Abstract
Targetable gene alterations have become increasingly important in the treatment of cancers. Thirty STK11-mutated lung cancers from 199 cases with molecular profiling performed during 2016–2024 were studied for clinical, morphologic, immunohistochemical (IHC) and molecular features. Of the 30 STK11-mutated lung cancers, 29 were lung adenocarcinomas (LADCs) and 1 was large cell neuroendocrine carcinoma (LCNEC). STK11 mutation was not found in other subtypes of lung cancers. Of the 29 STK11-mutated LADCs, 6 (21%) were mucinous and 23 (79%) were non-mucinous. Of the 19 non-mucinous LADCs with sufficient material for IHC, 9 (47%) displayed acinar/papillary/lepidic patterns, 8 (42%) were poorly differentiated (solid/trabecular/basaloid/complex glandular), and 2 (11%) had mixed solid and acinar patterns. The most common concurrent altered genes were KRAS (52%), followed by TP53 (38%), KEAP1 (34%), and DNA repair genes (BRCA2/ATM) (21%). A total of 6/15 (40%) LADCs with a KRAS mutation presented with mucinous morphology. Concurrent EGFR, ROS, or ALK alterations with STK11 mutation were rare or non-existent. Of the 3 LADCs with SMARCA4 deficiency, 2 were mucinous and 1 had basaloid/adenoid cystic-like features. All the cases were microsatellite stable (MSS). The majority (55%) had low TMB (<10). Most (86%) had PD-L1 TPS 0 or <5%. Among the 14 non-mucinous LADCs with IHC performed, 5 (36%) were TTF-1-negative and all displayed poorly differentiated morphology. Overall, 8/10 (80%) of poorly differentiated components in non-mucinous LADCs were negative for TTF-1. In contrast, all LADCs with better differentiated patterns (acini/papillary/lepidic) were positive for TTF-1. The majority (14/21, 67%) of patients with available follow-up presented with metastasis.
1. Introduction
Adenocarcinoma is the most common malignancy in the lung. Lung adenocarcinoma (LADC) can be further subclassified into mucinous and non-mucinous subtypes. Smoking is the most common risk factor for lung cancers [1], including LADC. With the advancement of molecular techniques and cancer research, targetable gene alterations have become increasingly important in the treatment of cancers. For example, EGFR-targeted therapy has become a cornerstone in the management of advanced LADCs [2,3].
Serine/threonine kinase 11 (STK11), encoding liver kinase B1 (LKB1), is a tumor suppressor gene [4,5]. Germline loss-of-function mutation in STK11 gives rise to Peutz-Jeghers syndrome [5,6], which is associated with a substantially increased lifetime risk of many cancers [5,7]. In the sporadic setting, STK11 mutations have also been found in a subset of LADCs [5,8,9,10]. Although STK11-mutated lung cancers have been sporadically studied, many aspects, including histopathologic features, remain to be elucidated.
In clinical pathology practice, differentiation of primary lung carcinoma versus metastatic adenocarcinoma mainly relies on immunohistochemical (IHC) marker study, in particular, TTF-1 and Napsin A expression in LADC [11]. TTF-1 is reported to be positive in ~90% non-mucinous LADC [12,13]. Whereas, for mucinous LADC, TTF-1 is often negative [14]. The TTF-1/Napsin A-negative non-mucinous LADCs have, so far, not been well characterized.
The aim of this study is to investigate the histomorphologic, IHC, clinical, and molecular features of STK11-mutated LADCs.
2. Materials and Methods
Clinical Cases and Tissue Samples
This study was approved by the USC Institutional Review Board (HS-24-00234). A total of 199 cases of primary and metastatic lung cancers from needle biopsy or resection specimens accessioned at our institution from 2016 to 2024 with comprehensive molecular profiling were found. STK11 mutation was identified in 30 cases (29 LADCs and 1 large cell neuroendocrine carcinoma (LCNEC)). The lung origin and adenocarcinoma subtype were confirmed by clinical and imaging studies, IHC (TTF-1/Napsin A), and/or mucicarmine stain. Other IHC markers (e.g., p40, CK7) commonly used in the workup for lung cancer and markers for exclusion of metastasis of non-lung origin were also performed when appropriate. Neuroendocrine carcinoma was confirmed by neuroendocrine markers (synaptophysin/chromogranin/CD56/INSM1).
Clinical and Pathologic Assessment
Given that the vast majority of STK11-mutated lung cancers were LADCs, this study mainly focused on STK11-mutated LADCs. All the 29 STK11-mutated LADCs were reviewed for their molecular features, IHC profile, and, when tissue was available for review, blindly by two pathologists for their morphologic growth patterns (defined by WHO). Patients’ demographic information, smoking history, and clinical follow-up data (from 7 months to 8 years) were also recorded.
