The Microbiome, Inflammation, and GVHD Axis: The Balance Between the “Gut” and the Bad
Abstract
1. Introduction
2. Pre-Clinical Studies
3. Clinical Studies
4. Antibiotics Impact in Microbiome
5. Molecular Microbiological Techniques and Their Impact on Microbiota Studies in HSCT
6. Mechanism of Action Between the Microbiome and GVHD
6.1. HLA Regulation by the Microbiome During GVHD
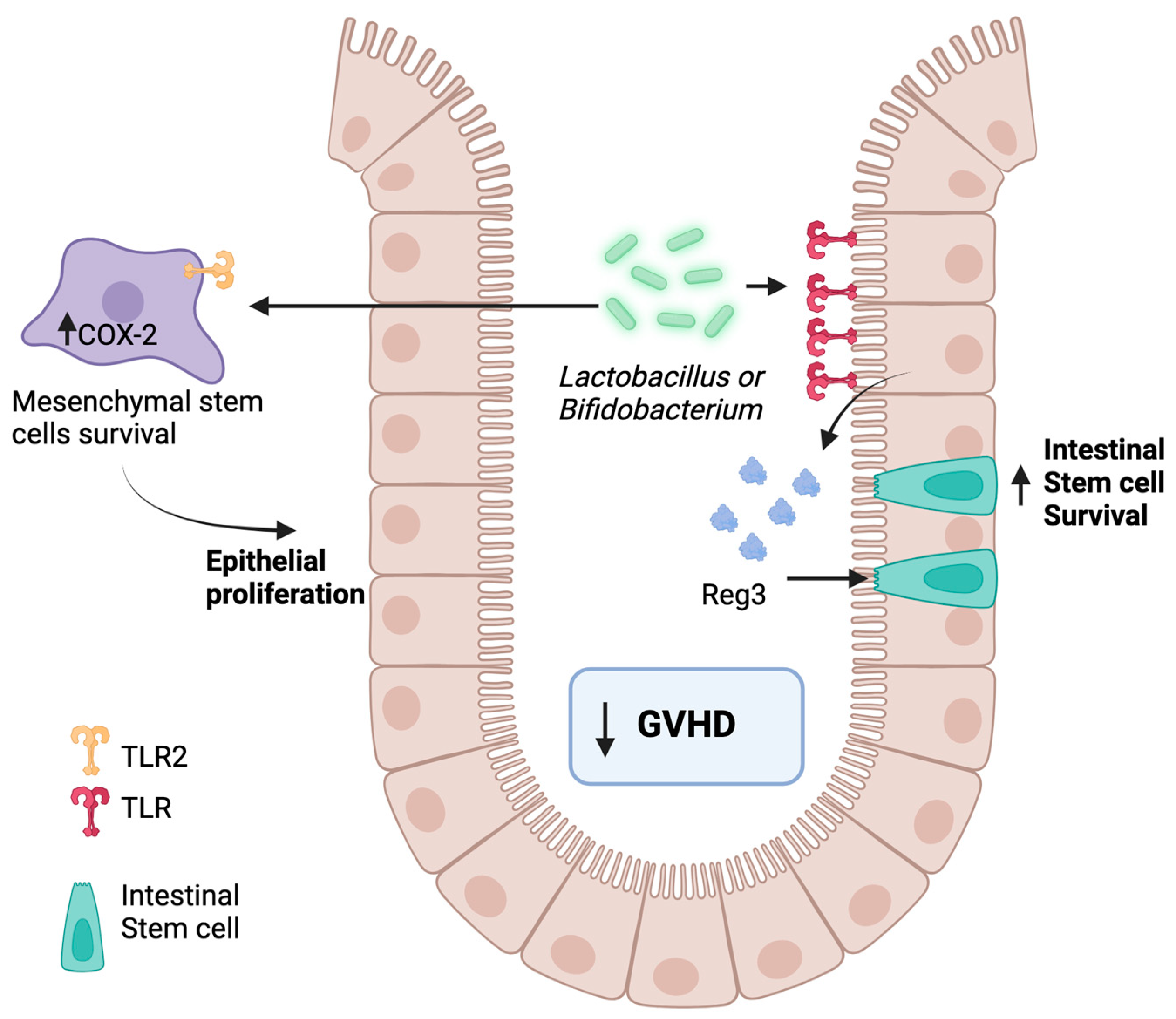
6.2. Toll-like Receptors’ Regulation by the Microbiome in GVHD
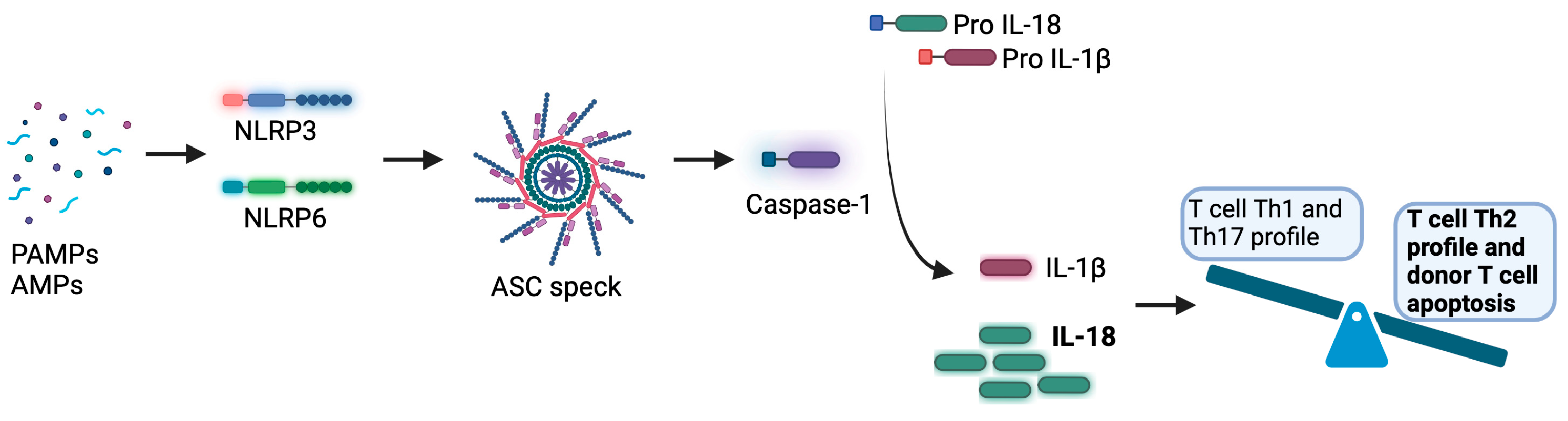
6.3. Inflammasome and Microbiome During GVHD
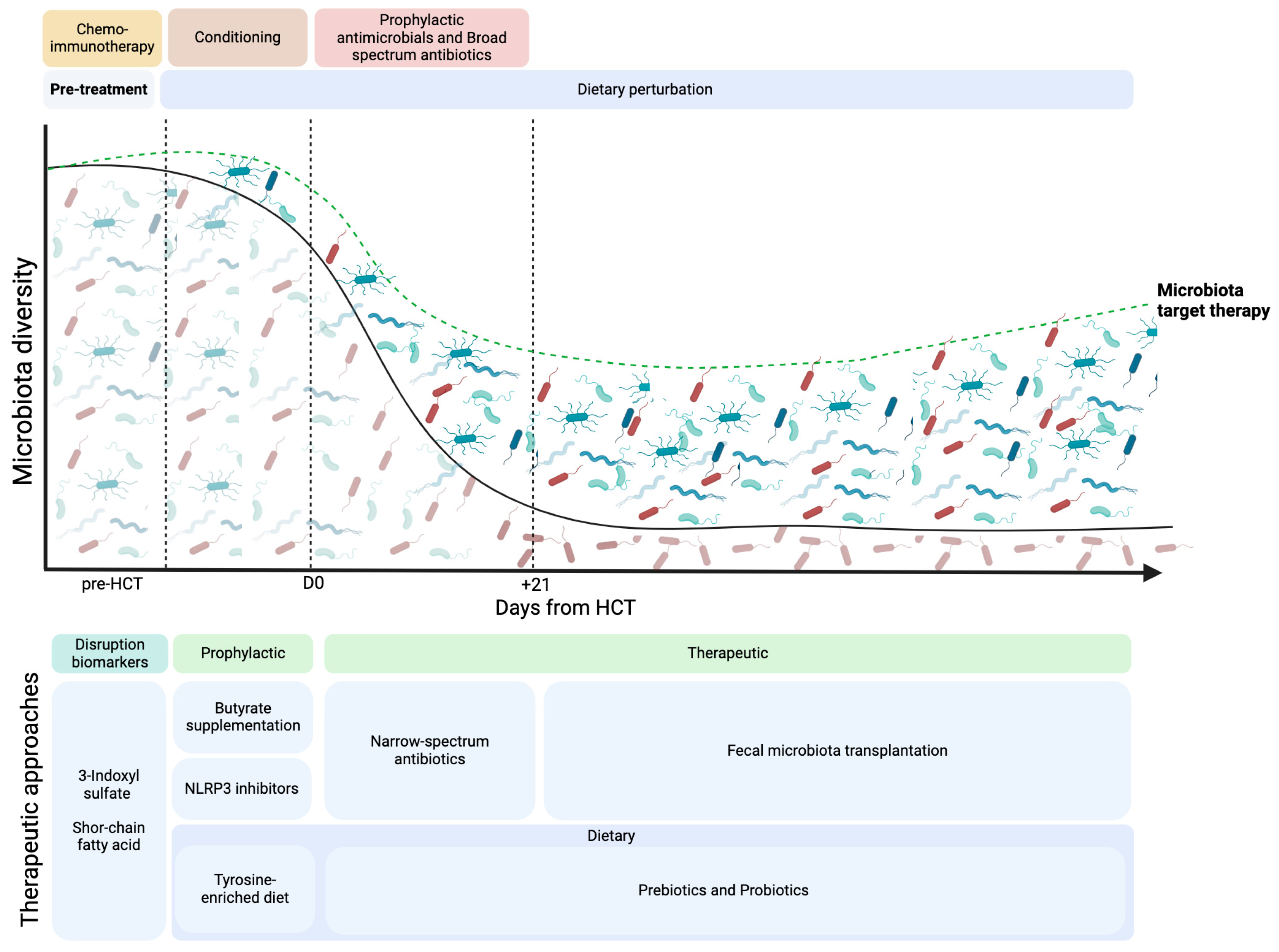
7. Prevention and Treatment
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buchbinder, D.; Nugent, D.J.; Brazauskas, R.; Wang, Z.; Aljurf, M.D.; Cairo, M.S.; Chow, R.; Duncan, C.; Eldjerou, L.K.; Gupta, V.; et al. Late effects in hematopoietic cell transplant recipients with acquired severe aplastic anemia: A report from the late effects working committee of the center for international blood and marrow transplant research. Biol. Blood Marrow Transpl. 2012, 18, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Cusatis, R.; Litovich, C.; Feng, Z.; Allbee-Johnson, M.; Kapfhammer, M.; Mattila, D.; Akinola, I.; Phelan, R.; Broglie, L.; Auletta, J.J.; et al. Current Trends and Outcomes in Cellular Therapy Activity in the United States, Including Prospective Patient-Reported Outcomes Data Collection in the Center for International Blood and Marrow Transplant Research Registry. Transpl. Cell Ther. 2024, 30, 917.e1–917.e12. [Google Scholar] [CrossRef]
- Søborg, A.; Reekie, J.; Sengeløv, H.; Da Cunha-Bang, C.; Lund, T.K.; Ekenberg, C.; Lodding, I.P.; Moestrup, K.S.; Lundgren, L.; Lundgren, J.D.; et al. Trends in underlying causes of death in allogeneic hematopoietic cell transplant recipients over the last decade. Eur. J. Haematol. 2024, 112, 802–809. [Google Scholar] [CrossRef]
- Gyurkocza, B.; Rezvani, A.; Storb, R.F. Allogeneic hematopoietic cell transplantation: The state of the art. Expert. Rev. Hematol. 2010, 3, 285–299. [Google Scholar] [CrossRef]
- Zeiser, R.; Blazar, B.R. Pathophysiology of Chronic Graft-versus-Host Disease and Therapeutic Targets. N. Engl. J. Med. 2017, 377, 2565–2579. [Google Scholar] [CrossRef] [PubMed]
- Schoemans, H.M.; Lee, S.J.; Ferrara, J.L.; Wolff, D.; Levine, J.E.; Schultz, K.R.; Shaw, B.E.; Flowers, M.E.; Ruutu, T.; Greinix, H.; et al. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transpl. 2018, 53, 1401–1415. [Google Scholar] [CrossRef]
- Malard, F.; Holler, E.; Sandmaier, B.M.; Huang, H.; Mohty, M. Acute graft-versus-host disease. Nat. Rev. Dis. Primers 2023, 9, 27. [Google Scholar] [CrossRef]
- Salomao, M.; Dorritie, K.; Mapara, M.Y.; Sepulveda, A. Histopathology of Graft-vs-Host Disease of Gastrointestinal Tract and Liver: An Update. Am. J. Clin. Pathol. 2016, 145, 591–603. [Google Scholar] [CrossRef]
- Perkey, E.; Maillard, I. New Insights into Graft-Versus-Host Disease and Graft Rejection. Annu. Rev. Pathol. 2018, 13, 219–245. [Google Scholar] [CrossRef]
- Zeiser, R.; Blazar, B.R. Acute Graft-versus-Host Disease—Biologic Process, Prevention, and Therapy. N. Engl. J. Med. 2017, 377, 2167–2179. [Google Scholar] [CrossRef]
- Koyama, M.; Kuns, R.D.; Olver, S.D.; Raffelt, N.C.; Wilson, Y.A.; Don, A.L.; Lineburg, K.E.; Cheong, M.; Robb, R.J.; Markey, K.A.; et al. Recipient nonhematopoietic antigen-presenting cells are sufficient to induce lethal acute graft-versus-host disease. Nat. Med. 2011, 18, 135–142. [Google Scholar] [CrossRef]
- Hill, G.R.; Ferrara, J.L. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: Rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood 2000, 95, 2754–2759. [Google Scholar] [CrossRef]
- Koyama, M.; Hill, G.R. The primacy of gastrointestinal tract antigen-presenting cells in lethal graft-versus-host disease. Blood 2019, 134, 2139–2148. [Google Scholar] [CrossRef] [PubMed]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70, S38–S44. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Galloway-Peña, J.R.; Smith, D.P.; Sahasrabhojane, P.; Ajami, N.J.; Wadsworth, W.D.; Daver, N.G.; Chemaly, R.F.; Marsh, L.; Ghantoji, S.S.; Pemmaraju, N.; et al. The role of the gastrointestinal microbiome in infectious complications during induction chemotherapy for acute myeloid leukemia. Cancer 2016, 122, 2186–2196. [Google Scholar] [CrossRef]
- Taur, Y.; Xavier, J.B.; Lipuma, L.; Ubeda, C.; Goldberg, J.; Gobourne, A.; Lee, Y.J.; Dubin, K.A.; Socci, N.D.; Viale, A.; et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin. Infect. Dis. 2012, 55, 905–914. [Google Scholar] [CrossRef]
- Jenq, R.R.; Ubeda, C.; Taur, Y.; Menezes, C.C.; Khanin, R.; Dudakov, J.A.; Liu, C.; West, M.L.; Singer, N.V.; Equinda, M.J.; et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J. Exp. Med. 2012, 209, 903–911. [Google Scholar] [CrossRef]
- Taur, Y.; Jenq, R.R.; Perales, M.A.; Littmann, E.R.; Morjaria, S.; Ling, L.; No, D.; Gobourne, A.; Viale, A.; Dahi, P.B.; et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 2014, 124, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Holler, E.; Butzhammer, P.; Schmid, K.; Hundsrucker, C.; Koestler, J.; Peter, K.; Zhu, W.; Sporrer, D.; Hehlgans, T.; Kreutz, M.; et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: Loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol. Blood Marrow Transpl. 2014, 20, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Jenq, R.R.; Taur, Y.; Devlin, S.M.; Ponce, D.M.; Goldberg, J.D.; Ahr, K.F.; Littmann, E.R.; Ling, L.; Gobourne, A.C.; Miller, L.C.; et al. Intestinal Blautia Is Associated with Reduced Death from Graft-versus-Host Disease. Biol. Blood Marrow Transpl. 2015, 21, 1373–1383. [Google Scholar] [CrossRef]
