Current Topics of the Mechanism of Intestinal Fibrosis in Crohn’s Disease
Abstract
1. Introduction
2. Clinical Problems Associated with Intestinal Stricture in Crohn’s Disease
3. Current Treatment Strategies for Intestinal Stricture of Patients with Crohn’s Disease
4. What Is Intestinal Fibrosis in Crohn’s Disease?
5. Cells and Molecules Involved in Intestinal Fibrosis
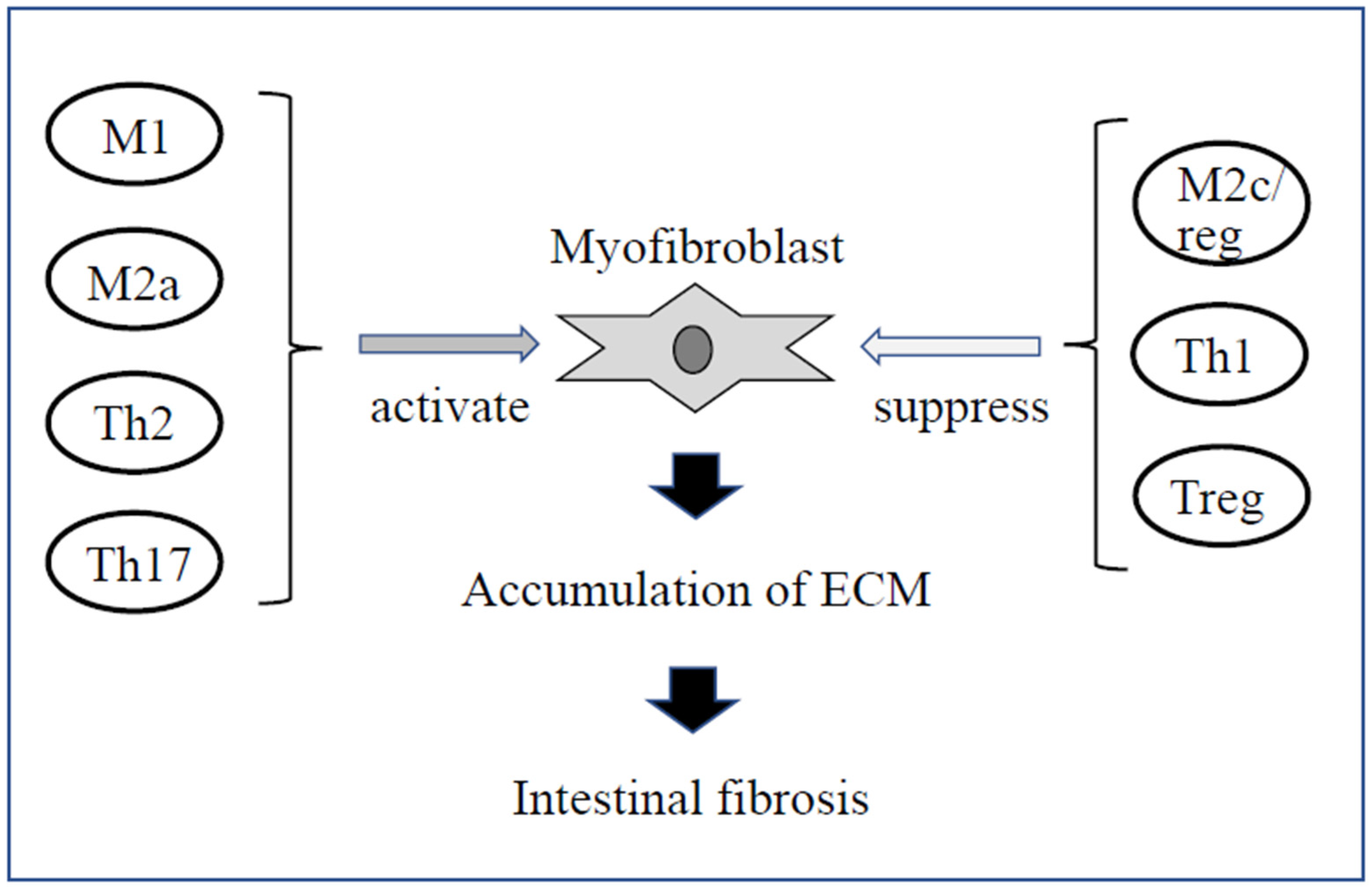
5.1. Mesenchymal Cells Such as Myofibroblasts
5.2. Immune Cells
6. Microbiota and Intestinal Fibrosis
7. New Therapeutic Strategies for Intestinal Fibrosis
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rieder, F.; Brenmoehl, J.; Leeb, S.; Schölmerich, J.; Rogler, G. Wound healing and fibrosis in intestinal disease. Gut 2007, 56, 130–139. [Google Scholar] [CrossRef]
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity 2019, 50, 992–1006. [Google Scholar] [CrossRef]
- Harbord, M.; Eliakim, R.; Bettenworth, D.; Karmiris, K.; Katsanos, K.; Kopylov, U.; Kucharzik, T.; Molnár, T.; Raine, T.; Sebastian, S.; et al. European Crohn’s and Colitis Organisation [ECCO]. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J. Crohns Colitis 2017, 11, 769–784. [Google Scholar] [CrossRef]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohns Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Van Assche, G.; Geboes, K.; Rutgeerts, P. Medical therapy for Crohn’s disease strictures. Inflamm. Bowel Dis. 2004, 10, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Louis, E.; Collard, A.; Oger, A.F.; Degroote, E.; Aboul Nasr El Yafi, F.A.; Belaiche, J. Behaviour of Crohn’s disease according to the Vienna classification: Changing pattern over the course of the disease. Gut 2001, 49, 777–782. [Google Scholar] [CrossRef]
- Cosnes, J.; Bourrier, A.; Nion-Larmurier, I.; Sokol, H.; Beaugerie, L.; Seksik, P. Factors affecting outcomes in Crohn’s disease over 15 years. Gut 2012, 61, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Thienpont, C.; D’Hoore, A.; Vermeire, S.; Demedts, I.; Bisschops, R.; Coremans, G.; Rutgeerts, P.; Van Assche, G. Long-term outcome of endoscopic dilatation in patients with Crohn’s disease is not affected by disease activity or medical therapy. Gut 2010, 59, 320–324. [Google Scholar]
- Rieder, F.; Fiocchi, C. Intestinal fibrosis in inflammatory bowel disease—Current knowledge and future perspectives. J. Crohns Colitis 2008, 2, 279–290. [Google Scholar] [CrossRef]
- Kotze, P.G.; Shen, B.; Lightner, A.; Yamamoto, T.; Spinelli, A.; Ghosh, S.; Panaccione, R. Modern management of perianal fistulas in Crohn’s disease: Future directions. Gut 2018, 67, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Ott, C.; Schölmerich, J. Extraintestinal manifestations and complications in IBD. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 585–595. [Google Scholar] [CrossRef]