Immunohistochemistry
IHC stains were performed on some of the cases based on clinical needs at the time of original case sign-out. The IHC stains were performed with a Bond III automated immunostaining platform (Leica Biosystems, Nussloch, Germany) using standard clinical protocols with appropriate positive and negative controls. Briefly, unstained glass slides of 5 µm thick tissue sections were baked and dewaxed following standard procedures. Heat-induced epitope retrieval was performed using BOND Epitope Retrieval Solution 1 (ER1) or BOND Epitope Retrieval Solution 2 (ER2) (Leica Biosystems) at 100 °C for 30 min. The antibodies used included TTF-1 (SPT24, Leica, Wetzlar, Germany), Napsin-A (TMU-Ad02, Biocare, Pacheco, USA), p40 (BC28, Biocare), CK7 (RN7, Leica), and neuroendocrine markers (Synaptophysin (27G12, Leica), Chromogranin (5H7, Leica), CD56 (CD564, Leica), and INSM1 (A-8, Santa Cruz, USA), among others. The IHC stains were recorded as positive (at least 10% of tumor cells with moderate or strong stain) or negative for each case, as well as for the poorly differentiated and better differentiated components when both were present in the same tumor.
Gene Sequencing and Molecular Analysis
The genetic alterations, including tumor mutation burden (TMB), microsatellite stability (MSS), and PD-L1 tumor percentage score (TPS), were analyzed by Caris Molecular Intelligence comprehensive tumor profiling services (Caris Life Sciences, Irving, USA) [15]. Briefly, Caris’s MI Profile Tumor Seek Hybrid was used along with the IHC in this study. IlluminaNovaSeq 6000 sequencers (San Diego, USA) were used to perform whole exome sequencing (WES) and whole transcriptome sequencing (WTS), which could detect 23,000+ genes, with more than 700 of these genes being documented as clinically relevant. Sequencing results were analyzed by Caris NGS bioinformatic pipeline, and reports were provided by Report Generation software (v4.4). Microsatellite instability status was detected in this assay. Tumor-only MSI status by NGS was determined by the direct analysis of known microsatellite regions sequenced. Tumor mutational burden (TMB) was determined by the number of non-synonymous, somatic mutations per one megabase (Mb) of sequenced DNA in the tumor sample. The cut-offs for low TMB and high TMB were <10 and ≥10 mut/Mb, respectively. IHC antibodies were also used in combination with the MI Tumor Seek Hybrid assay. PD-L1 22c3 (Dako, Santa Clara, USA) IHC was used for tumor proportion score (TPS) calculation. The TPS was grouped into three categories based on membranous staining of viable tumor cells: less than 1%, 1–49%, and at least 50%, which corresponded to negative, low positive, and high positive, respectively. It needed complete or partial membranous staining of viable tumor cells. The results were then compiled into a final report. More specific details of the methods used, including the bioinformatics pipeline, can be potentially obtained by directly contacting Caris.
3. Results
Clinical And Morpho-Pathologic Features of STK11-Mutated LADCs
Of the 199 lung cancer cases with molecular profiling performed, 112 were LADCs, 68 squamous cell carcinomas, 7 adenosquamous carcinomas, 4 adenoid cystic carcinomas, 4 large cell neuroendocrine carcinomas (LCNEC), 2 small cell carcinomas, and 2 spindle cell/pleomorphic carcinomas. Of the 199 cases, 30 (15%) had the STK11 mutation. Of the 30 cases with STK11 mutation, 29 were LADCs, accounting for 26% of the 112 LADCs studied, and 1 was LCNEC. None of the squamous cell carcinomas, adenosquamous carcinomas, adenoid cystic carcinomas, or pleomorphic carcinomas showed STK11 mutation. Patients’ ages ranged from 59 to 92 years old (mean 74 ± 9) with male predominance (the ratio of male to female was 4:1). Most patients (24/30, 80%) were current or former smokers, and almost all (28/30, 93%) had more than 10 years of smoking history.
Of the 29 STK11-mutated LADCs, 6 (21%) were mucinous and 23 (79%) were non-mucinous. Of the 23 non-mucinous LADCs, 19 contained adequate tissue for assessment of growth patterns. Of these 19 cases, 9 (47%) displayed acinar/papillary/lepidic growth patterns, 8 (42%) showed poorly differentiated (solid/nested/trabecular/basaloid/complex glandular/cribriform patterns), and 2 (2/19, 11%) had mixed solid and acinar patterns.