- Simms-Waldrip, T.R.; Sunkersett, G.; Coughlin, L.A.; Savani, M.R.; Arana, C.; Kim, J.; Kim, M.; Zhan, X.; Greenberg, D.E.; Xie, Y.; et al. Antibiotic-Induced Depletion of Anti-inflammatory Clostridia Is Associated with the Development of Graft-versus-Host Disease in Pediatric Stem Cell Transplantation Patients. Biol. Blood Marrow Transpl. 2017, 23, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Doki, N.; Suyama, M.; Sasajima, S.; Ota, J.; Igarashi, A.; Mimura, I.; Morita, H.; Fujioka, Y.; Sugiyama, D.; Nishikawa, H.; et al. Clinical impact of pre-transplant gut microbial diversity on outcomes of allogeneic hematopoietic stem cell transplantation. Ann. Hematol. 2017, 96, 1517–1523. [Google Scholar] [CrossRef]
- Golob, J.L.; Pergam, S.A.; Srinivasan, S.; Fiedler, T.L.; Liu, C.; Garcia, K.; Mielcarek, M.; Ko, D.; Aker, S.; Marquis, S.; et al. Stool Microbiota at Neutrophil Recovery Is Predictive for Severe Acute Graft vs Host Disease After Hematopoietic Cell Transplantation. Clin. Infect. Dis. 2017, 65, 1984–1991. [Google Scholar] [CrossRef]
- Peled, J.U.; Devlin, S.M.; Staffas, A.; Lumish, M.; Khanin, R.; Littmann, E.R.; Ling, L.; Kosuri, S.; Maloy, M.; Slingerland, J.B.; et al. Intestinal Microbiota and Relapse After Hematopoietic-Cell Transplantation. J. Clin. Oncol. 2017, 35, 1650–1659. [Google Scholar] [CrossRef]
- Han, L.; Jin, H.; Zhou, L.; Zhang, X.; Fan, Z.; Dai, M.; Lin, Q.; Huang, F.; Xuan, L.; Zhang, H.; et al. Intestinal Microbiota at Engraftment Influence Acute Graft-Versus-Host Disease via the Treg/Th17 Balance in Allo-HSCT Recipients. Front. Immunol. 2018, 9, 669. [Google Scholar] [CrossRef]
- Stein-Thoeringer, C.K.; Nichols, K.B.; Lazrak, A.; Docampo, M.D.; Slingerland, A.E.; Slingerland, J.B.; Clurman, A.G.; Armijo, G.; Gomes, A.L.C.; Shono, Y.; et al. Lactose drives Enterococcus expansion to promote graft-versus-host disease. Science 2019, 366, 1143–1149. [Google Scholar] [CrossRef]
- Margolis, E.B.; Alfaro, G.M.; Sun, Y.; Dallas, R.H.; Allison, K.J.; Ferrolino, J.; Ross, H.S.; Davis, A.E.; Jia, Q.; Turner, P.; et al. Microbiota Predict Infections and Acute Graft-Versus-Host Disease After Pediatric Allogeneic Hematopoietic Stem Cell Transplantation. J. Infect. Dis. 2023, 228, 627–636. [Google Scholar] [CrossRef]
- Connell, M.S.; Wilson, R. The Treatment of X-Irradiated Germfree Cfw and C3H Mice with Isologous and Homologous Bone Marrow. Life Sci. 1965, 4, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.M.; Wilson, R.; Bealmear, P.M. Mortality and gross pathology of secondary disease in germfree mouse radiation chimeras. Radiat. Res. 1971, 45, 577–588. [Google Scholar] [CrossRef] [PubMed]
- van Bekkum, D.W.; Roodenburg, J.; Heidt, P.J.; van der Waaij, D. Mitigation of secondary disease of allogeneic mouse radiation chimeras by modification of the intestinal microflora. J. Natl. Cancer Inst. 1974, 52, 401–404. [Google Scholar] [CrossRef]
- Lampert, I.A.; Moore, R.H.; Huby, R.; Cohen, J. Observations on the role of endotoxin in graft-versus-host disease. Prog. Clin. Biol. Res. 1988, 272, 351–359. [Google Scholar] [PubMed]
- Vriesendorp, H.M.; Heidt, P.J.; Zurcher, C. Gastrointestinal decontamination of dogs treated with total body irradiation and bone marrow transplantation. Exp. Hematol. 1981, 9, 904–916. [Google Scholar]
- van Bekkum, D.W.; Knaan, S. Role of bacterial microflora in development of intestinal lesions from graft-versus-host reaction. J. Natl. Cancer Inst. 1977, 58, 787–790. [Google Scholar] [CrossRef]
- Shouval, R.; Waters, N.R.; Gomes, A.L.C.; Zuanelli Brambilla, C.; Fei, T.; Devlin, S.M.; Nguyen, C.L.; Markey, K.A.; Dai, A.; Slingerland, J.B.; et al. Conditioning Regimens are Associated with Distinct Patterns of Microbiota Injury in Allogeneic Hematopoietic Cell Transplantation. Clin. Cancer Res. 2023, 29, 165–173. [Google Scholar] [CrossRef]
- Weber, D.; Jenq, R.R.; Peled, J.U.; Taur, Y.; Hiergeist, A.; Koestler, J.; Dettmer, K.; Weber, M.; Wolff, D.; Hahn, J.; et al. Microbiota Disruption Induced by Early Use of Broad-Spectrum Antibiotics Is an Independent Risk Factor of Outcome after Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transpl. 2017, 23, 845–852. [Google Scholar] [CrossRef]
- Yeshurun, M.; Rozovski, U.; Shargian, L.; Pasvolsky, O.; van der Werf, S.; Tridello, G.; Knelange, N.; Mikulska, M.; Styczynski, J.; Averbuch, D.; et al. Infection prevention practices among EBMT hematopoietic cell transplant centers: The EBMT Infectious Disease Working Party survey. Bone Marrow Transpl. 2023, 58, 414–423. [Google Scholar] [CrossRef]
- Gardner, J.C.; Courter, J.D.; Dandoy, C.E.; Davies, S.M.; Teusink-Cross, A. Safety and Efficacy of Prophylactic Levofloxacin in Pediatric and Adult Hematopoietic Stem Cell Transplantation Patients. Transpl. Cell Ther. 2022, 28, 167.e1–167.e5. [Google Scholar] [CrossRef]
- Oliveira, A.L.; de Souza, M.; Carvalho-Dias, V.M.; Ruiz, M.A.; Silla, L.; Tanaka, P.Y.; Simões, B.P.; Trabasso, P.; Seber, A.; Lotfi, C.J.; et al. Epidemiology of bacteremia and factors associated with multi-drug-resistant gram-negative bacteremia in hematopoietic stem cell transplant recipients. Bone Marrow Transpl. 2007, 39, 775–781. [Google Scholar] [CrossRef]
- Egan, G.; Robinson, P.D.; Martinez, J.P.D.; Alexander, S.; Ammann, R.A.; Dupuis, L.L.; Fisher, B.T.; Lehrnbecher, T.; Phillips, B.; Cabral, S.; et al. Efficacy of antibiotic prophylaxis in patients with cancer and hematopoietic stem cell transplantation recipients: A systematic review of randomized trials. Cancer Med. 2019, 8, 4536–4546. [Google Scholar] [CrossRef]
- Deshpande, A.; Pasupuleti, V.; Thota, P.; Pant, C.; Rolston, D.D.; Sferra, T.J.; Hernandez, A.V.; Donskey, C.J. Community-associated Clostridium difficile infection and antibiotics: A meta-analysis. J. Antimicrob. Chemother. 2013, 68, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Letendre, C.; Enot, D.; Chénard-Poirier, M.; Mehraj, V.; Séguin, N.C.; Guenda, K.; Gagnon, K.; Woerther, P.L.; Ghez, D.; et al. The influence of gut-decontamination prophylactic antibiotics on acute graft-versus-host disease and survival following allogeneic hematopoietic stem cell transplantation. Oncoimmunology 2017, 6, e1258506. [Google Scholar] [CrossRef]