- Cosnes, J.; Cattan, S.; Blain, A.; Beaugerie, L.; Carbonnel, F.; Parc, R.; Gendre, J.P. Long-term evolution of disease behavior of Crohn’s disease. Inflamm. Bowel Dis. 2002, 8, 244–250. [Google Scholar] [CrossRef]
- Rieder, F.; Zimmermann, E.M.; Remzi, F.H.; Sandborn, W.J. Crohn’s disease complicated by strictures: A systematic review. Gut 2013, 62, 1072–1084. [Google Scholar] [CrossRef]
- Rieder, F.; Lawrance, I.C.; Leite, A.; Sans, M. Predictors of fibrostenotic Crohn’s disease. Inflamm. Bowel Dis. 2011, 17, 2000–2007. [Google Scholar] [CrossRef]
- Rieder, F.; Fiocchi, C.; Rogler, G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology 2017, 152, 340–350. [Google Scholar] [CrossRef]
- Chang, C.W.; Wong, J.M.; Tung, C.C.; Shih, I.L.; Wang, H.Y.; Wei, S.C. Intestinal stricture in Crohn’s disease. Intest. Res. 2015, 13, 19–26. [Google Scholar] [CrossRef]
- Maconi, G.; Sampietro, G.M.; Cristaldi, M.; Danelli, P.G.; Russo, A.; Bianchi Porro, G.; Taschieri, A.M. Preoperative characteristics and postoperative behavior of bowel wall on risk of recurrence after conservative surgery in Crohn’s disease: A prospective study. Ann. Surg. 2001, 233, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Speca, S.; Giusti, I.; Rieder, F.; Latella, G. Cellular and molecular mechanisms of intestinal fibrosis. World J. Gastroenterol. 2012, 18, 3635–3661. [Google Scholar] [CrossRef] [PubMed]
- Lawrance, I.C.; Rogler, G.; Bamias, G.; Breynaert, C.; Florholmen, J.; Pellino, G.; Reif, S.; Speca, S.; Latella, G. Cellular and Molecular Mediators of Intestinal Fibrosis. J. Crohns Colitis 2017, 11, 1491–1503. [Google Scholar] [CrossRef]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Von Lampe, B.; Barthel, B.; Coupland, S.E.; Riecken, E.O.; Rosewicz, S. Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut 2000, 47, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Arpino, V.; Brock, M.; Gill, S.E. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015, 44–46, 247–254. [Google Scholar] [CrossRef]
- Valatas, V.; Filidou, E.; Drygiannakis, I.; Kolios, G. Stromal and immune cells in gut fibrosis: The myofibroblast and the scarface. Ann. Gastroenterol. 2017, 30, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Pucilowska, J.B.; Williams, K.L.; Lund, P.K. Fibrogenesis. IV. Fibrosis and inflammatory bowel disease: Cellular mediators and animal models. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G653–G659. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Fiocchi, C. Intestinal fibrosis in IBD—A dynamic, multifactorial process. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 228–235. [Google Scholar] [CrossRef]