TMB, Other Concurring Gene Alterations, And Histopathologic Correlation for STK11-Mutated LADCs
TMB for these STK11-mutated LADCs ranged from 2 to 17 (mean 8.6 ± 3.8). A total of 16/29 (55%) had low TMB (<10), and 13 (45%) had moderate to high TMB (≥10). A total of 15/29 (52%) LADCs had a KRAS mutation, of which 6/15 (40%) presented with mucinous morphology.
Tumor suppressor genes were the second most common concurring alterations: 11/29 (38%) with TP53 mutation, 1 with MDM2 amplification, and 1 with APC alteration.
KEAP1 alterations were present in 10/29 (34%) LADCs. None of the LADCs with combined STK11 and KEAP1 mutations was associated with mucinous morphology, even in the setting of a KRAS mutation.
DNA repair gene (BRCA2, ATM) alterations were not uncommon and seen in 6/29 (21%) cases. DNA repair gene alteration and tumor suppressor gene (TP53/MDM2/APC) alterations appeared to be mutually exclusive. Concurrent EGFR and STK11 alterations were exceptionally uncommon and seen in only 1/30 (3.3%) cases. No ROS or ALK alterations were found in this series.
Of note, there were 3 LADCs with SMARCA4 deficiency, 2 of which were mucinous and 1 basaloid/adenoid cystic-like.
MSI, PD-L1, and IHC Features of STK11-Mutated LADCs
All 30 STK11-mutated lung cancer cases were microsatellite stable (MSS).
Of the 29 STK11-mutated LADCs, 20/29 (69%) had PD-L1 TPS 0; 7/29 (24%) had PD-L1 TPS 1–49%; the remaining 2 LADCs (7%) had high (≥50%) PD-L1 TPS (90% and 100%, respectively). Both cases with high PD-L1 TPS had ATM and KEAP1 alterations.
Among the 15 cases with IHC performed, TTF-1/Napsin A negativity was seen in 1 LCNEC and 6/15 (40%) of LADCs (1 mucinous and 5 non-mucinous). The growth pattern of the 5 TTF-1/Napsin A negative non-mucinous LADCs included solid/trabecular/basaloid patterns (Figure 1). Interestingly, 2 cases showed mixed positive TTF-1/Napsin A in better differentiated acinar/papillary patterns and negative TTF-1/Napsin A in poorly differentiated (solid/nested) areas (Figure 2). Overall, of the 10 STK11-mutated LADCs containing poorly differentiated (solid/nested/trabecular/basaloid/complex glandular) patterns, 8 (80%) exhibited TTF-1/Napsin A negativity in poorly differentiated components. In contrast, all the LADCs with better differentiated patterns (acini/papillary/lepidic) were positive for TTF-1/Napsin A (Figure 3). All cases, regardless of growth pattern, were diffusely CK7 positive. Of the 6 mucinous LADCs, 3 had IHC, of which 1 was TTF-1/Napsin A negative, and 2 were TTF-1/Napsin A positive.
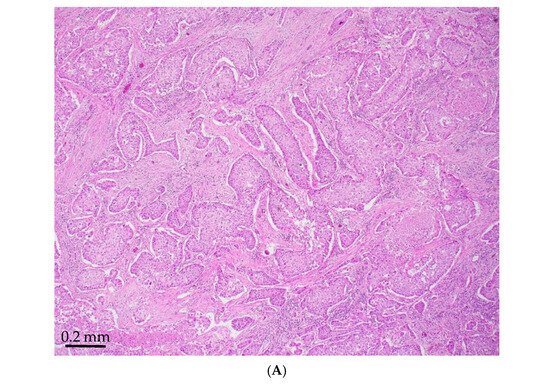
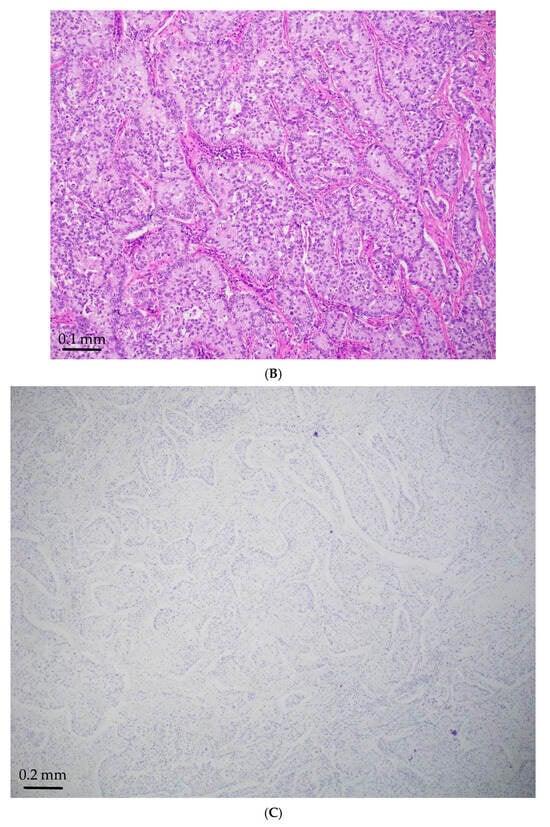
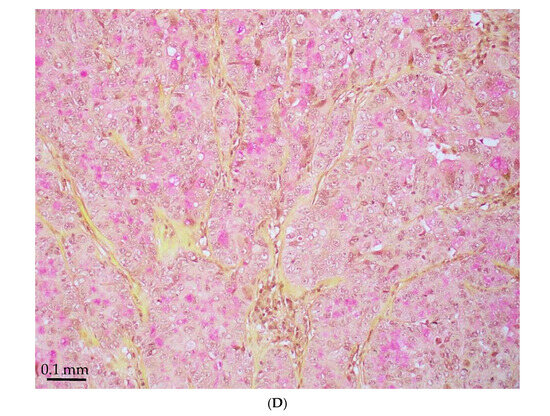
Figure 1.