- Shono, Y.; Docampo, M.D.; Peled, J.U.; Perobelli, S.M.; Velardi, E.; Tsai, J.J.; Slingerland, A.E.; Smith, O.M.; Young, L.F.; Gupta, J.; et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci. Transl. Med. 2016, 8, 339ra371. [Google Scholar] [CrossRef]
- Hussen, N.H.A.; Qadir, S.H.; Rahman, H.S.; Hamalaw, Y.Y.; Kareem, P.S.S.; Hamza, B.A. Long-term toxicity of fluoroquinolones: A comprehensive review. Drug Chem. Toxicol. 2024, 47, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Buckner, C.D.; Clift, R.A.; Sanders, J.E.; Meyers, J.D.; Counts, G.W.; Farewell, V.T.; Thomas, E.D. Protective environment for marrow transplant recipients: A prospective study. Ann. Intern. Med. 1978, 89, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Navari, R.M.; Buckner, C.D.; Clift, R.A.; Storb, R.; Sanders, J.E.; Stewart, P.; Sullivan, K.M.; Williams, B.; Counts, G.W.; Meyers, J.D.; et al. Prophylaxis of infection in patients with aplastic anemia receiving allogeneic marrow transplants. Am. J. Med. 1984, 76, 564–572. [Google Scholar] [CrossRef]
- Storb, R.; Prentice, R.L.; Buckner, C.D.; Clift, R.A.; Appelbaum, F.; Deeg, J.; Doney, K.; Hansen, J.A.; Mason, M.; Sanders, J.E.; et al. Graft-versus-host disease and survival in patients with aplastic anemia treated by marrow grafts from HLA-identical siblings. Beneficial effect of a protective environment. N. Engl. J. Med. 1983, 308, 302–307. [Google Scholar] [CrossRef]
- Petersen, F.B.; Buckner, C.D.; Clift, R.A.; Lee, S.; Nelson, N.; Counts, G.W.; Meyers, J.D.; Sanders, J.E.; Stewart, P.S.; Bensinger, W.I.; et al. Laminar air flow isolation and decontamination: A prospective randomized study of the effects of prophylactic systemic antibiotics in bone marrow transplant patients. Infection 1986, 14, 115–121. [Google Scholar] [CrossRef]
- Petersen, F.B.; Buckner, C.D.; Clift, R.A.; Nelson, N.; Counts, G.W.; Meyers, J.D.; Thomas, E.D. Infectious complications in patients undergoing marrow transplantation: A prospective randomized study of the additional effect of decontamination and laminar air flow isolation among patients receiving prophylactic systemic antibiotics. Scand. J. Infect. Dis. 1987, 19, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Beelen, D.W.; Haralambie, E.; Brandt, H.; Linzenmeier, G.; Müller, K.D.; Quabeck, K.; Sayer, H.G.; Graeven, U.; Mahmoud, H.K.; Schaefer, U.W. Evidence that sustained growth suppression of intestinal anaerobic bacteria reduces the risk of acute graft-versus-host disease after sibling marrow transplantation. Blood 1992, 80, 2668–2676. [Google Scholar] [CrossRef] [PubMed]
- Beelen, D.W.; Elmaagacli, A.; Müller, K.D.; Hirche, H.; Schaefer, U.W. Influence of intestinal bacterial decontamination using metronidazole and ciprofloxacin or ciprofloxacin alone on the development of acute graft-versus-host disease after marrow transplantation in patients with hematologic malignancies: Final results and long-term follow-up of an open-label prospective randomized trial. Blood 1999, 93, 3267–3275. [Google Scholar]
- Tanaka, J.S.; Young, R.R.; Heston, S.M.; Jenkins, K.; Spees, L.P.; Sung, A.D.; Corbet, K.; Thompson, J.C.; Bohannon, L.; Martin, P.L.; et al. Anaerobic Antibiotics and the Risk of Graft-versus-Host Disease after Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transpl. 2020, 26, 2053–2060. [Google Scholar] [CrossRef] [PubMed]
- Gavriilaki, M.; Sakellari, I.; Anagnostopoulos, A.; Gavriilaki, E. The Impact of Antibiotic-Mediated Modification of the Intestinal Microbiome on Outcomes of Allogeneic Hematopoietic Cell Transplantation: Systematic Review and Meta-Analysis. Biol. Blood Marrow Transpl. 2020, 26, 1738–1746. [Google Scholar] [CrossRef]
- Kimura, S.; Akahoshi, Y.; Nakano, H.; Ugai, T.; Wada, H.; Yamasaki, R.; Ishihara, Y.; Kawamura, K.; Sakamoto, K.; Ashizawa, M.; et al. Antibiotic prophylaxis in hematopoietic stem cell transplantation. A meta-analysis of randomized controlled trials. J. Infect. 2014, 69, 13–25. [Google Scholar] [CrossRef]
- Lehrnbecher, T.; Averbuch, D.; Castagnola, E.; Cesaro, S.; Ammann, R.A.; Garcia-Vidal, C.; Kanerva, J.; Lanternier, F.; Mesini, A.; Mikulska, M.; et al. 8th European Conference on Infections in Leukaemia: 2020 guidelines for the use of antibiotics in paediatric patients with cancer or post-haematopoietic cell transplantation. Lancet Oncol. 2021, 22, e270–e280. [Google Scholar] [CrossRef]
- Lehrnbecher, T.; Fisher, B.T.; Phillips, B.; Alexander, S.; Ammann, R.A.; Beauchemin, M.; Carlesse, F.; Castagnola, E.; Davis, B.L.; Dupuis, L.L.; et al. Guideline for Antibacterial Prophylaxis Administration in Pediatric Cancer and Hematopoietic Stem Cell Transplantation. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 71, 226–236. [Google Scholar] [CrossRef]
- Taplitz, R.A.; Kennedy, E.B.; Flowers, C.R. Antimicrobial Prophylaxis for Adult Patients with Cancer-Related Immunosuppression: ASCO and IDSA Clinical Practice Guideline Update Summary. J. Oncol. Pract. 2018, 14, 692–695. [Google Scholar] [CrossRef]
- Baden, L.R.; Swaminathan, S.; Almyroudis, N.G.; Angarone, M.; Baluch, A.; Barros, N.; Buss, B.; Cohen, S.; Cooper, B.; Chiang, A.D.; et al. Prevention and Treatment of Cancer-Related Infections, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2024, 22, 617–644. [Google Scholar] [CrossRef]
- Nesher, L.; Rolston, K.V.I. Febrile Neutropenia in Transplant Recipients. In Principles and Practice of Transplant Infectious Diseases; Safdar, A., Ed.; Springer: New York, NY, USA, 2019; pp. 185–198. [Google Scholar]
- Freifeld, A.G.; Bow, E.J.; Sepkowitz, K.A.; Boeckh, M.J.; Ito, J.I.; Mullen, C.A.; Raad, I.I.; Rolston, K.V.; Young, J.A.; Wingard, J.R. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2011, 52, e56–e93. [Google Scholar] [CrossRef] [PubMed]