- Fiocchi, C.; Lund, P.K. Themes in fibrosis and gastrointestinal inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G677–G683. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, G.; Elias, M.; Zhu, Y.; Wang, J. The role of cytokine and immune responses in intestinal fibrosis. J. Dig. Dis. 2020, 21, 308–314. [Google Scholar] [CrossRef]
- Mikami, Y.; Takada, Y.; Hagihara, Y.; Kanai, T. Innate lymphoid cells in organ fibrosis. Cytokine Growth Factor Rev. 2018, 42, 27–36. [Google Scholar] [CrossRef]
- Jun, Y.K.; Kwon, S.H.; Yoon, H.T.; Park, H.; Soh, H.; Lee, H.J.; Im, J.P.; Kim, J.S.; Kim, J.W.; Koh, S.J. Toll-like receptor 4 regulates intestinal fibrosis via cytokine expression and epithelial-mesenchymal transition. Sci. Rep. 2020, 10, 19867. [Google Scholar] [CrossRef]
- Huaux, F.; Arras, M.; Tomasi, D.; Barbarin, V.; Delos, M.; Coutelier, J.P.; Vink, A.; Phan, S.H.; Renauld, J.C.; Lison, D. A profibrotic function of IL-12p40 in experimental pulmonary fibrosis. J. Immunol. 2002, 169, 2653–2661. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.L.; Koh, T.J. Phenotypic transitions of macrophages orchestrate tissue repair. Am. J. Pathol. 2013, 183, 1352–1363. [Google Scholar] [CrossRef]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef]
- Witherel, C.E.; Abebayehu, D.; Barker, T.H.; Spiller, K.L. Macrophage and Fibroblast Interactions in Biomaterial-Mediated Fibrosis. Adv. Healthc. Mater. 2019, 8, e1801451. [Google Scholar] [CrossRef] [PubMed]
- Adhyatmika, A.; Putri, K.S.; Beljaars, L.; Melgert, B.N. The Elusive Antifibrotic Macrophage. Front. Med. 2015, 2, 81. [Google Scholar] [CrossRef][Green Version]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Barron, L.; Wynn, T.A. Fibrosis is regulated by Th2 and Th17 responses and by dynamic interactions between fibroblasts and macrophages. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G723–G728. [Google Scholar] [CrossRef] [PubMed]
- Gieseck, R.L., III; Wilson, M.S.; Wynn, T.A. Type 2 immunity in tissue repair and fibrosis. Nat. Rev. Immunol. 2018, 18, 62–76. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, S. T Cells in Fibrosis and Fibrotic Diseases. Front. Immunol. 2020, 11, 1142. [Google Scholar] [CrossRef]
