Mpox Insights: From Structure to Human Cell Interaction
Abstract
1. Introduction
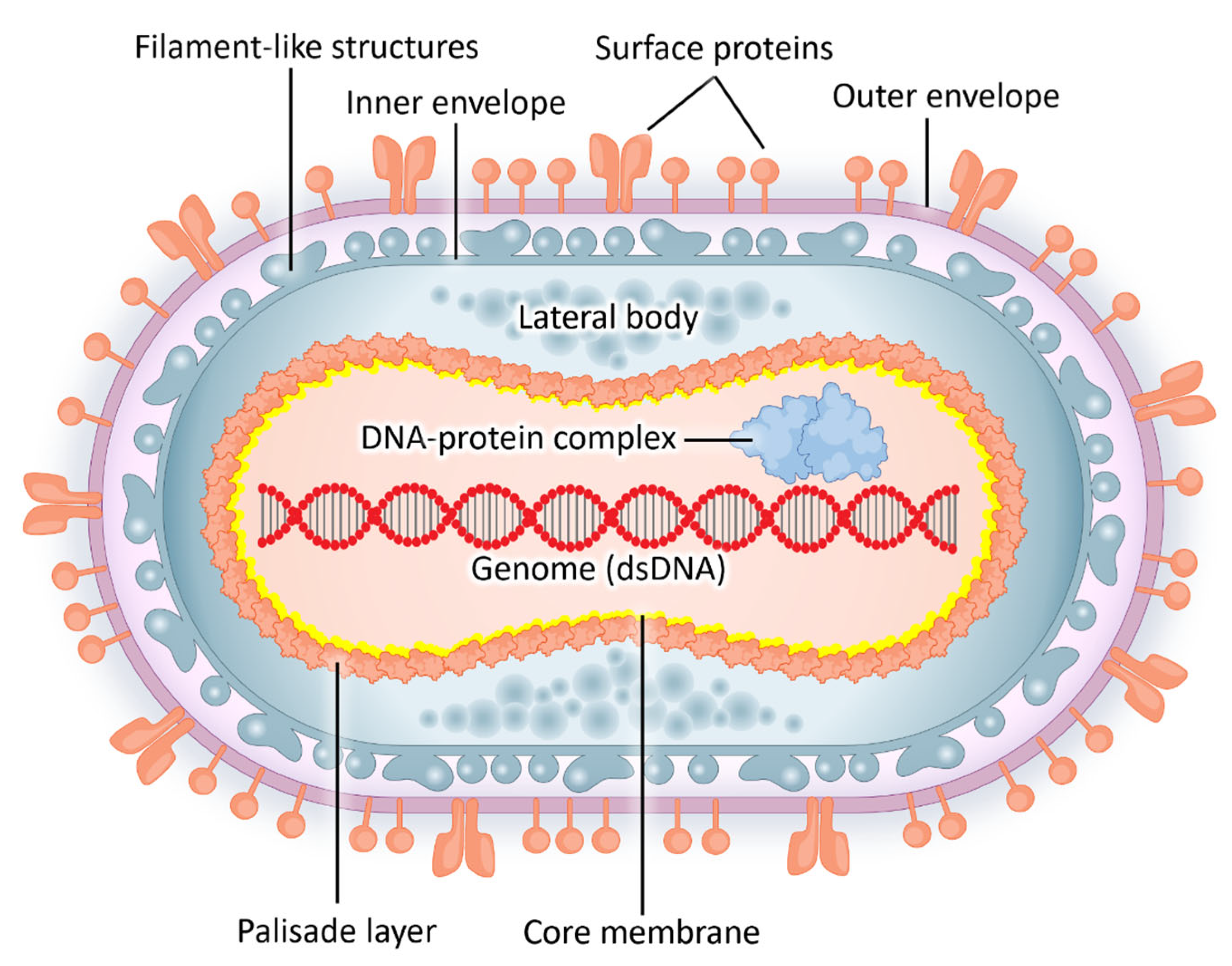
2. Epidemiology and Viral Structure
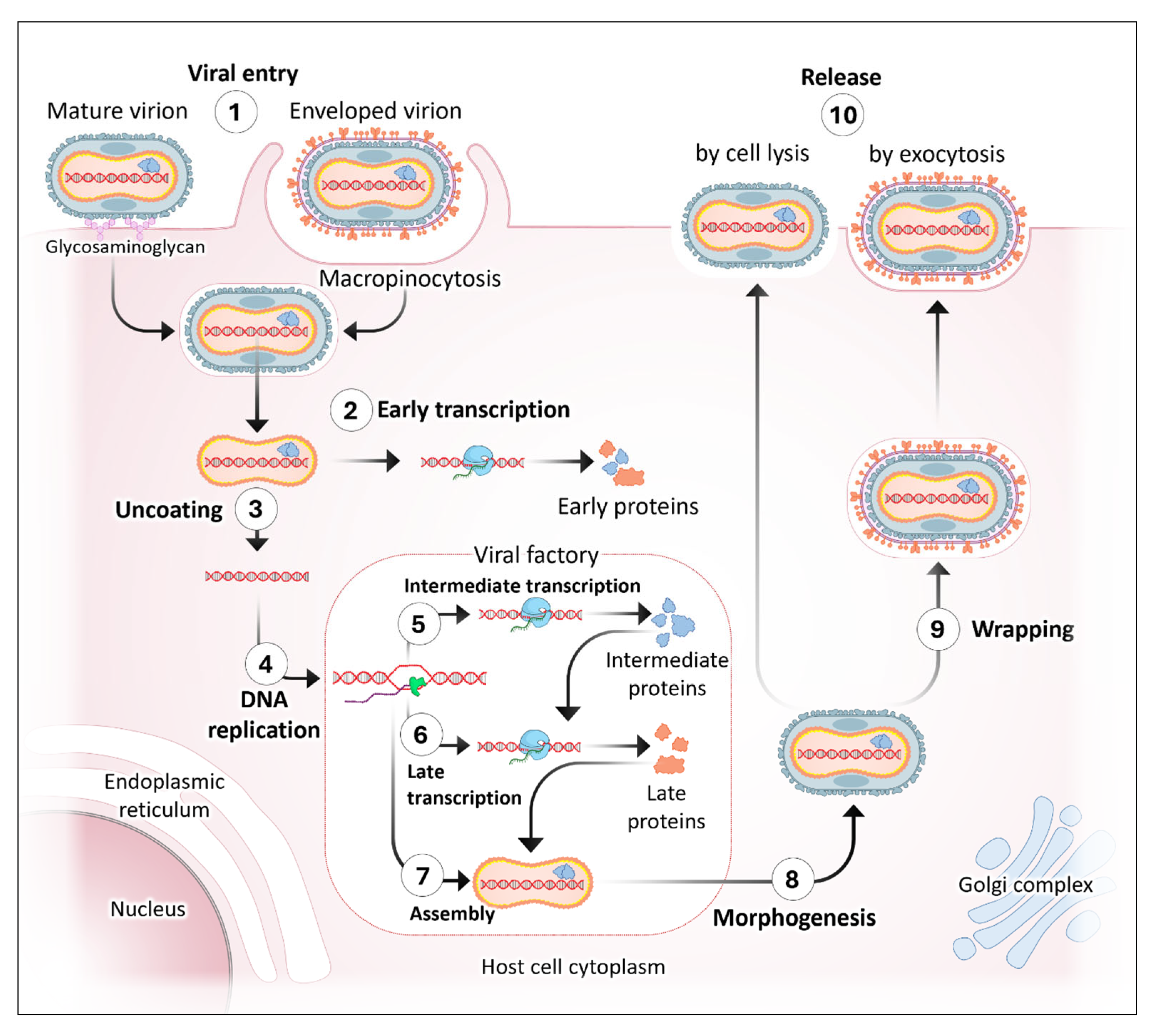
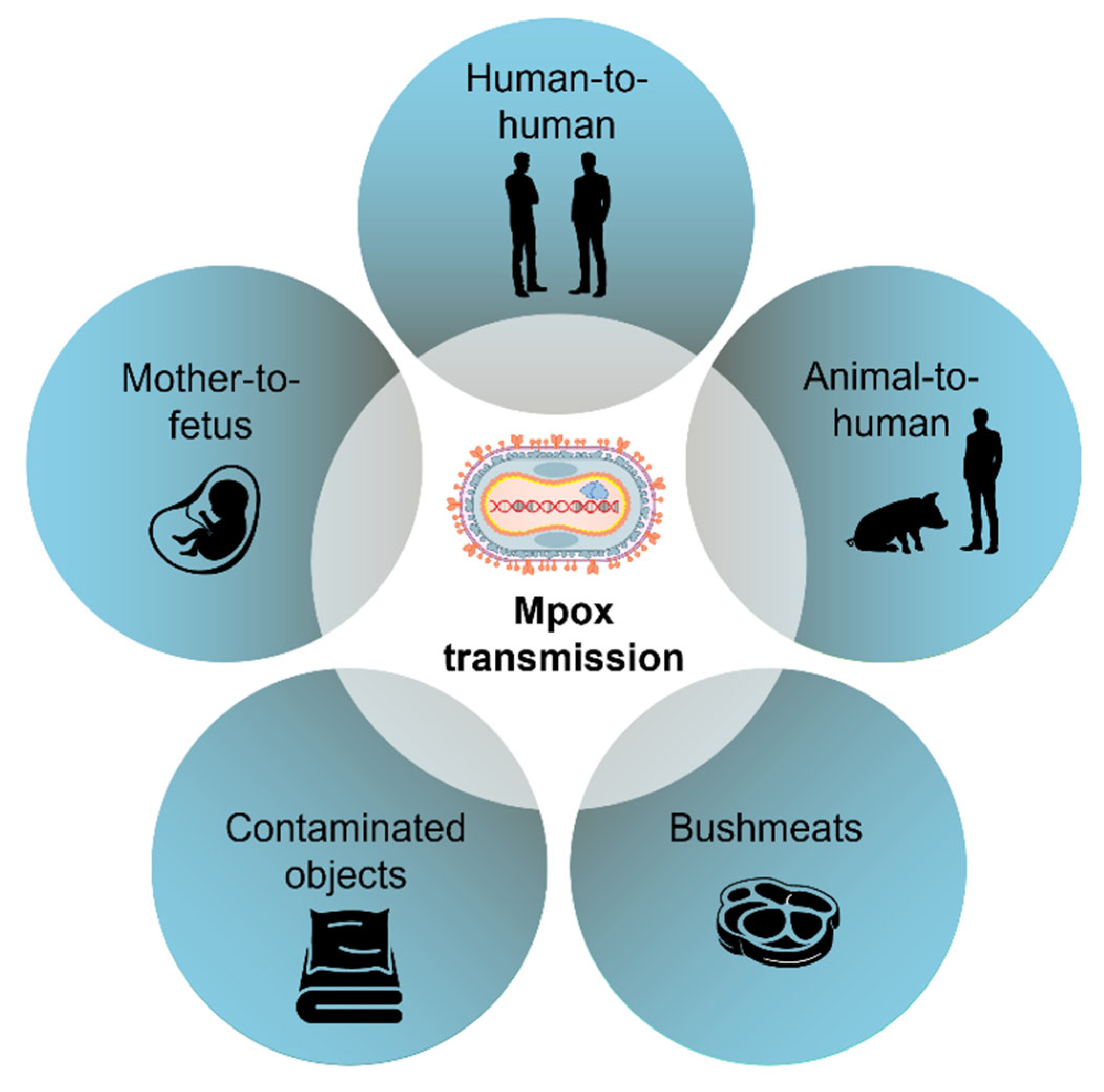