- Averbuch, D.; Orasch, C.; Cordonnier, C.; Livermore, D.M.; Mikulska, M.; Viscoli, C.; Gyssens, I.C.; Kern, W.V.; Klyasova, G.; Marchetti, O.; et al. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: Summary of the 2011 4th European Conference on Infections in Leukemia. Haematologica 2013, 98, 1826–1835. [Google Scholar] [CrossRef]
- Rosa, R.G.; Goldani, L.Z. Cohort study of the impact of time to antibiotic administration on mortality in patients with febrile neutropenia. Antimicrob. Agents Chemother. 2014, 58, 3799–3803. [Google Scholar] [CrossRef]
- Contejean, A.; Maillard, A.; Canouï, E.; Kernéis, S.; Fantin, B.; Bouscary, D.; Parize, P.; Garcia-Vidal, C.; Charlier, C. Advances in antibacterial treatment of adults with high-risk febrile neutropenia. J. Antimicrob. Chemother. 2023, 78, 2109–2120. [Google Scholar] [CrossRef] [PubMed]
- Hayase, E.; Hayase, T.; Jamal, M.A.; Miyama, T.; Chang, C.C.; Ortega, M.R.; Ahmed, S.S.; Karmouch, J.L.; Sanchez, C.A.; Brown, A.N.; et al. Mucus-degrading Bacteroides link carbapenems to aggravated graft-versus-host disease. Cell 2022, 185, 3705–3719.e14. [Google Scholar] [CrossRef] [PubMed]
- Hanks, C.R.; Slain, D.; Kanate, A.S.; Wen, S.; Cumpston, A. Impact of anti-anaerobic antibiotic activity on graft-versus-host disease in allogeneic hematopoietic stem cell transplant (HSCT) recipients at an institution that utilizes antibiotic cycling. Transpl. Infect. Dis. 2021, 23, e13676. [Google Scholar] [CrossRef]
- Rashidi, A.; Gao, F.; Fredricks, D.N.; Pergam, S.A.; Mielcarek, M.; Milano, F.; Sandmaier, B.M.; Lee, S.J. Analysis of Antibiotic Exposure and Development of Acute Graft-vs-Host Disease Following Allogeneic Hematopoietic Cell Transplantation. JAMA Netw. Open 2023, 6, e2317188. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, J.C.; Jørgensen, M.; Moestrup, K.S.; Ilett, E.E.; Zucco, A.G.; Marandi, R.Z.; Julian, M.N.; Paredes, R.; Lundgren, J.D.; Sengeløv, H.; et al. Impact of Antibiotic Treatment on the Gut Microbiome and its Resistome in Hematopoietic Stem Cell Transplant Recipients. J. Infect. Dis. 2023, 228, 28–36. [Google Scholar] [CrossRef]
- Ciernikova, S.; Kasperova, B.; Drgona, L.; Smolkova, B.; Stevurkova, V.; Mego, M. Targeting the gut microbiome: An emerging trend in hematopoietic stem cell transplantation. Blood Rev. 2021, 48, 100790. [Google Scholar] [CrossRef]
- Shannon, C.E. The mathematical theory of communication. 1963. MD Comput. 1997, 14, 306–317. [Google Scholar]
- Kim, B.R.; Shin, J.; Guevarra, R.; Lee, J.H.; Kim, D.W.; Seol, K.H.; Lee, J.H.; Kim, H.B.; Isaacson, R. Deciphering Diversity Indices for a Better Understanding of Microbial Communities. J. Microbiol. Biotechnol. 2017, 27, 2089–2093. [Google Scholar] [CrossRef] [PubMed]
- Malard, F.; Gasc, C.; Plantamura, E.; Doré, J. High gastrointestinal microbial diversity and clinical outcome in graft-versus-host disease patients. Bone Marrow Transpl. 2018, 53, 1493–1497. [Google Scholar] [CrossRef] [PubMed]
- Ilett, E.E.; Jørgensen, M.; Noguera-Julian, M.; Nørgaard, J.C.; Daugaard, G.; Helleberg, M.; Paredes, R.; Murray, D.D.; Lundgren, J.; MacPherson, C.; et al. Associations of the gut microbiome and clinical factors with acute GVHD in allogeneic HSCT recipients. Blood Adv. 2020, 4, 5797–5809. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.B.; Ponce, D.M.; Dai, A.; Devlin, S.M.; Gomes, A.L.C.; Moore, G.F.; Slingerland, J.; Shouval, R.; Armijo, G.K.; DeWolf, S.; et al. Preservation of the fecal microbiome is associated with reduced severity of graft-versus-host disease. Blood 2022, 140, 2385–2397. [Google Scholar] [CrossRef]
- Eriguchi, Y.; Takashima, S.; Oka, H.; Shimoji, S.; Nakamura, K.; Uryu, H.; Shimoda, S.; Iwasaki, H.; Shimono, N.; Ayabe, T.; et al. Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting Paneth cell production of α-defensins. Blood 2012, 120, 223–231. [Google Scholar] [CrossRef]
- Peled, J.U.; Gomes, A.L.C.; Devlin, S.M.; Littmann, E.R.; Taur, Y.; Sung, A.D.; Weber, D.; Hashimoto, D.; Slingerland, A.E.; Slingerland, J.B.; et al. Microbiota as Predictor of Mortality in Allogeneic Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2020, 382, 822–834. [Google Scholar] [CrossRef]
- Koyama, M.; Mukhopadhyay, P.; Schuster, I.S.; Henden, A.S.; Hülsdünker, J.; Varelias, A.; Vetizou, M.; Kuns, R.D.; Robb, R.J.; Zhang, P.; et al. MHC Class II Antigen Presentation by the Intestinal Epithelium Initiates Graft-versus-Host Disease and Is Influenced by the Microbiota. Immunity 2019, 51, 885–898.e887. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, D.; Liu, C.; Kaosaard, K.; Semple, K.; Anasetti, C.; Yu, X.Z. Prevention of GVHD while sparing GVL effect by targeting Th1 and Th17 transcription factor T-bet and RORγt in mice. Blood 2011, 118, 5011–5020. [Google Scholar] [CrossRef]
- Koyama, M.; Hill, G.R. Alloantigen presentation and graft-versus-host disease: Fuel for the fire. Blood 2016, 127, 2963–2970. [Google Scholar] [CrossRef] [PubMed]
- Hess, N.J.; Brown, M.E.; Capitini, C.M. GVHD Pathogenesis, Prevention and Treatment: Lessons From Humanized Mouse Transplant Models. Front. Immunol. 2021, 12, 723544. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Vodanovic-Jankovic, S.; Johnson, B.; Keller, M.; Komorowski, R.; Drobyski, W.R. Absence of regulatory T-cell control of TH1 and TH17 cells is responsible for the autoimmune-mediated pathology in chronic graft-versus-host disease. Blood 2007, 110, 3804–3813. [Google Scholar] [CrossRef]
- Yi, T.; Chen, Y.; Wang, L.; Du, G.; Huang, D.; Zhao, D.; Johnston, H.; Young, J.; Todorov, I.; Umetsu, D.T.; et al. Reciprocal differentiation and tissue-specific pathogenesis of Th1, Th2, and Th17 cells in graft-versus-host disease. Blood 2009, 114, 3101–3112. [Google Scholar] [CrossRef]