STK11-mutated LADC with poorly differentiated growth patterns. (A) Solid pattern (H&E, magnification ×150). (B) Peripheral palisading basaloid pattern (H&E, magnification ×300). (C) Negative for TTF-1 (magnification ×150). (D) Positive for Mucicarmine (magnification ×300).
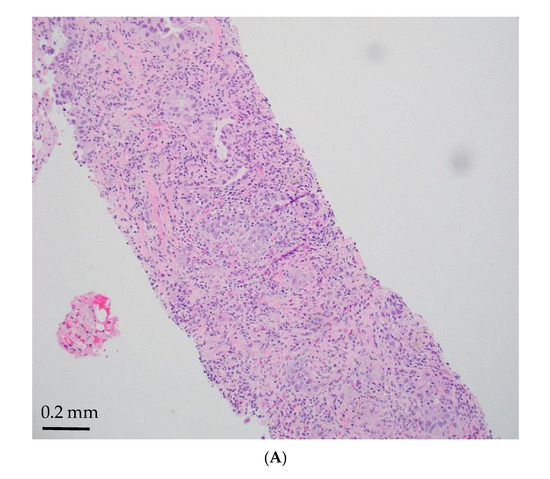
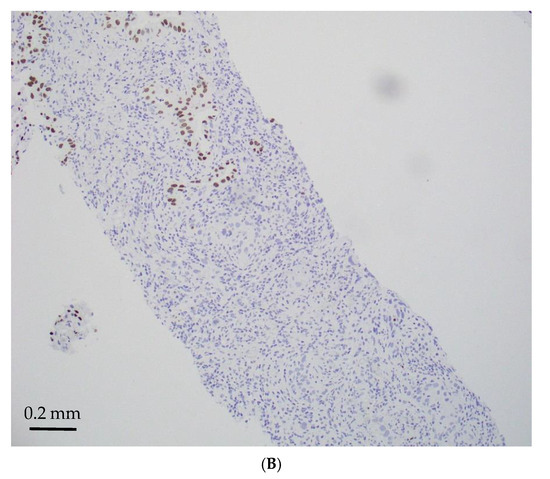
Figure 2.
STK11-mutated LADCs with mixed solid and acinar patterns (magnification ×150). (A) Mixed solid (lower) and acinar (upper) patterns (H&E); (B) TTF-1 negative in solid pattern (lower) and positive in acinar pattern (upper).
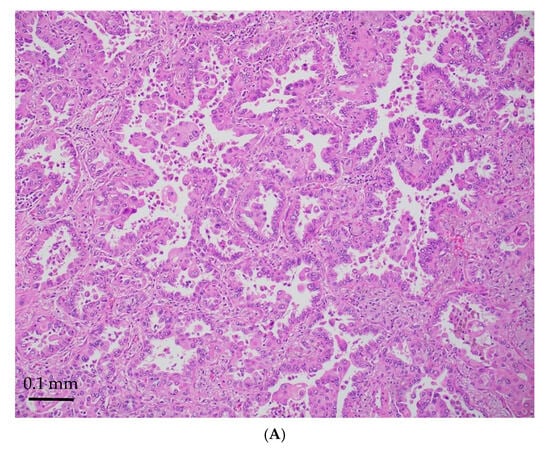
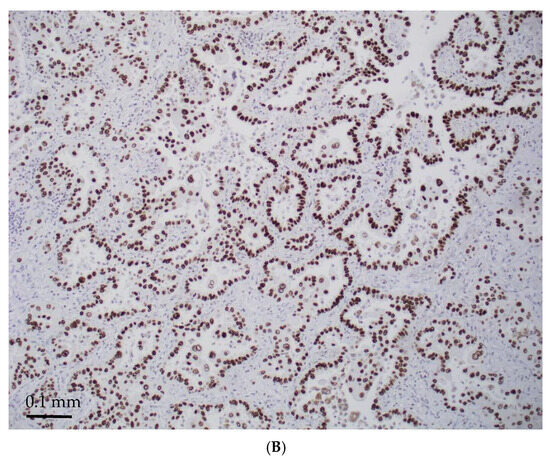
Figure 3.