3. Human Cell Interaction and Viral Transmission
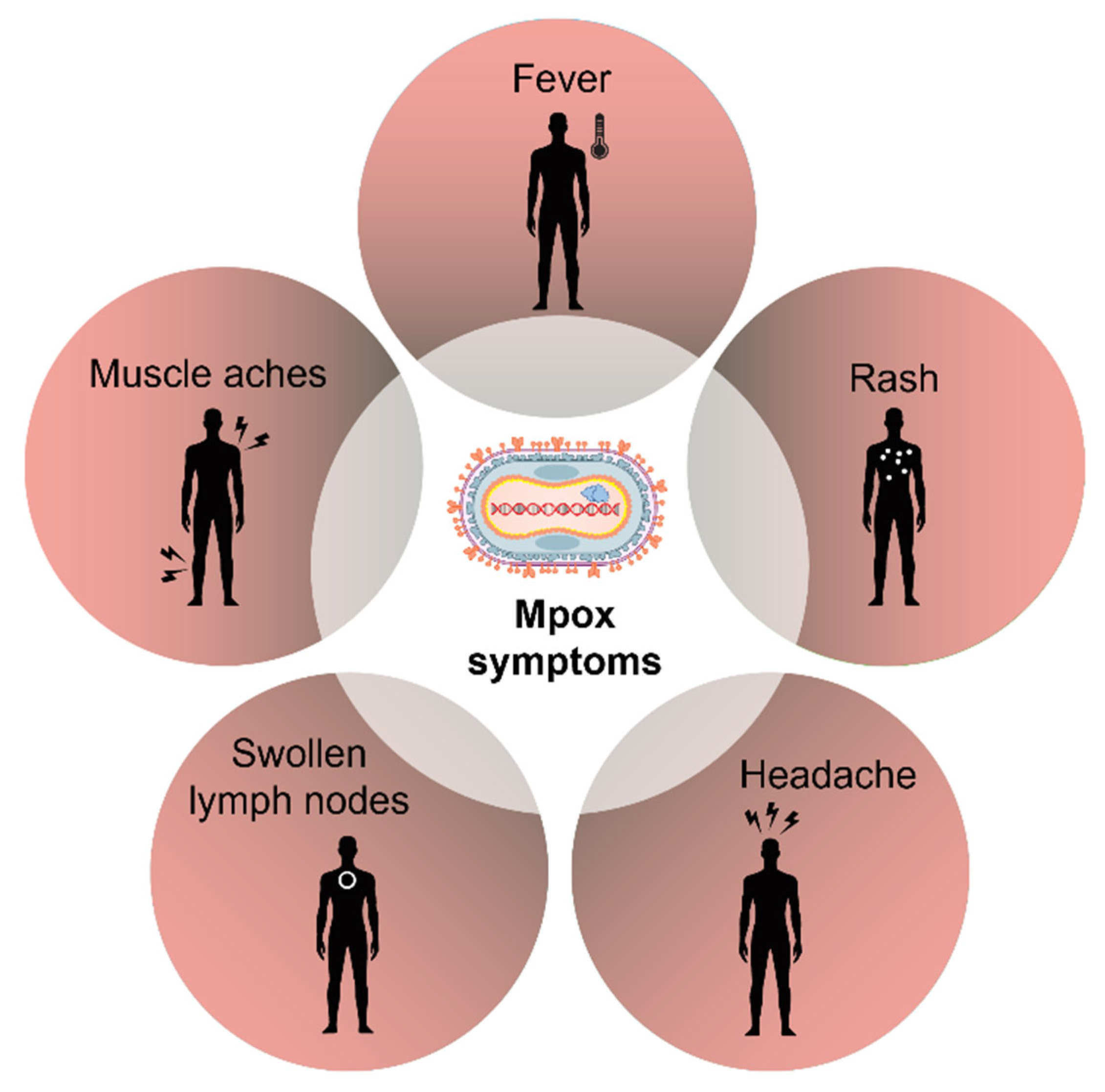
4. Symptoms and Treatment
4.1. Vaccines for Mpox
4.2. Clinical Trial Findings on Tecovirimat for Mpox
4.3. Severe Mpox in People with Advanced HIV
5. Public Health Importance and Challenges in Mpox Studies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The European Centre for Disease Prevention and Control. Rapid Risk Assessment on the Monkeypox Outbreak; The European Centre for Disease Prevention and Control: Solna, Sweden, 2022; Available online: https://www.ecdc.europa.eu/en/news-events/ecdc-releases-first-update-its-rapid-risk-assessment-monkeypox-outbreak (accessed on 4 July 2025).
