur. Ann. Allergy Clin. Immunol. 2023, 55, 51–56. [Google Scholar] [CrossRef]
- Novak, N.; Cabanillas, B. Viruses and asthma: The role of common respiratory viruses in asthma and its potential meaning for SARS-CoV-2. Immunology 2020, 161, 83–93. [Google Scholar] [CrossRef]
- Global, regional, and national incidence and mortality burden of non-COVID-19 lower respiratory infections and aetiologies, 1990–2021: A systematic analysis from the Global Burden of Disease Study 2021. Lancet Infect. Dis. 2024, 24, 974–1002. [CrossRef]
- Kuandyk Sabitova, A.; Ortega, M.A.; Ntegwa, M.J.; Sarria-Santamera, A. Impact of the COVID-19 pandemic on access to and delivery of maternal and child healthcare services in low-and middle-income countries: A systematic review of the literature. Front. Public Health 2024, 12, 1346268. [Google Scholar] [CrossRef]

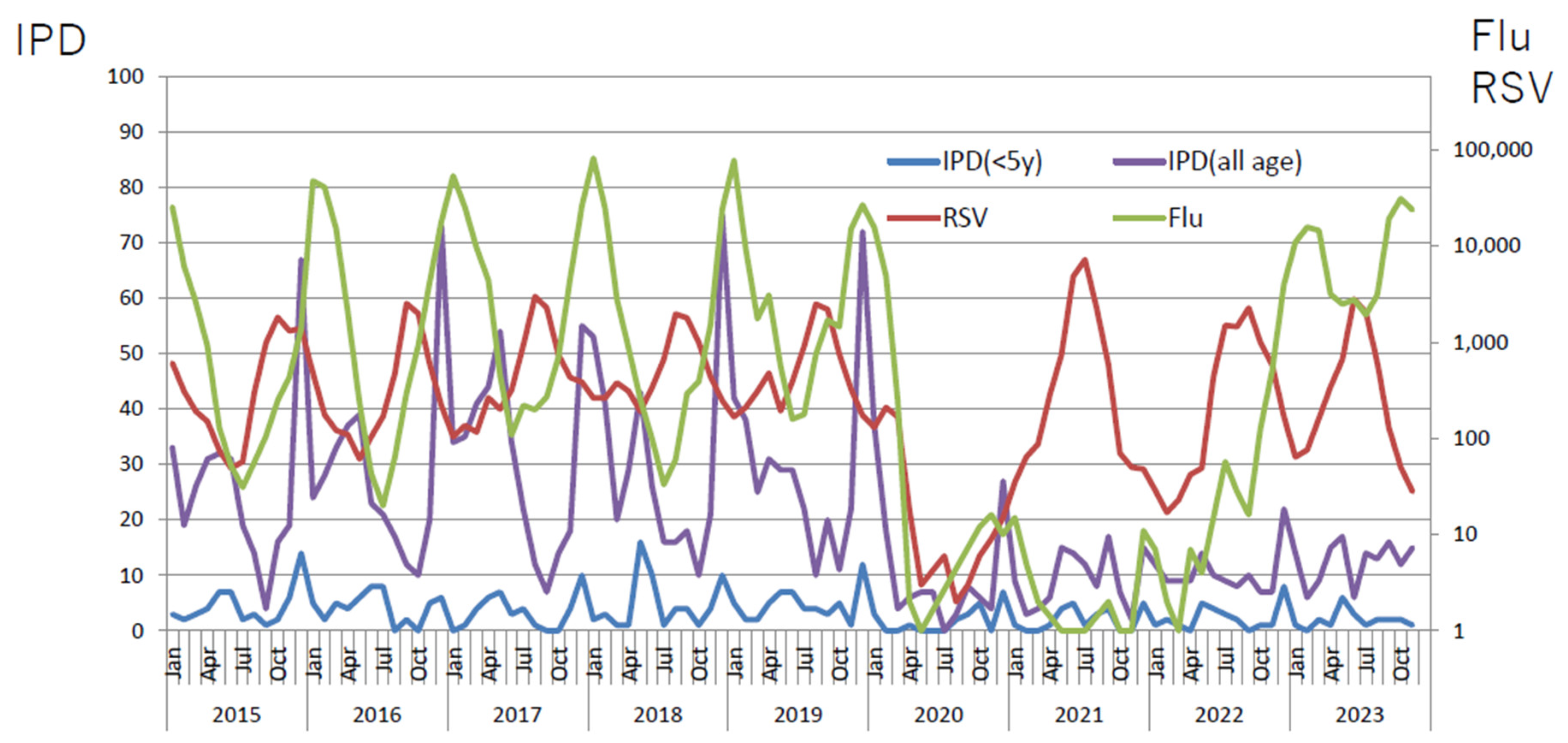
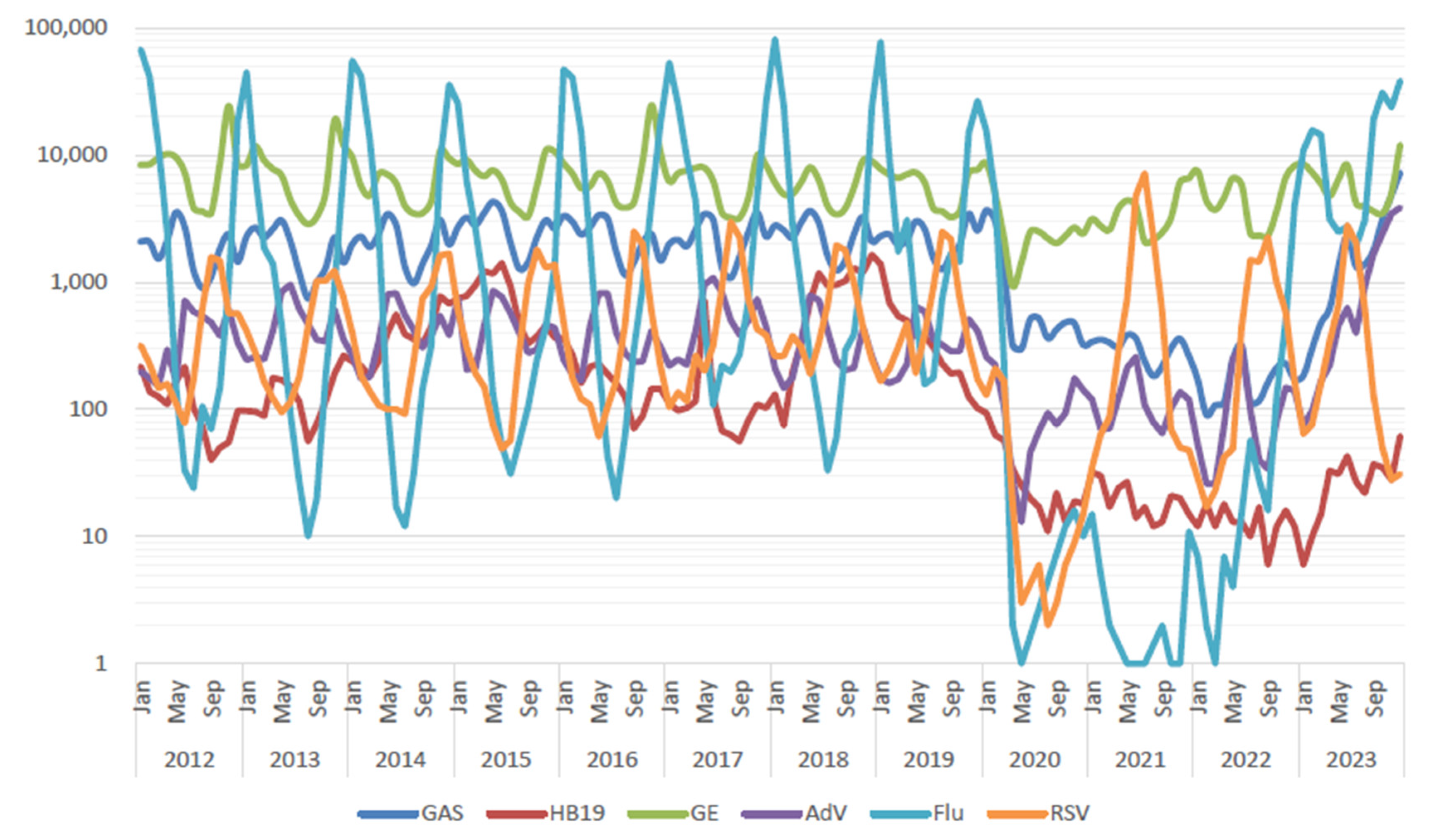
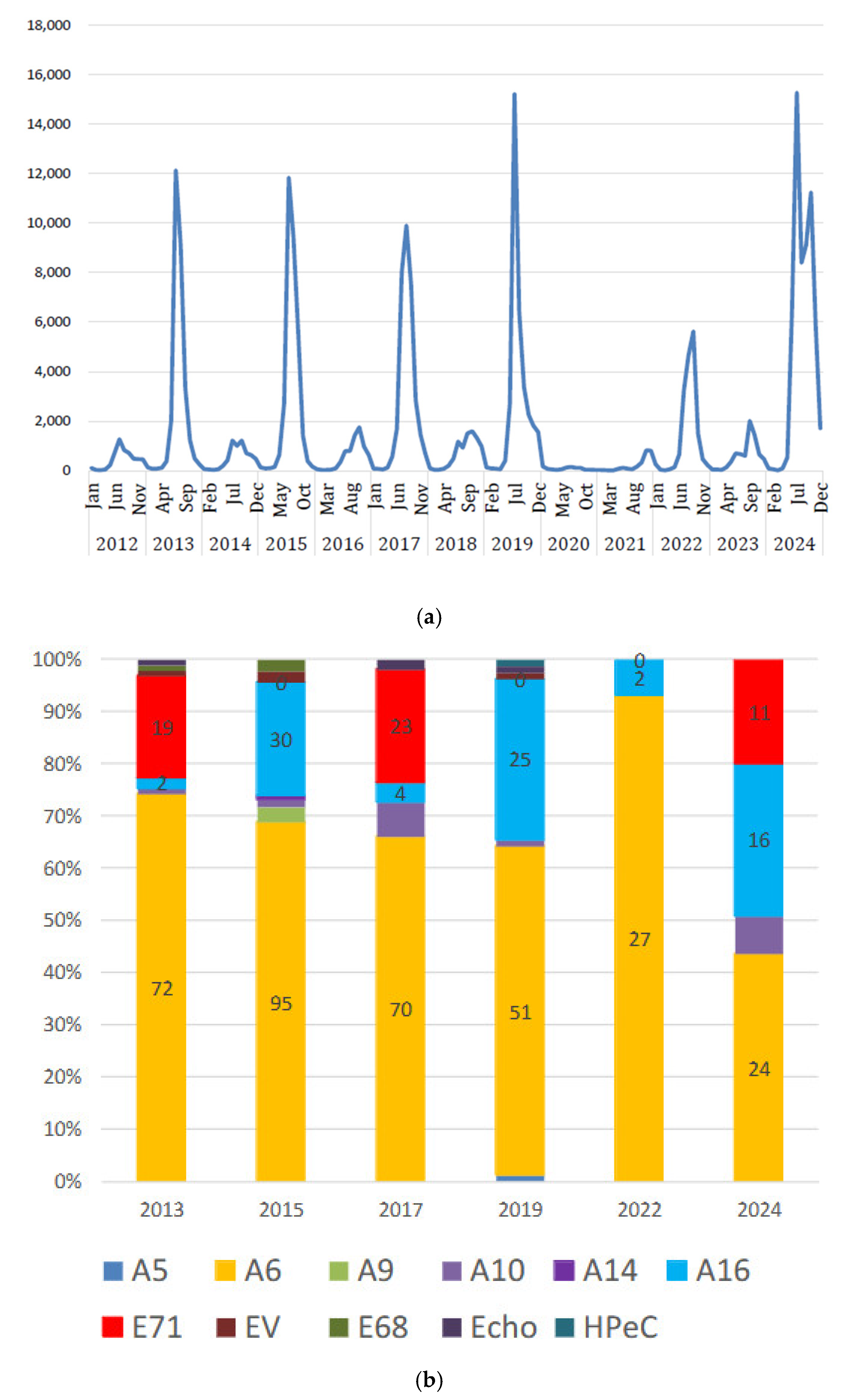
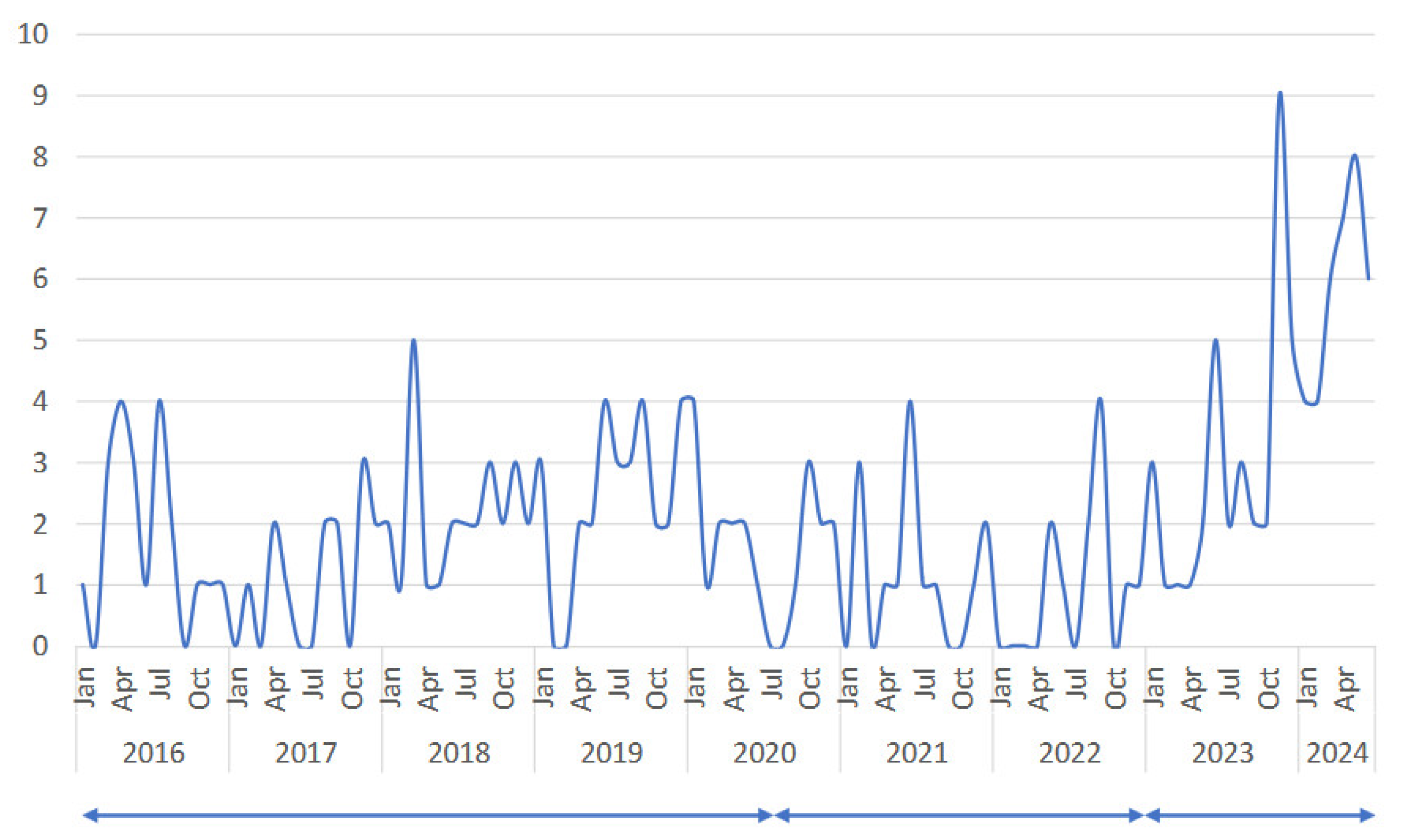
| n | age (y) | onset to admission (days) | hospital stay (days) | WBC (/μL) | CRP (mg/dL) | LDH (U/L) | use of steroid | PCR test | pneumoniae on Chest X-ray | ||