STK11-mutated LADCs with better differentiated patterns (acinar/papillary) (magnification ×300). H&E stain (A) and diffusely TTF-1 positive (B).
Of the 2 STK11-mutated mucinous LADCs with SMARCA4 deficiency, 1 was TTF-1 positive, and 1 was TTF-1 negative. The 1 non-mucinous LADC with SMARCA4 deficiency showed a basaloid/adenoid cystic-like pattern and was TTF-1 negative.
Clinical Outcome of Patients With STK11-Mutated LADCs
Of the STK11-mutated LADC patients with available follow-up, the majority (14/21, 67%) presented with metastases.
The molecular, morphologic, and IHC findings, as well as the clinical follow-up, are summarized in Table 1.

Table 1.
Molecular and clinicopathologic features of STK11-mutated LADC.
4. Discussion
This study demonstrated that the vast majority (97%) of STK11-mutated lung cancers were LADC. STK11 mutation occurred in 26% of the LADCs, which, after taking into consideration the potential difference in the study population, is similar to the 17–20% reported in the TCGA (The Cancer Genome Atlas) database [16]. Like general LADC, STK11-mutated LADC was strongly associated with smoking history, and predominantly in male patients. STK11 mutations were found in both mucinous and non-mucinous LADCs. The most common concurrent altered gene with the STK11 mutation was KRAS, followed by TP53, KEAP1, and DNA repair genes (BRCA 1/2 and ATM). Tumor suppressor and DNA repair gene alterations appeared to be mutually exclusive. The targetable gene alterations EGFR, ROS, and ALK were rare or not found to concur with the STK11 mutation. Except in a rare case of LCNEC, the STK11 mutation was not found in non-LADC tumors.
The histomorphology of STK11-mutated LADCs was variable. Concurrence of STK11 with KRAS mutation could be either mucinous or non-mucinous phenotypes. All the STK11-mutated mucinous LADCs had concurrent KRAS alteration; however, concurrent STK11 and KRAS mutation did not always present with mucinous morphology. Interestingly, LADCs with concurrent STK11 and KEAP1 mutations (10 of 10 cases) never exhibited mucinous morphology, even in cases with concurrent KRAS mutations (4 of 4 cases).
Immunohistochemically, about half of the STK11-mutated non-mucinous LADCs were TTF-1/Napsin A negative, much more common than the non-mucinous LADCs in general (~10%), of which all were poorly differentiated with a solid/trabecular/basaloid growth pattern.
It has been reported that KRAS mutation silences NKX2.1/TTF-1 by methylation of its promoter, leading to downregulation of TTF-1 [17]. Loss of TTF-1 in KRAS-mutant cells is related to increased activation of the ERK signaling pathway, which promotes cancer cell proliferation and migration and is associated with mucinous LADC [18]. This event might account for the TTF-1 negativity in KRAS-mutated mucinous LADCs, as well as some KRAS-mutated non-mucinous LADCs, as seen in this study. These features are also important for practicing pathologists to recognize that, when TTF-1/Napsin A negative, non-mucinous, poorly differentiated LADCs are encountered, STK11-mutated LADCs should be considered. In addition, given the common association of negative TTF-1 with poorly differentiated morphology, loss of TTF-1 may be used as a predictor for aggressive behavior.
SMARCA4-deficient lung cancer can be classified as thoracic SMARCA4-deficient tumors or SMARCA4-deficient non-small cell lung cancer. Histologically, SMARCA4-deficient thoracic tumors are often poorly differentiated or undifferentiated. In contrast, SMARCA4-deficient non-small cell lung cancer exhibits a broad spectrum of morphological features [19,20,21,22]. As shown in this study, SMACR4-deficient LADC with STK11 co-mutation can present with mucinous or basaloid morphology.
The frequent association of STK11 mutation with TP53 and DNA gene repair mutations, as well as poor differentiation in morphology, is in keeping with the aggressive nature and poor prognosis of these tumors.