- Apea, V.; Titanji, B.K.; Dakin, F.H.; Hayes, R.; Smuk, M.; Kawu, H.; Waters, L.; Levy, I.; Kuritzkes, D.R.; Gandhi, M.; et al. International healthcare workers’ experiences and perceptions of the 2022 multi-country mpox outbreak. PLoS Glob Public Health 2025, 5, e0003704. [Google Scholar] [CrossRef]
- The Centers for Disease Control and Prevention. Clinical Considerations for Mpox in Immunocompromised People; The Centers for Disease Control and Prevention: Atlanta, GA, USA, 2024. Available online: https://www.cdc.gov/mpox/hcp/clinical-care/immunocompromised-people.html (accessed on 4 July 2025).
- Wawina-Bokalanga, T.; Merritt, S.; Kinganda-Lusamaki, E.; Jansen, D.; Halbrook, M.; O’Toole, Á.; Pukuta-Simbu, E.; Vakaniaki, E.H.; Ola-Mpumbe, R.; Kwete-Mbokama, P.; et al. Epidemiology and phylogenomic characterisation of two distinct mpox outbreaks in Kinshasa, DR Congo, involving a new subclade Ia lineage: A retrospective, observational study. Lancet 2025, 406, 63–75. [Google Scholar] [CrossRef]
- Levitt, C.V.; Tran, Q.K.; Hraky, H.; Mazer-Amirshahi, M.; Pourmand, A. Emergency department approach to monkeypox. World J. Emerg. Med. 2023, 14, 341–348. [Google Scholar] [CrossRef]
- Olawade, D.B.; Wada, O.Z.; Fidelis, S.C.; Oluwole, O.S.; Alisi, C.S.; Orimabuyaku, N.F.; Clement David-Olawade, A. Strengthening Africa’s response to Mpox (monkeypox): Insights from historical outbreaks and the present global spread. Sci. One Health 2024, 3, 100085. [Google Scholar] [CrossRef] [PubMed]
- Hutin, Y.J.; Williams, R.J.; Malfait, P.; Pebody, R.; Loparev, V.N.; Ropp, S.L.; Rodriguez, M.; Knight, J.C.; Tshioko, F.K.; Khan, A.S.; et al. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg Infect Dis 2001, 7, 434–438. [Google Scholar] [CrossRef] [PubMed]
- United Nations Children’s Fund. Democratic Republic of the Congo Humanitarian Situation Report No. 7 (Mpox), 31 March 2025; United Nations Children’s Fund: New York, NY, USA, 2025; Available online: https://www.unicef.org/documents/democratic-republic-congo-humanitarian-situation-report-no-7-mpox-31-march-2025?utm_source=chatgpt.com (accessed on 4 July 2025).