- Koyama, M.; Hippe, D.S.; Srinivasan, S.; Proll, S.C.; Miltiadous, O.; Li, N.; Zhang, P.; Ensbey, K.S.; Hoffman, N.G.; Schmidt, C.R.; et al. Intestinal microbiota controls graft-versus-host disease independent of donor-host genetic disparity. Immunity 2023, 56, 1876–1893.e1878. [Google Scholar] [CrossRef]
- Ellison, C.A.; Natuik, S.A.; McIntosh, A.R.; Scully, S.A.; Danilenko, D.M.; Gartner, J.G. The role of interferon-gamma, nitric oxide and lipopolysaccharide in intestinal graft-versus-host disease developing in F1-hybrid mice. Immunology 2003, 109, 440–449. [Google Scholar] [CrossRef]
- Round, J.L.; Lee, S.M.; Li, J.; Tran, G.; Jabri, B.; Chatila, T.A.; Mazmanian, S.K. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 2011, 332, 974–977. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; Atarashi, K.; Manel, N.; Brodie, E.L.; Shima, T.; Karaoz, U.; Wei, D.; Goldfarb, K.C.; Santee, C.A.; Lynch, S.V.; et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009, 139, 485–498. [Google Scholar] [CrossRef]
- van der Velden, W.J.; Herbers, A.H.; Netea, M.G.; Blijlevens, N.M. Mucosal barrier injury, fever and infection in neutropenic patients with cancer: Introducing the paradigm febrile mucositis. Br. J. Haematol. 2014, 167, 441–452. [Google Scholar] [CrossRef]
- Cui, M.; Xiao, H.; Li, Y.; Zhang, S.; Dong, J.; Wang, B.; Zhu, C.; Jiang, M.; Zhu, T.; He, J.; et al. Sexual Dimorphism of Gut Microbiota Dictates Therapeutics Efficacy of Radiation Injuries. Adv. Sci. 2019, 6, 1901048. [Google Scholar] [CrossRef]
- Cario, E.; Podolsky, D.K. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect. Immun. 2000, 68, 7010–7017. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.T.; Vora, P.; Faure, E.; Thomas, L.S.; Arnold, E.T.; Arditi, M. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J. Immunol. 2001, 167, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Otte, J.M.; Cario, E.; Podolsky, D.K. Mechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology 2004, 126, 1054–1070. [Google Scholar] [CrossRef]
- Wu, J.; Gan, Y.; Li, M.; Chen, L.; Liang, J.; Zhuo, J.; Luo, H.; Xu, N.; Wu, X.; Wu, Q.; et al. Patchouli alcohol attenuates 5-fluorouracil-induced intestinal mucositis via TLR2/MyD88/NF-kB pathway and regulation of microbiota. Biomed. Pharmacother. 2020, 124, 109883. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, Y.; Huang, Z.; Dong, W.; Deng, Y.; Wang, F.; Li, M.; Yuan, J. Administration of probiotic mixture DM#1 ameliorated 5-fluorouracil-induced intestinal mucositis and dysbiosis in rats. Nutrition 2017, 33, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Justino, P.F.C.; Franco, A.X.; Pontier-Bres, R.; Monteiro, C.E.S.; Barbosa, A.L.R.; Souza, M.; Czerucka, D.; Soares, P.M.G. Modulation of 5-fluorouracil activation of toll-like/MyD88/NF-κB/MAPK pathway by Saccharomyces boulardii CNCM I-745 probiotic. Cytokine 2020, 125, 154791. [Google Scholar] [CrossRef]
- Gibson, R.J.; Coller, J.K.; Wardill, H.R.; Hutchinson, M.R.; Smid, S.; Bowen, J.M. Chemotherapy-induced gut toxicity and pain: Involvement of TLRs. Support. Care Cancer 2016, 24, 2251–2258. [Google Scholar] [CrossRef]
- Ey, B.; Eyking, A.; Gerken, G.; Podolsky, D.K.; Cario, E. TLR2 mediates gap junctional intercellular communication through connexin-43 in intestinal epithelial barrier injury. J. Biol. Chem. 2009, 284, 22332–22343. [Google Scholar] [CrossRef]
- Ey, B.; Eyking, A.; Klepak, M.; Salzman, N.H.; Göthert, J.R.; Rünzi, M.; Schmid, K.W.; Gerken, G.; Podolsky, D.K.; Cario, E. Loss of TLR2 worsens spontaneous colitis in MDR1A deficiency through commensally induced pyroptosis. J. Immunol. 2013, 190, 5676–5688. [Google Scholar] [CrossRef]
- Ciorba, M.A.; Riehl, T.E.; Rao, M.S.; Moon, C.; Ee, X.; Nava, G.M.; Walker, M.R.; Marinshaw, J.M.; Stappenbeck, T.S.; Stenson, W.F. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut 2012, 61, 829–838. [Google Scholar] [CrossRef]
- Hossain, M.S.; Jaye, D.L.; Pollack, B.P.; Farris, A.B.; Tselanyane, M.L.; David, E.; Roback, J.D.; Gewirtz, A.T.; Waller, E.K. Flagellin, a TLR5 agonist, reduces graft-versus-host disease in allogeneic hematopoietic stem cell transplantation recipients while enhancing antiviral immunity. J. Immunol. 2011, 187, 5130–5140. [Google Scholar] [CrossRef] [PubMed]
- Vaishnava, S.; Behrendt, C.L.; Ismail, A.S.; Eckmann, L.; Hooper, L.V. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc. Natl. Acad. Sci. USA 2008, 105, 20858–20863. [Google Scholar] [CrossRef]
- Wang, L.; Fouts, D.E.; Stärkel, P.; Hartmann, P.; Chen, P.; Llorente, C.; DePew, J.; Moncera, K.; Ho, S.B.; Brenner, D.A.; et al. Intestinal REG3 Lectins Protect against Alcoholic Steatohepatitis by Reducing Mucosa-Associated Microbiota and Preventing Bacterial Translocation. Cell Host Microbe 2016, 19, 227–239. [Google Scholar] [CrossRef]
- Loonen, L.M.; Stolte, E.H.; Jaklofsky, M.T.; Meijerink, M.; Dekker, J.; van Baarlen, P.; Wells, J.M. REG3γ-deficient mice have altered mucus distribution and increased mucosal inflammatory responses to the microbiota and enteric pathogens in the ileum. Mucosal Immunol. 2014, 7, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Kim, Y.H.; Jeong, S.; Greenson, J.K.; Chaudhry, M.S.; Hoepting, M.; Anderson, E.R.; van den Brink, M.R.; Peled, J.U.; Gomes, A.L.; et al. Survival signal REG3α prevents crypt apoptosis to control acute gastrointestinal graft-versus-host disease. J. Clin. Investig. 2018, 128, 4970–4979. [Google Scholar] [CrossRef] [PubMed]
- Holler, E. Cytokines, viruses, and graft-versus-host disease. Curr. Opin. Hematol. 2002, 9, 479–484. [Google Scholar] [CrossRef]
- Jankovic, D.; Ganesan, J.; Bscheider, M.; Stickel, N.; Weber, F.C.; Guarda, G.; Follo, M.; Pfeifer, D.; Tardivel, A.; Ludigs, K.; et al. The Nlrp3 inflammasome regulates acute graft-versus-host disease. J. Exp. Med. 2013, 210, 1899–1910. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Radujkovic, A.; Kordelas, L.; Dai, H.; Schult, D.; Majer-Lauterbach, J.; Beelen, D.; Müller-Tidow, C.; Dreger, P.; Luft, T. Interleukin-18 and outcome after allogeneic stem cell transplantation: A retrospective cohort study. EBioMedicine 2019, 49, 202–212. [Google Scholar] [CrossRef]