In addition to MSS, except for cases with concurrent ATM and STK11 mutations, the low PD-L1 TPS in most STK11-mutated LADCs may imply poor responses to PD-L1/immune checkpoint inhibitors (ICI), which seems to be in line with the results of other studies [16,23,24,25].
STK11 is a tumor suppressor gene. One of its roles is acting as an inhibitor of mTOR signaling. When STK11 is mutated, the mTOR pathway becomes hyperactivated, resulting in increased cell growth and proliferation. Additionally, STK11 inactivation creates a pro-growth and inert tumor immune microenvironment, including reduced density of infiltrating cytotoxic CD8+ T lymphocytes, a low expression of PD-L1 in the tumor, an immune-suppressive neutrophil-enriched niche, and inactivation of the STING (stimulator of interferon genes) pathway. These events can contribute to not only tumor development/progression but also immune evasion and resistance to ICI [26,27,28]. The combination of STK11, KRAS, and/or KEAP1 mutations has been shown to confer worse prognosis and survival outcomes [29,30,31,32,33].
Given the low expression of PD-L1 and inert immune microenvironment in STK11-mutated LADCs (with or without KRAS and/or KEAP1 co-mutation), new strategies for treatment are being explored for these patients. Combining two ICIs (e.g., anti-PD-L1 and anti-CTLA-4) with chemotherapy has shown greater effectiveness than either treatment alone in retrospective studies [27,28,29]. Blocking IL-6 has shown some success in preclinical models by decreasing tumor proliferation and improving T-cell function [27,28]. For patients with the specific KRAS p.G12C subtype, targeted inhibitors like sotorasib and adagrasib are available. KEAP1 mutations activate the NRF2 pathway [30,31]. As the activation of the NRF2 pathway is a key driver of resistance, inhibition of NRF2, particularly in combination with PD-L1 inhibition, has shown promising results in some cancer types [27,29].
Of note, our study showed that almost half (45%) of the STK11-mutated LADCs had TMB ≥ 10. These patients with high TMB may have better responses to ICI than patients with low TMB. In addition, the frequent DNA repair gene mutations (BRCA2 and ATM, 21%) may qualify these patients for treatment with a PARP inhibitor. Therefore, further stratification of these patients in a personalized manner may be beneficial in achieving optimal outcomes. Future studies using recently developed tools, such as Multi-omics Immuno-Oncology Biologic Research (IOBR), to uncover the immune microenvironment in these tumors and design new immunotherapeutic regimens are warranted [34].
In conclusion, STK11-mutated LADCs have unique attributes. Recognition of this molecular entity will not only have prognostic and therapeutic implications but may also have diagnostic value in daily practice. Testing for STK11 mutation may be included in the existing panel of routine molecular tests for pulmonary adenocarcinomas. More recently, STK11 IHC staining has become available, leading to more convenient biomarker assessment [26,27,28,29,30,31,32,33,34,35].
This study is limited by the relatively small sample size, lack of robust clinical follow-up data, potential case selection bias (e.g., referral for testing, sample adequacy), and the retrospective nature of the study design, as well as assay/threshold differences that could have affected prevalence, PD-L1, and TMB distributions. Large cohorts and more comprehensive studies are warranted to validate the results.
Author Contributions
Conceptualization, G.-Q.X.; Methodology, J.J. and G.-Q.X.; Validation, J.J., W.D.W. and G.-Q.X.; Formal analysis, J.J. and G.-Q.X.; Data curation, J.J. and G.-Q.X.; Writing—original draft, J.J. and G.-Q.X.; Writing—review & editing, J.J., W.D.W. and G.-Q.X.; Supervision, G.-Q.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the USC Institutional Review Board (HS-24-00234). The date for ethical approval is 22 September 2022, and recertified on 11 July 2025.