- Karagoz, A.; Tombuloglu, H.; Alsaeed, M.; Tombuloglu, G.; AlRubaish, A.A.; Mahmoud, A.; Smajlovic, S.; Cordic, S.; Rabaan, A.A.; Alsuhaimi, E. Monkeypox (mpox) virus: Classification, origin, transmission, genome organization, antiviral drugs, and molecular diagnosis. J. Infect. Public Health 2023, 16, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Sagdat, K.; Batyrkhan, A.; Kanayeva, D. Exploring monkeypox virus proteins and rapid detection techniques. Front. Cell Infect. Microbiol. 2024, 14, 1414224. [Google Scholar] [CrossRef]
- Lu, J.; Xing, H.; Wang, C.; Tang, M.; Wu, C.; Ye, F.; Yin, L.; Yang, Y.; Tan, W.; Shen, L. Mpox (formerly monkeypox): Pathogenesis, prevention, and treatment. Signal Transduct. Target. Ther. 2023, 8, 458. [Google Scholar] [CrossRef]
- Abduljalil, J.M.; Elfiky, A.A.; Elgohary, A.M. Exploration of natural compounds against the human mpox virus DNA-dependent RNA polymerase in silico. J. Infect. Public Health 2023, 16, 996–1003. [Google Scholar] [CrossRef]
- Meem, S.S.; Proma, A.Y.; Bhuiyan, M.A.; Dewan, S.M.R. The pressing need for study on the effects of Mpox on the progression of vascular inflammation: A well-timed call. Health Sci. Rep. 2024, 7, e2223. [Google Scholar] [CrossRef]
- Kataria, R.; Kaur, S.; Kaundal, R. Deciphering the complete human-monkeypox virus interactome: Identifying immune responses and potential drug targets. Front. Immunol. 2023, 14, 1116988. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Chung, C.S.; Heine, H.G.; Chang, W. Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J. Virol. 2000, 74, 3353–3365. [Google Scholar] [CrossRef] [PubMed]
- Parnian, R.; Heydarifard, F.; Mousavi, F.S.; Heydarifard, Z.; Zandi, M. Innate Immune Response to Monkeypox Virus Infection: Mechanisms and Immune Escape. J. Innate Immun. 2024, 16, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Liu, Y.; Dou, D.; Su, B. The unique immune evasion mechanisms of the mpox virus and their implication for developing new vaccines and immunotherapies. Virol. Sin. 2024, 39, 709–718. [Google Scholar] [CrossRef]
- Branda, F.; Romano, C.; Ciccozzi, M.; Giovanetti, M.; Scarpa, F.; Ciccozzi, A.; Maruotti, A. Mpox: An Overview of Pathogenesis, Diagnosis, and Public Health Implications. J. Clin. Med. 2024, 13, 2234. [Google Scholar] [CrossRef]
- Griffin, B.D.; Corredor, J.C.; Pei, Y.; Nagy, E. Downregulation of Cell Surface Major Histocompatibility Complex Class I Expression Is Mediated by the Left-End Transcription Unit of Fowl Adenovirus 9. Viruses 2021, 13, 2211. [Google Scholar] [CrossRef]
- Lum, F.M.; Torres-Ruesta, A.; Tay, M.Z.; Lin, R.T.P.; Lye, D.C.; Renia, L.; Ng, L.F.P. Monkeypox: Disease epidemiology, host immunity and clinical interventions. Nat. Rev. Immunol. 2022, 22, 597–613. [Google Scholar] [CrossRef]
- Elsheikh, R.; Makram, A.M.; Vasanthakumaran, T.; Tomar, S.; Shamim, K.; Tranh, N.D.; Elsheikh, S.S.; Van, N.T.; Huy, N.T. Monkeypox: A comprehensive review of a multifaceted virus. Infect. Med. 2023, 2, 74–88. [Google Scholar] [CrossRef]
- Qiu, J. An animal source of mpox emerges—And it’s a squirrel. Nature 2025, 640, 575–576. [Google Scholar] [CrossRef]
- Reynolds, M.G.; Carroll, D.S.; Karem, K.L. Factors affecting the likelihood of monkeypox’s emergence and spread in the post-smallpox era. Curr. Opin. Virol. 2012, 2, 335–343. [Google Scholar] [CrossRef]