- Antin, J.H.; Weisdorf, D.; Neuberg, D.; Nicklow, R.; Clouthier, S.; Lee, S.J.; Alyea, E.; McGarigle, C.; Blazar, B.R.; Sonis, S.; et al. Interleukin-1 blockade does not prevent acute graft-versus-host disease: Results of a randomized, double-blind, placebo-controlled trial of interleukin-1 receptor antagonist in allogeneic bone marrow transplantation. Blood 2002, 100, 3479–3482. [Google Scholar] [CrossRef]
- Reddy, P.; Teshima, T.; Kukuruga, M.; Ordemann, R.; Liu, C.; Lowler, K.; Ferrara, J.L. Interleukin-18 regulates acute graft-versus-host disease by enhancing Fas-mediated donor T cell apoptosis. J. Exp. Med. 2001, 194, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Min, C.K.; Maeda, Y.; Lowler, K.; Liu, C.; Clouthier, S.; Lofthus, D.; Weisiger, E.; Ferrara, J.L.; Reddy, P. Paradoxical effects of interleukin-18 on the severity of acute graft-versus-host disease mediated by CD4+ and CD8+ T-cell subsets after experimental allogeneic bone marrow transplantation. Blood 2004, 104, 3393–3399. [Google Scholar] [CrossRef]
- Reddy, P.; Teshima, T.; Hildebrandt, G.; Williams, D.L.; Liu, C.; Cooke, K.R.; Ferrara, J.L. Pretreatment of donors with interleukin-18 attenuates acute graft-versus-host disease via STAT6 and preserves graft-versus-leukemia effects. Blood 2003, 101, 2877–2885. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zuo, K.; Liu, Z.; Xu, L.; Yang, X. Disordered GPR43/NLRP3 expression in peripheral leukocytes of patients with atrial fibrillation is associated with intestinal short chain fatty acids levels. Eur. J. Med. Res. 2024, 29, 233. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Docampo, M.D.; Riwes, M.; Peltier, D.; Toubai, T.; Henig, I.; Wu, S.J.; Kim, S.; Taylor, A.; Brabbs, S.; et al. Microbial metabolite sensor GPR43 controls severity of experimental GVHD. Nat. Commun. 2018, 9, 3674. [Google Scholar] [CrossRef]
- Elinav, E.; Strowig, T.; Kau, A.L.; Henao-Mejia, J.; Thaiss, C.A.; Booth, C.J.; Peaper, D.R.; Bertin, J.; Eisenbarth, S.C.; Gordon, J.I.; et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 2011, 145, 745–757. [Google Scholar] [CrossRef]
- Levy, M.; Thaiss, C.A.; Zeevi, D.; Dohnalová, L.; Zilberman-Schapira, G.; Mahdi, J.A.; David, E.; Savidor, A.; Korem, T.; Herzig, Y.; et al. Microbiota-Modulated Metabolites Shape the Intestinal Microenvironment by Regulating NLRP6 Inflammasome Signaling. Cell 2015, 163, 1428–1443. [Google Scholar] [CrossRef]
- Holler, E.; Rogler, G.; Herfarth, H.; Brenmoehl, J.; Wild, P.J.; Hahn, J.; Eissner, G.; Schölmerich, J.; Andreesen, R. Both donor and recipient NOD2/CARD15 mutations associate with transplant-related mortality and GvHD following allogeneic stem cell transplantation. Blood 2004, 104, 889–894. [Google Scholar] [CrossRef]
- Holler, E.; Rogler, G.; Brenmoehl, J.; Hahn, J.; Herfarth, H.; Greinix, H.; Dickinson, A.M.; Socié, G.; Wolff, D.; Fischer, G.; et al. Prognostic significance of NOD2/CARD15 variants in HLA-identical sibling hematopoietic stem cell transplantation: Effect on long-term outcome is confirmed in 2 independent cohorts and may be modulated by the type of gastrointestinal decontamination. Blood 2006, 107, 4189–4193. [Google Scholar] [CrossRef]
- Weber, D.; Oefner, P.J.; Hiergeist, A.; Koestler, J.; Gessner, A.; Weber, M.; Hahn, J.; Wolff, D.; Stämmler, F.; Spang, R.; et al. Low urinary indoxyl sulfate levels early after transplantation reflect a disrupted microbiome and are associated with poor outcome. Blood 2015, 126, 1723–1728. [Google Scholar] [CrossRef]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Swimm, A.; Giver, C.R.; DeFilipp, Z.; Rangaraju, S.; Sharma, A.; Ulezko Antonova, A.; Sonowal, R.; Capaldo, C.; Powell, D.; Qayed, M.; et al. Indoles derived from intestinal microbiota act via type I interferon signaling to limit graft-versus-host disease. Blood 2018, 132, 2506–2519. [Google Scholar] [CrossRef] [PubMed]
- Markey, K.A.; Schluter, J.; Gomes, A.L.C.; Littmann, E.R.; Pickard, A.J.; Taylor, B.P.; Giardina, P.A.; Weber, D.; Dai, A.; Docampo, M.D.; et al. The microbe-derived short-chain fatty acids butyrate and propionate are associated with protection from chronic GVHD. Blood 2020, 136, 130–136. [Google Scholar] [CrossRef]
- Mathewson, N.D.; Jenq, R.; Mathew, A.V.; Koenigsknecht, M.; Hanash, A.; Toubai, T.; Oravecz-Wilson, K.; Wu, S.R.; Sun, Y.; Rossi, C.; et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat. Immunol. 2016, 17, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Vossen, J.M.; Guiot, H.F.; Lankester, A.C.; Vossen, A.C.; Bredius, R.G.; Wolterbeek, R.; Bakker, H.D.; Heidt, P.J. Complete suppression of the gut microbiome prevents acute graft-versus-host disease following allogeneic bone marrow transplantation. PLoS ONE 2014, 9, e105706. [Google Scholar] [CrossRef]
- Weber, D.; Oefner, P.J.; Dettmer, K.; Hiergeist, A.; Koestler, J.; Gessner, A.; Weber, M.; Stämmler, F.; Hahn, J.; Wolff, D.; et al. Rifaximin preserves intestinal microbiota balance in patients undergoing allogeneic stem cell transplantation. Bone Marrow Transpl. 2016, 51, 1087–1092. [Google Scholar] [CrossRef]
- Kaleko, M.; Bristol, J.A.; Hubert, S.; Parsley, T.; Widmer, G.; Tzipori, S.; Subramanian, P.; Hasan, N.; Koski, P.; Kokai-Kun, J.; et al. Development of SYN-004, an oral beta-lactamase treatment to protect the gut microbiome from antibiotic-mediated damage and prevent Clostridium difficile infection. Anaerobe 2016, 41, 58–67. [Google Scholar] [CrossRef]
- Li, X.; Lin, Y.; Li, X.; Xu, X.; Zhao, Y.; Xu, L.; Gao, Y.; Li, Y.; Tan, Y.; Qian, P.; et al. Tyrosine supplement ameliorates murine aGVHD by modulation of gut microbiome and metabolome. EBioMedicine 2020, 61, 103048. [Google Scholar] [CrossRef]