Informed Consent Statement
There is no need patient’s consent because the use of archived/paraffin-embedded tissue and pts consent is waived.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Burney, L.E. Smoking and lung cancer: A statement of the Public Health Service. J. Am. Med. Assoc. 1959, 171, 1829–1837. [Google Scholar] [CrossRef] [PubMed]
- Giaccone, G.; Rodriguez, J.A. EGFR inhibitors: What have we learned from the treatment of lung cancer? Nat. Clin. Pract. Oncol. 2005, 2, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Ladanyi, M.; Pao, W. Lung adenocarcinoma: Guiding EGFR-targeted therapy and beyond. Mod. Pathol. 2008, 21 (Suppl 2), S16–S22. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Zhao, Z.Y.; Li, B.R.; Yang, F.; Li, J.; Jin, X.W.; Wang, H.; Yu, E.D.; Sun, S.H.; Ning, S.B. The altered activity of P53 signaling pathway by STK11 gene mutations and its cancer phenotype in Peutz-Jeghers syndrome. BMC Med. Genet. 2018, 19, 141. [Google Scholar] [CrossRef]
- Zyla, R.E.; Hahn, E.; Hodgson, A. Gene of the month: STK11. J. Clin. Pathol. 2021, 74, 681–685. [Google Scholar] [CrossRef]
- Jenne, D.E.; Reomann, H.; Nezu, J.I.; Friedel, W.; Loff, S.; Jeschke, R.; Müller, O.; Back, W.; Zimmer, M. Peutz-Jeghers syndrome is caused by mutations in a novel serine threoninekinase. Nat. Genet. 1998, 18, 38–43. [Google Scholar] [CrossRef]
- Daniell, J.; Plazzer, J.P.; Perera, A.; Macrae, F. An exploration of genotype-phenotypelink between Peutz-Jeghers syndrome and STK11: A review. Fam. Cancer 2018, 17, 421–427. [Google Scholar] [CrossRef]
- Sanchez-Cespedes, M.; Parrella, P.; Esteller, M.; Nomoto, S.; Trink, B.; Engles, J.M.; Westra, W.H.; Herman, J.G.; Sidransky, D. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002, 62, 3659–3662. [Google Scholar]
- Collisson, E.A.; Campbell, J.D.; Brooks, A.N.; Berger, A.H.; Lee, W.; Chmielecki, J.; Beer, D.G.; Cope, L.; Creighton, C.J.; Danilova, L.; et al. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014, 511, 543–550. [Google Scholar] [CrossRef]
- Ji, H.; Ramsey, M.R.; Hayes, D.N.; Fan, C.; McNamara, K.; Kozlowski, P.; Torrice, C.; Wu, M.C.; Shimamura, T.; Perera, S.A.; et al. Lkb1 modulates lung cancer differentiation and metastasis. Nature 2007, 448, 807–810. [Google Scholar] [CrossRef]
- Jagirdar, J. Application of immunohistochemistry to the diagnosis of primary and metastatic carcinoma to the lung. Arch. Pathol. Lab. Med. 2008, 132, 384–396. [Google Scholar] [CrossRef]
- Vidarsdottir, H.; Tran, L.; Nodin, B.; Jirström, K.; Planck, M.; Mattsson, J. Comparison of Three Different TTF-1 Clones in Resected Primary Lung Cancer and Epithelial Pulmonary Metastases. Am. J. Clin. Pathol. 2018, 150, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Matoso, A.; Singh, K.; Jacob, R.; Greaves, W.O.; Tavares, R.; Noble, L.; Resnick, M.B.; DeLellis, R.A.; Wang, L.J. Comparison of thyroid transcription factor-1 expression by 2 monoclonal antibodies in pulmonary and nonpulmonary primary tumors. Appl. Immunohistochem. Mol. Morphol. 2010, 18, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, R.; Li, Y.; Pan, Y.; Hu, H.; Zhang, Y.; Li, H.; Shen, L.; Yu, Y.; Sun, Y.; et al. Negative thyroid transcription factor 1 expression defines an unfavorable subgroup of lung adenocarcinomas. J. Thorac. Oncol. 2015, 10, 1444–1450. [Google Scholar] [CrossRef]
- Caris Molecular Intelligence: Technical Information. Available online: https://www.carismolecularintelligence.com/wp-content/uploads/2017/03/Profile (accessed on 3 February 2025).
- Malhotra, J.; Ryan, B.; Patel, M.; Chan, N.; Guo, Y.; Aisner, J.; Jabbour, S.K.; Pine, S. Clinical outcomes and immune phenotypes associated with STK11 co-occurring mutations in non-small cell lung cancer. J. Thorac. Dis. 2022, 14, 1772–1783. [Google Scholar] [CrossRef]
- Maeda, Y.; Tsuchiya, T.; Hao, H.; Tompkins, D.H.; Xu, Y.; Mucenski, M.L.; Du, L.; Keiser, A.R.; Fukazawa, T.; Naomoto, Y.; et al. Kras(G12D) and NKX2.1 haploinsufficiency induce mucinous adenocarcinoma of the lung. J. Clin. Investig. 2012, 122, 4388–4400. [Google Scholar]
- Hwang, D.H.; Sholl, L.M.; Rojas-Rudilla, V.; Hall, D.L.; Shivdasani, P.; Garcia, E.P.; MacConaill, L.E.; Vivero, M.; Hornick, J.L.; Kuo, F.C.; et al. KRAS and NKX2-1 Mutations in Invasive Mucinous Adenocarcinoma of the Lung. J. Thorac. Oncol. 2016, 11, 496–503. [Google Scholar] [CrossRef]
- Longo, V.; Catino, A.; Montrone, M.; Montagna, E.S.; Pesola, F.; Marech, I. Treatment of Thoracic SMARCA4-Deficient Undifferentiated Tumors: Where We Are and Where We Will Go. Int. J. Mol. Sci. 2024, 25, 3237. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, G.; Han, W.; Jiang, H. The role of SMARCA4 in lung cancer. Sci. Rep. 2025, 15, 28605. [Google Scholar] [CrossRef]
- Le Loarer, F.; Watson, S.; Pierron, G.; de Montpreville, V.T.; Ballet, S.; Firmin, N.; Auguste, A.; Pissaloux, D.; Boyault, S.; Paindavoine, S.; et al. SMARCA4 inactivation defines a group of undifferentiated thoracic malignancies transcriptionally related to BAF-deficient sarcomas. Nat. Genet. 2015, 47, 1200–1205. [Google Scholar] [CrossRef]
- WHO Classification of Tumours, 5th ed.; Thoracic Tumors; World Health Organization: Geneva, Switzerland, 2021; Volume 5, pp. 111–114.