- Zhan, S.; Li, N.; Liu, C.; Mao, R.; Wu, D.; Li, T.; Chen, M.; Zhuang, X.; Zeng, Z. Intestinal Fibrosis and Gut Microbiota: Clues from Other Organs. Front. Microbiol. 2021, 12, 694967. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Liu, S.; Zhang, Y.; Zhang, D. Toll-like Receptors and Inflammatory Bowel Disease. Front. Microbiol. 2018, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Jacob, N.; Jacobs, J.P.; Kumagai, K.; Ha, C.W.Y.; Kanazawa, Y.; Lagishetty, V.; Altmayer, K.; Hamill, A.M.; Von Arx, A.; Sartor, R.B.; et al. Inflammation-independent TL1A-mediated intestinal fibrosis is dependent on the gut microbiome. Mucosal Immunol. 2018, 11, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhang, X.; Chen, J.; Ichikawa, R.; Wallace, K.; Pothoulakis, C.; Koon, H.W.; Targan, S.R.; Shih, D.Q. Sustained Tl1a (Tnfsf15) Expression on Both Lymphoid and Myeloid Cells Leads to Mild Spontaneous Intestinal Inflammation and Fibrosis. Eur. J. Microbiol. Immunol. 2013, 3, 11–20. [Google Scholar] [CrossRef]
- Palmela, C.; Chevarin, C.; Xu, Z.; Torres, J.; Sevrin, G.; Hirten, R.; Barnich, N.; Ng, S.C.; Colombel, J.F. Adherent-invasive Escherichia coli in inflammatory bowel disease. Gut 2018, 67, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Darfeuille-Michaud, A.; Boudeau, J.; Bulois, P.; Neut, C.; Glasser, A.L.; Barnich, N.; Bringer, M.A.; Swidsinski, A.; Beaugerie, L.; Colombel, J.F. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology 2004, 127, 412–421. [Google Scholar] [CrossRef]
- Small, C.L.; Reid-Yu, S.A.; McPhee, J.B.; Coombes, B.K. Persistent infection with Crohn’s disease-associated adherent-invasive Escherichia coli leads to chronic inflammation and intestinal fibrosis. Nat. Commun. 2013, 4, 1957. [Google Scholar] [CrossRef]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Ha, C.W.Y.; Martin, A.; Sepich-Poore, G.D.; Shi, B.; Wang, Y.; Gouin, K.; Humphrey, G.; Sanders, K.; Ratnayake, Y.; Chan, K.S.L.; et al. Translocation of Viable Gut Microbiota to Mesenteric Adipose Drives Formation of Creeping Fat in Humans. Cell 2020, 183, 666–683. [Google Scholar] [CrossRef]
- Manichanh, C.; Rigottier-Gois, L.; Bonnaud, E.; Gloux, K.; Pelletier, E.; Frangeul, L.; Nalin, R.; Jarrin, C.; Chardon, P.; Marteau, P.; et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 2006, 55, 205–211. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.D.; Wang, Y.D. The Relationship Between Gut Microbiota and Inflammatory Diseases: The Role of Macrophages. Front. Microbiol. 2020, 11, 1065. [Google Scholar] [CrossRef]
- Danese, S.; Bonovas, S.; Lopez, A.; Fiorino, G.; Sandborn, W.J.; Rubin, D.T.; Kamm, M.A.; Colombel, J.F.; Sands, B.E.; Vermeire, S.; et al. Identification of Endpoints for Development of Antifibrosis Drugs for Treatment of Crohn’s Disease. Gastroenterology 2018, 155, 76–87. [Google Scholar] [CrossRef]
- Rieder, F.; Kessler, S.; Sans, M.; Fiocchi, C. Animal models of intestinal fibrosis: New tools for the understanding of pathogenesis and therapy of human disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G786–G801. [Google Scholar] [CrossRef]