- Wu, K.; Yuan, Y.; Yu, H.; Dai, X.; Wang, S.; Sun, Z.; Wang, F.; Fei, H.; Lin, Q.; Jiang, H.; et al. The gut microbial metabolite trimethylamine N-oxide aggravates GVHD by inducing M1 macrophage polarization in mice. Blood 2020, 136, 501–515. [Google Scholar] [CrossRef]
- Andersen, S.; Staudacher, H.; Weber, N.; Kennedy, G.; Varelias, A.; Banks, M.; Bauer, J. Pilot study investigating the effect of enteral and parenteral nutrition on the gastrointestinal microbiome post-allogeneic transplantation. Br. J. Haematol. 2020, 188, 570–581. [Google Scholar] [CrossRef]
- Yoshifuji, K.; Inamoto, K.; Kiridoshi, Y.; Takeshita, K.; Sasajima, S.; Shiraishi, Y.; Yamashita, Y.; Nisaka, Y.; Ogura, Y.; Takeuchi, R.; et al. Prebiotics protect against acute graft-versus-host disease and preserve the gut microbiota in stem cell transplantation. Blood Adv. 2020, 4, 4607–4617. [Google Scholar] [CrossRef] [PubMed]
- Iyama, S.; Sato, T.; Tatsumi, H.; Hashimoto, A.; Tatekoshi, A.; Kamihara, Y.; Horiguchi, H.; Ibata, S.; Ono, K.; Murase, K.; et al. Efficacy of Enteral Supplementation Enriched with Glutamine, Fiber, and Oligosaccharide on Mucosal Injury following Hematopoietic Stem Cell Transplantation. Case Rep. Oncol. 2014, 7, 692–699. [Google Scholar] [CrossRef]
- Cohen, S.A.; Woodfield, M.C.; Boyle, N.; Stednick, Z.; Boeckh, M.; Pergam, S.A. Incidence and outcomes of bloodstream infections among hematopoietic cell transplant recipients from species commonly reported to be in over-the-counter probiotic formulations. Transpl. Infect. Dis. 2016, 18, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Rangarajan, S.; Borate, U. A cautionary tale for probiotic use in hematopoietic SCT patients-Lactobacillus acidophilus sepsis in a patient with mantle cell lymphoma undergoing hematopoietic SCT. Bone Marrow Transpl. 2013, 48, 461–462. [Google Scholar] [CrossRef] [PubMed]
- Sadanand, A.; Newland, J.G.; Bednarski, J.J. Safety of Probiotics Among High-Risk Pediatric Hematopoietic Stem Cell Transplant Recipients. Infect. Dis. Ther. 2019, 8, 301–306. [Google Scholar] [CrossRef]
- Ladas, E.J.; Bhatia, M.; Chen, L.; Sandler, E.; Petrovic, A.; Berman, D.M.; Hamblin, F.; Gates, M.; Hawks, R.; Sung, L.; et al. The safety and feasibility of probiotics in children and adolescents undergoing hematopoietic cell transplantation. Bone Marrow Transpl. 2016, 51, 262–266. [Google Scholar] [CrossRef]
- Yazdandoust, E.; Hajifathali, A.; Roshandel, E.; Zarif, M.N.; Pourfathollah, A.A.; Parkhideh, S.; Mehdizadeh, M.; Amini-Kafiabad, S. Gut microbiota intervention by pre and probiotics can induce regulatory T cells and reduce the risk of severe acute GVHD following allogeneic hematopoietic stem cell transplantation. Transpl. Immunol. 2023, 78, 101836. [Google Scholar] [CrossRef]
- Nigam, M.; Panwar, A.S.; Singh, R.K. Orchestrating the fecal microbiota transplantation: Current technological advancements and potential biomedical application. Front. Med. Technol. 2022, 4, 961569. [Google Scholar] [CrossRef]
- Sbahi, H.; Di Palma, J.A. Faecal microbiota transplantation: Applications and limitations in treating gastrointestinal disorders. BMJ Open Gastroenterol. 2016, 3, e000087. [Google Scholar] [CrossRef]
- Yang, R.; Chen, Z.; Cai, J. Fecal microbiota transplantation: Emerging applications in autoimmune diseases. J. Autoimmun. 2023, 141, 103038. [Google Scholar] [CrossRef]
- Kaito, S.; Toya, T.; Yoshifuji, K.; Kurosawa, S.; Inamoto, K.; Takeshita, K.; Suda, W.; Kakihana, K.; Honda, K.; Hattori, M.; et al. Fecal microbiota transplantation with frozen capsules for a patient with refractory acute gut graft-versus-host disease. Blood Adv. 2018, 2, 3097–3101. [Google Scholar] [CrossRef] [PubMed]
- Spindelboeck, W.; Schulz, E.; Uhl, B.; Kashofer, K.; Aigelsreiter, A.; Zinke-Cerwenka, W.; Mulabecirovic, A.; Kump, P.K.; Halwachs, B.; Gorkiewicz, G.; et al. Repeated fecal microbiota transplantations attenuate diarrhea and lead to sustained changes in the fecal microbiota in acute, refractory gastrointestinal graft-versus-host-disease. Haematologica 2017, 102, e210–e213. [Google Scholar] [CrossRef] [PubMed]
- Taur, Y.; Coyte, K.; Schluter, J.; Robilotti, E.; Figueroa, C.; Gjonbalaj, M.; Littmann, E.R.; Ling, L.; Miller, L.; Gyaltshen, Y.; et al. Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant. Sci. Transl. Med. 2018, 10, eaap9489. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Zhou, Y.; Gao, J.; Jiao, Y.; Zhu, B.; Wu, D.; Qi, X. Safety and Efficacy of Fecal Microbiota Transplantation for Grade IV Steroid Refractory GI-GvHD Patients: Interim Results From FMT2017002 Trial. Front. Immunol. 2021, 12, 678476. [Google Scholar] [CrossRef]
- Rashidi, A.; Ebadi, M.; Rehman, T.U.; Elhusseini, H.; Kazadi, D.; Halaweish, H.; Khan, M.H.; Hoeschen, A.; Cao, Q.; Luo, X.; et al. Randomized Double-Blind Phase II Trial of Fecal Microbiota Transplantation Versus Placebo in Allogeneic Hematopoietic Cell Transplantation and AML. J. Clin. Oncol. 2023, 41, 5306–5319. [Google Scholar] [CrossRef] [PubMed]
- Brennan, T.V.; Lin, L.; Huang, X.; Cardona, D.M.; Li, Z.; Dredge, K.; Chao, N.J.; Yang, Y. Heparan sulfate, an endogenous TLR4 agonist, promotes acute GVHD after allogeneic stem cell transplantation. Blood 2012, 120, 2899–2908. [Google Scholar] [CrossRef]
- Hill, G.R.; Koyama, M. Cytokines and costimulation in acute graft-versus-host disease. Blood 2020, 136, 418–428. [Google Scholar] [CrossRef]
- Toubai, T.; Mathewson, N.D.; Magenau, J.; Reddy, P. Danger Signals and Graft-versus-host Disease: Current Understanding and Future Perspectives. Front. Immunol. 2016, 7, 539. [Google Scholar] [CrossRef]
- Magenau, J.; Jaglowski, S.; Uberti, J.; Farag, S.S.; Riwes, M.M.; Pawarode, A.; Anand, S.; Ghosh, M.; Maciejewski, J.; Braun, T.; et al. A phase 2 trial of CD24Fc for prevention of graft-versus-host disease. Blood 2024, 143, 21–31. [Google Scholar] [CrossRef]