- Zaucha, G.M.; Jahrling, P.B.; Geisbert, T.W.; Swearengen, J.R.; Hensley, L. The pathology of experimental aerosolized monkeypox virus infection in cynomolgus monkeys (Macaca fascicularis). Lab. Investig. 2001, 81, 1581–1600. [Google Scholar] [CrossRef]
- McCollum, A.M.; Shelus, V.; Hill, A.; Traore, T.; Onoja, B.; Nakazawa, Y.; Doty, J.B.; Yinka-Ogunleye, A.; Petersen, B.W.; Hutson, C.L.; et al. Epidemiology of Human Mpox—Worldwide, 2018–2021. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 68–72. [Google Scholar] [CrossRef]
- Adebisi, Y.A.; Ezema, S.M.; Bolarinwa, O.; Bassey, A.E.; Ogunkola, I.O. Sex Workers and the Mpox Response in Africa. J. Infect. Dis. 2024, 230, 786–788. [Google Scholar] [CrossRef] [PubMed]
- Sampson, M.M.; Magee, G.; Schrader, E.A.; Dantuluri, K.L.; Bukhari, A.; Passaretti, C.; Temming, L.; Leonard, M.; Philips, J.B.; Weinrib, D. Mpox (Monkeypox) Infection During Pregnancy. Obs. Gynecol. 2023, 141, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A.; Pittman, P.R. Mpox (Monkeypox) in Pregnancy: Viral Clade Differences and Their Associations with Varying Obstetrical and Fetal Outcomes. Viruses 2023, 15, 1649. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox Virus Infection in Humans across 16 Countries—April–June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef]
- Miller, M.J.; Cash-Goldwasser, S.; Marx, G.E.; Schrodt, C.A.; Kimball, A.; Padgett, K.; Noe, R.S.; McCormick, D.W.; Wong, J.M.; Labuda, S.M.; et al. Severe Monkeypox in Hospitalized Patients—United States, August 10–October 10, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1412–1417. [Google Scholar] [CrossRef]
- Fox, T.; Gould, S.; Princy, N.; Rowland, T.; Lutje, V.; Kuehn, R. Therapeutics for treating mpox in humans. Cochrane Database Syst. Rev. 2023, 3, CD015769. [Google Scholar] [CrossRef]
- Rao, A.K.; Schrodt, C.A.; Minhaj, F.S.; Waltenburg, M.A.; Cash-Goldwasser, S.; Yu, Y.; Petersen, B.W.; Hutson, C.; Damon, I.K. Interim Clinical Treatment Considerations for Severe Manifestations of Mpox—United States, February 2023. MMWR Morb Mortal. Wkly. Rep. 2023, 72, 232–243. [Google Scholar] [CrossRef]
- Saldana, C.S.; Kelley, C.F.; Aldred, B.M.; Cantos, V.D. Mpox and HIV: A Narrative Review. Curr. HIV/AIDS Rep. 2023, 20, 261–269. [Google Scholar] [CrossRef]
- Raadsen, M.P.; Dahlke, C.; Fathi, A.; Hardtke, S.; Klüver, M.; Krähling, V.; Gerresheim, G.K.; Mayer, L.; Mykytyn, A.Z.; Weskamm, L.M.; et al. Safety, immunogenicity, and optimal dosing of a modified vaccinia Ankara-based vaccine against MERS-CoV in healthy adults: A phase 1b, double-blind, randomised placebo-controlled clinical trial. Lancet Infect. Dis. 2025, 25, 231–242. [Google Scholar] [CrossRef]
- Ali, R.; Alonga, J.; Biampata, J.L.; Kombozi Basika, M.; Maljkovic Berry, I.; Bisento, N.; Blum, E.; Bonnett, T.; Cone, K.; Crozier, I.; et al. Tecovirimat for Clade I MPXV Infection in the Democratic Republic of Congo. N. Engl. J. Med. 2025, 392, 1484–1496. [Google Scholar] [CrossRef]
- Chenchula, S.; Atal, S.; Ghanta, M.K.; Uppugunduri, C.R.; Karunakaran, S.; Amerneni, K.C.; Sarma, P.; Prakash, S.; Amerneni, L.S.; Padmavathi, R.; et al. Emerging variants of Mpox virus and tecovirimat resistance: Genomic insights and implications for treatment strategies. Virology 2025, 608, 110532. [Google Scholar] [CrossRef]
- The Centers for Disease Control and Prevention. Preventing Mpox; The Centers for Disease Control and Prevention: Atlanta, GA, USA, 2024. Available online: https://www.cdc.gov/mpox/prevention/index.html (accessed on 4 July 2025).