- Ma, Y.; Guan, Q.; Bai, A.; Weiss, C.R.; Hillman, C.L.; Ma, A.; Zhou, G.; Qing, G.; Peng, Z. Targeting TGF-beta1 by employing a vaccine ameliorates fibrosis in a mouse model of chronic colitis. Inflamm. Bowel Dis. 2010, 16, 1040–1050. [Google Scholar] [CrossRef]
- Fraser, E.; Hoyles, R.K. Therapeutic advances in idiopathic pulmonary fibrosis. Clin. Med. 2016, 16, 42–51. [Google Scholar] [CrossRef]
- Meier, R.; Lutz, C.; Cosín-Roger, J.; Fagagnini, S.; Bollmann, G.; Hünerwadel, A.; Mamie, C.; Lang, S.; Tchouboukov, A.; Weber, F.E.; et al. Decreased Fibrogenesis After Treatment with Pirfenidone in a Newly Developed Mouse Model of Intestinal Fibrosis. Inflamm. Bowel Dis. 2016, 22, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.V.; Balani, P.; Lopez, A.R.; Nobleza, C.M.N.; Siddiqui, M.; Khan, S. A Review of Pirfenidone as an Anti-Fibrotic in Idiopathic Pulmonary Fibrosis and Its Probable Role in Other Diseases. Cureus 2021, 13, e12482. [Google Scholar] [CrossRef] [PubMed]
- Goffin, L.; Fagagnini, S.; Vicari, A.; Mamie, C.; Melhem, H.; Weder, B.; Lutz, C.; Lang, S.; Scharl, M.; Rogler, G.; et al. Anti-MMP-9 Antibody: A Promising Therapeutic Strategy for Treatment of Inflammatory Bowel Disease Complications with Fibrosis. Inflamm. Bowel Dis. 2016, 22, 2041–2057. [Google Scholar] [CrossRef]
- Weder, B.; Mamie, C.; Rogler, G.; Clarke, S.; McRae, B.; Ruiz, P.A.; Hausmann, M. BCL2 Regulates Differentiation of Intestinal Fibroblasts. Inflamm. Bowel Dis. 2018, 24, 1953–1966. [Google Scholar] [CrossRef]
- Nishida, A.; Hidaka, K.; Kanda, T.; Imaeda, H.; Shioya, M.; Inatomi, O.; Bamba, S.; Kitoh, K.; Sugimoto, M.; Andoh, A. Increased Expression of Interleukin-36, a Member of the Interleukin-1 Cytokine Family, in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Scheibe, K.; Backert, I.; Wirtz, S.; Hueber, A.; Schett, G.; Vieth, M.; Probst, H.C.; Bopp, T.; Neurath, M.F.; Neufert, C. IL-36R signalling activates intestinal epithelial cells and fibroblasts and promotes mucosal healing in vivo. Gut 2017, 66, 823–838. [Google Scholar] [CrossRef] [PubMed]
- Scheibe, K.; Kersten, C.; Schmied, A.; Vieth, M.; Primbs, T.; Carlé, B.; Knieling, F.; Claussen, J.; Klimowicz, A.C.; Zheng, J.; et al. Inhibiting Interleukin 36 Receptor Signaling Reduces Fibrosis in Mice With Chronic Intestinal Inflammation. Gastroenterology 2019, 156, 1082–1097. [Google Scholar] [CrossRef]
- Russell, S.E.; Horan, R.M.; Stefanska, A.M.; Carey, A.; Leon, G.; Aguilera, M.; Statovci, D.; Moran, T.; Fallon, P.G.; Shanahan, F.; et al. IL-36α expression is elevated in ulcerative colitis and promotes colonic inflammation. Mucosal Immunol. 2016, 9, 1193–1204. [Google Scholar] [CrossRef]