- Gatlik, E.; Mehes, B.; Voltz, E.; Sommer, U.; Tritto, E.; Lestini, G.; Liu, X.; Pal, P.; Velinova, M.; Denney, W.S.; et al. First-in-human safety, tolerability, and pharmacokinetic results of DFV890, an oral low-molecular-weight NLRP3 inhibitor. Clin. Transl. Sci. 2024, 17, e13789. [Google Scholar] [CrossRef]
- Parmar, D.V.; Kansagra, K.A.; Momin, T.; Patel, H.B.; Jansari, G.A.; Bhavsar, J.; Shah, C.; Patel, J.M.; Ghoghari, A.; Barot, A.; et al. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of the Oral NLRP3 Inflammasome Inhibitor ZYIL1: First-in-Human Phase 1 Studies (Single Ascending Dose and Multiple Ascending Dose). Clin. Pharmacol. Drug Dev. 2023, 12, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manero, G.; Ooi, M.; Lao, Z.; Gill, H.; Abaza, Y.; Stahl, M.; Haque, T.; DeZern, A.E.; Greenberg, P.L.; Pelletier, M.; et al. Safety and Preliminary Efficacy of DFV890 in Adult Patients with Myeloid Diseases: A Phase 1b Study. Blood 2023, 142, 3250. [Google Scholar] [CrossRef]
- Klück, V.; Jansen, T.; Janssen, M.; Comarniceanu, A.; Efdé, M.; Tengesdal, I.W.; Schraa, K.; Cleophas, M.C.P.; Scribner, C.L.; Skouras, D.B.; et al. Dapansutrile, an oral selective NLRP3 inflammasome inhibitor, for treatment of gout flares: An open-label, dose-adaptive, proof-of-concept, phase 2a trial. Lancet Rheumatol. 2020, 2, e270–e280. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Kunder, R.; Chu, T.; Hains, A.; Nguyen, A.; McBride, J.M.; Zhong, Y.; Santagostino, S.; Wilson, M.; Trenchak, A.; et al. First-in-human phase 1 trial evaluating safety, pharmacokinetics, and pharmacodynamics of NLRP3 inflammasome inhibitor, GDC-2394, in healthy volunteers. Clin. Transl. Sci. 2023, 16, 1653–1666. [Google Scholar] [CrossRef]
- Ostrand-Rosenberg, S.; Fenselau, C. Myeloid-Derived Suppressor Cells: Immune-Suppressive Cells That Impair Antitumor Immunity and Are Sculpted by Their Environment. J. Immunol. 2018, 200, 422–431. [Google Scholar] [CrossRef]
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 2021, 21, 485–498. [Google Scholar] [CrossRef]
- Koehn, B.H.; Saha, A.; McDonald-Hyman, C.; Loschi, M.; Thangavelu, G.; Ma, L.; Zaiken, M.; Dysthe, J.; Krepps, W.; Panthera, J.; et al. Danger-associated extracellular ATP counters MDSC therapeutic efficacy in acute GVHD. Blood 2019, 134, 1670–1682. [Google Scholar] [CrossRef]

| Patient No, Population | Pathology | HSCT Characteristics + | Antimicrobial Prophylaxis | Microbiota Findings | Outcomes | Ref |
|---|---|---|---|---|---|---|
| n = 18, adults | AML/MDS/MPN 72%, NHL 28% | MAC 22%, RIC 56%, NMA 22% PBSCs 33%, UCB 67% | Vancomycin, fluoroquinolone, metronidazole |
| Increased microbial “chaos” early after allogeneic BMT as a potential risk factor for subsequent GVHD. | [20] |
| n = 80, adults | Leukemia 31%, lymphoma 42%, MM 8%, MDS 19% |
| Ciprofloxacin and IV vancomycin if viridians streptococci ++ |
| OS 3y: Low 36%, intermediate 60%, and high diversity 67% (p = 0.019). | [21] |
| n = 31, adults | AML 45%, ALL 16%, Ly 13%, MM 9%, SMD 19%, CML 3%, AA 3% |
| Ciprofloxacin and metronidazole |
|
| [22] |
| n = 64, adults. | NHL 37%, AML 38%, ALL 10%, HL 5%, CLL 6%, MDS 4% |
| Ciprofloxacin and IV vancomycin ++ |
|
| [23] |
| n = 15, children | SCA 27%, AML 20%, ALL 13%, HLH 13%, DBA 7%, AA 7%, FA 7%, SCID 7% |
| Levofloxacin prophylaxis (53%) |
|
| [24] |
| n = 107, adults | ALL 16%, AML 46%, MDS 21%, other 17% |
|
|
|
| [25] |
| n = 66, adults | ALL 6%, AML 26%, CLL 6%, HL 3%, MDS 36%, MM 3%, NHL 15% |
| Not reported |
|
| [26] |
| n = 541, adults | AML 36%, MDS 16%, NHL 17%, MM 11%, ALL 8%, other neoplasia 17% | MAC 59%, RIC 30%, NMA 11% PBSCs/BM 32%, CBU 18%, T cell depletion 50%. Donor related 30%, unrelated 70% | Not reported | Stool samples collected after HSCT and 2 y of follow up.
|
| [27] |
| n = 141, adults | AML 60%, ALL 35%, MDS 5% |
| Oral sulfamethoxazole and norfloxacin |
|
| [28] |
| n = 1324, adults | AML 36%, MDS/MPN 18%, NHL 17%, ALL 9%, MM 9%, CLL 3%, CML 2%, HL 2%, AA 1%, Other 3% |
| Not reported |
|
| [29] |
| n = 1362, adults | AML 36%, MPN/MDS 19%, NHL 17%, ALL 9%, MM 8%, CLL 2%, HL 2%, CML 2%, AA 1%, other 3%. | BM 8%, CBU 15%, PBSCs unmodified 43%, PBSCs T cell depleted 33% MAC 57%, RIC 34%, NMA 9% |
|
|
| [30] |
| n = 74, children | AML 59%, ALL 39%, HL 3%, LDS 3%, other neoplasia 3%. | Haplo 61%, MUD 26%, MSD 12%, MMUD 1% BM 36.5%, PBSCs 63.5% RIC 67%, MAC 33%, NMA 1% T cell depletion ex vivo 51.4% | No oral decontamination or antibacterial prophylaxis peri-HSCT. |
|
| [31] |
| Vendor | MHC-II Inducer | MHC-II Suppressor | Ref |
|---|---|---|---|
| JAX Mice |
|
| [79] |
|
| ||
| |||
| Taconic and Charles River |
| [79] | |
|
|
| Vendor | MHC-II Inducer | MHC-II Suppressor | Ref |
|---|---|---|---|
| JAX Mice |
|
| [79] |
| Taconic and Charles River |
|
| [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinzon-Leal, P.; Gutierrez-Barbosa, H.; Medina-Moreno, S.; Zapata, J.C. The Microbiome, Inflammation, and GVHD Axis: The Balance Between the “Gut” and the Bad. Immuno 2025, 5, 10. https://doi.org/10.3390/immuno5010010
Pinzon-Leal P, Gutierrez-Barbosa H, Medina-Moreno S, Zapata JC. The Microbiome, Inflammation, and GVHD Axis: The Balance Between the “Gut” and the Bad. Immuno. 2025; 5(1):10. https://doi.org/10.3390/immuno5010010
Chicago/Turabian StylePinzon-Leal, Paula, Hernando Gutierrez-Barbosa, Sandra Medina-Moreno, and Juan C. Zapata. 2025. "The Microbiome, Inflammation, and GVHD Axis: The Balance Between the “Gut” and the Bad" Immuno 5, no. 1: 10. https://doi.org/10.3390/immuno5010010
APA StylePinzon-Leal, P., Gutierrez-Barbosa, H., Medina-Moreno, S., & Zapata, J. C. (2025). The Microbiome, Inflammation, and GVHD Axis: The Balance Between the “Gut” and the Bad. Immuno, 5(1), 10. https://doi.org/10.3390/immuno5010010