- Bisanzio, D.; Davis, A.E.; Talbird, S.E.; Van Effelterre, T.; Metz, L.; Gaudig, M.; Mathieu, V.O.; Brogan, A.J. Targeted preventive vaccination campaigns to reduce Ebola outbreaks: An individual-based modeling study. Vaccine 2023, 41, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, Z.; Sheng, S.; Song, R.; Jin, R. The Current State and Progress of Mpox Vaccine Research. China CDC Wkly. 2024, 6, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Matusali, G.; Petruccioli, E.; Cimini, E.; Colavita, F.; Bettini, A.; Tartaglia, E.; Sbarra, S.; Meschi, S.; Lapa, D.; Francalancia, M.; et al. Evaluation of Cross-Immunity to the Mpox Virus Due to Historic Smallpox Vaccination. Vaccines 2023, 11, 1541. [Google Scholar] [CrossRef] [PubMed]
| Vaccine | Types | Use | Approval | Clinical Trials |
|---|---|---|---|---|
| JYNNEOS (Imvamune or Imvanex) | Replication-restricted vaccinia virus Ankara (MVA-BN). | Recommended for both pre-exposure and post-exposure prophylaxis. | U.S. FDA for the prevention of both smallpox and mpox. | Several clinical trials have been conducted to evaluate safety, immunogenicity, and efficacy, particularly in immunocompromised persons, children, and those with HIV [34]. |
| ACAM2000 | Live, replicating vaccinia virus. | Not recommended for individuals with weakened immune systems, eczema, or pregnancy. | FDA-approved for smallpox | Limited clinical trial data were available, but there were observational insights from the 2022 mpox outbreak. |
| Drugs | Mechanisms | Approvals | Clinical Trials |
|---|---|---|---|
| Tecovirimat (TPOXX) | Inhibits the viral envelope protein VP37, blocking virus release from infected cells. | FDA-approved for smallpox; authorized under EA-IND (Expanded Access Investigational New Drug) protocol for mpox. | PLATINUM Trial (UK): Study evaluating efficacy in non-hospitalized mpox patients. STOMP Trial (US): Investigating safety and effectiveness in diverse populations, including HIV patients. |
| Brincidofovir (CMX001 or Tembexa) | Oral nucleotide analog that inhibits viral DNA polymerase. | FDA-approved for smallpox infection (FDA, 2021) | Limited use in mpox showed variable outcomes; some patients experienced liver toxicity and discontinued treatment. Further studies are needed to validate its efficacy and safety profile for mpox. |
| Cidofovir (Vistide) | Broad-spectrum antiviral administered intravenously. | FDA-approved for the treatment of cytomegalovirus (CMV) | Data is not available on the effectiveness of cidofovir in the treatment of MPXV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaman, M.S.; Sizemore, R.C.; Rodriguez, D.; Lopez, E.; Alam, S.M.G.; Tufa, S.; Lopez-Alvarenga, J.C.; Akimbekov, N.S.; Razzaque, M.S. Mpox Insights: From Structure to Human Cell Interaction. J. Mol. Pathol. 2025, 6, 29. https://doi.org/10.3390/jmp6040029
Zaman MS, Sizemore RC, Rodriguez D, Lopez E, Alam SMG, Tufa S, Lopez-Alvarenga JC, Akimbekov NS, Razzaque MS. Mpox Insights: From Structure to Human Cell Interaction. Journal of Molecular Pathology. 2025; 6(4):29. https://doi.org/10.3390/jmp6040029
Chicago/Turabian StyleZaman, Md S., Robert C. Sizemore, Draven Rodriguez, Emilio Lopez, S. M. Golam Alam, Suleyman Tufa, Juan C. Lopez-Alvarenga, Nuraly S. Akimbekov, and Mohammed S. Razzaque. 2025. "Mpox Insights: From Structure to Human Cell Interaction" Journal of Molecular Pathology 6, no. 4: 29. https://doi.org/10.3390/jmp6040029
APA StyleZaman, M. S., Sizemore, R. C., Rodriguez, D., Lopez, E., Alam, S. M. G., Tufa, S., Lopez-Alvarenga, J. C., Akimbekov, N. S., & Razzaque, M. S. (2025). Mpox Insights: From Structure to Human Cell Interaction. Journal of Molecular Pathology, 6(4), 29. https://doi.org/10.3390/jmp6040029