- Kuhn, A.; Haust, M.; Ruland, V.; Weber, R.; Verde, P.; Felder, G.; Ohmann, C.; Gensch, K.; Ruzicka, T. Effect of bosentan on skin fibrosis in patients with systemic sclerosis: A prospective, open-label, non-comparative trial. Rheumatology 2010, 49, 1336–1345. [Google Scholar] [CrossRef][Green Version]
- Couluris, M.; Kinder, B.W.; Xu, P.; Gross-King, M.; Krischer, J.; Panos, R.J. Treatment of idiopathic pulmonary fibrosis with losartan: A pilot project. Lung 2012, 190, 523–527. [Google Scholar] [CrossRef] [PubMed]
- el-Agroudy, A.E.; Hassan, N.A.; Foda, M.A.; Ismail, A.M.; el-Sawy, E.A.; Mousa, O.; Ghoneim, M.A. Effect of angiotensin II receptor blocker on plasma levels of TGF-beta 1 and interstitial fibrosis in hypertensive kidney transplant patients. Am. J. Nephrol. 2003, 23, 300–306. [Google Scholar] [CrossRef]
- De, B.K.; Bandyopadhyay, K.; Das, T.K.; Das, D.; Biswas, P.K.; Majumdar, D.; Mandal, S.K.; Ray, S.; Dasgupta, S. Portal pressure response to losartan compared with propranolol in patients with cirrhosis. Am. J. Gastroenterol. 2003, 98, 1371–1376. [Google Scholar] [CrossRef] [PubMed]

| Clinical course | Age at diagnosis <40 years |
| Anal lesions | |
| Steroid therapy for initial treatment | |
| Small intestinal lesions | |
| Smoking | |
| Long term disease duration | |
| Deep ulcers in the intestinal tract | |
| Genetic findings | Janus-associated kinase 2 (JAK2) |
| ATG16L1 | |
| NOD2/CARD15 | |
| TNF superfamily 15 (TNFSF15) | |
| 5T5T (MMP3) | |
| rs1363670 |
| Fibrosis | IL-1 |
| IL-4 | |
| IL-13 | |
| IL-17 | |
| TGF-β1 | |
| TNF-α | |
| CTGF | |
| FGF | |
| IGF | |
| Anti-fibrosis | IFN-γ |
| Il-10 | |
| IL-12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honzawa, Y.; Yamamoto, S.; Okabe, M.; Seno, H.; Nakase, H. Current Topics of the Mechanism of Intestinal Fibrosis in Crohn’s Disease. Immuno 2021, 1, 574-582. https://doi.org/10.3390/immuno1040040
Honzawa Y, Yamamoto S, Okabe M, Seno H, Nakase H. Current Topics of the Mechanism of Intestinal Fibrosis in Crohn’s Disease. Immuno. 2021; 1(4):574-582. https://doi.org/10.3390/immuno1040040
Chicago/Turabian StyleHonzawa, Yusuke, Shuji Yamamoto, Makoto Okabe, Hiroshi Seno, and Hiroshi Nakase. 2021. "Current Topics of the Mechanism of Intestinal Fibrosis in Crohn’s Disease" Immuno 1, no. 4: 574-582. https://doi.org/10.3390/immuno1040040
APA StyleHonzawa, Y., Yamamoto, S., Okabe, M., Seno, H., & Nakase, H. (2021). Current Topics of the Mechanism of Intestinal Fibrosis in Crohn’s Disease. Immuno, 1(4), 574-582. https://doi.org/10.3390/immuno1040040