| For hypercytokinemia | for wheeze | ||||||||||
| 2015–2016 | 44 | 6.6 ± 3.4 | 7.8 ± 3.0 | 4.5 ± 2.2 | 8315 ± 3454 | 3.4 ± 3.2 | 460 ± 150 | 8 | 6 | 29 | 35 |
| 2024 | 54 | 5.9 ± 4.1 | 6.6 ± 2.9 | 4.7 ± 3.0 | 9539 ± 4643 | 3.5 ± 3.2 | 436 ± 177 | 23 | 12 | 50 | 31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagasawa, M. Verification of Immune Debts in Children Caused by the COVID-19 Pandemic from an Epidemiological and Clinical Perspective. Immuno 2025, 5, 5. https://doi.org/10.3390/immuno5010005
Nagasawa M. Verification of Immune Debts in Children Caused by the COVID-19 Pandemic from an Epidemiological and Clinical Perspective. Immuno. 2025; 5(1):5. https://doi.org/10.3390/immuno5010005
Chicago/Turabian StyleNagasawa, Masayuki. 2025. "Verification of Immune Debts in Children Caused by the COVID-19 Pandemic from an Epidemiological and Clinical Perspective" Immuno 5, no. 1: 5. https://doi.org/10.3390/immuno5010005
APA StyleNagasawa, M. (2025). Verification of Immune Debts in Children Caused by the COVID-19 Pandemic from an Epidemiological and Clinical Perspective. Immuno, 5(1), 5. https://doi.org/10.3390/immuno5010005