- Biton, J.; Mansuet-Lupo, A.; Pécuchet, N.; Alifano, M.; Ouakrim, H.; Arrondeau, J.; Boudou-Rouquette, P.; Goldwasser, F.; Leroy, K.; Goc, J.; et al. TP53, STK11, and EGFR Mutations Predict Tumor Immune Profile and the Response to Anti-PD-1 in Lung Adenocarcinoma. Clin. Cancer Res. 2018, 24, 5710–5723. [Google Scholar] [CrossRef]
- Mograbi, B.; Heeke, S.; Hofman, P. The importance of STK11/LKB1 assessment in non-small cell lung carcinomas. Diagnostics 2021, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Deng, Y.; Huang, B.; Chen, X. Prognostic implications of STK11 with different mutation status and its relationship with tumor-infiltrating immune cells in non-small cell lung cancer. Front. Immunol. 2024, 15, 1387896. [Google Scholar] [CrossRef] [PubMed]
- Sumbly, V.; Landry, I. Unraveling the Role of STK11/LKB1 in Non-small Cell Lung Cancer. Cureus 2022, 14, e21078. [Google Scholar] [CrossRef] [PubMed]
- Pons-Tostivint, E.; Lugat, A.; Fontenau, J.F.; Denis, M.G.; Bennouna, J. STK11/LKB1 Modulation of the Immune Response in Lung Cancer: From Biology to Therapeutic Impact. Cells 2021, 10, 3129. [Google Scholar] [CrossRef]
- Gutiérrez-Babativa, L.; Wagner-Gutiérrez, N.; Rojas, L.; Zuluaga, J.; Arrieta, O.; Cardona, A.F. Overcoming immunotherapy resistance in non-small cell lung cancer: A narrative review of related factors. Immunotherapy 2025, 17, 823–833. [Google Scholar] [CrossRef]
- Knetki-Wróblewska, M.; Wojas-Krawczyk, K.; Krawczyk, P.; Krzakowski, M. Emerging insights into STK11, KEAP1 and KRAS mutations: Implications for immunotherapy in patients with advanced non-small cell lung cancer. Transl. Lung Cancer Res. 2024, 13, 3718–3730. [Google Scholar] [CrossRef]
- Boeschen, M.; Kuhn, C.K.; Wirtz, H.; Seyfarth, H.J.; Frille, A.; Lordick, F.; Hacker, U.T.; Obeck, U.; Stiller, M.; Bläker, H.; et al. Comparative bioinformatic analysis of KRAS, STK11 and KEAP1 (co-)mutations in non-small cell lung cancer with a special focus on KRAS G12C. Lung Cancer 2023, 184, 107361. [Google Scholar] [CrossRef]
- Skoulidis, F.; Goldberg, M.E.; Greenawalt, D.M.; Hellmann, M.D.; Awad, M.M.; Gainor, J.F.; Schrock, A.B.; Hartmaier, R.J.; Trabucco, S.E.; Gay, L.; et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer Discov. 2018, 8, 822–835. [Google Scholar] [CrossRef]
- Manolakos, P.; Ward, L.D. A Critical Review of the Prognostic and Predictive Implications of KRAS and STK11 Mutations and Co-Mutations in Metastatic Non-Small Lung Cancer. J. Pers. Med. 2023, 13, 1010–1031. [Google Scholar] [CrossRef]
- Dziadziuszko, R. STK11 and KEAP1 Mutations in Lung Adenocarcinoma: Solving the Puzzle Continues. J. Thorac. Oncol. 2022, 17, 351–352. [Google Scholar] [CrossRef]
- Fang, Y.; Kong, Y.; Rong, G.; Luo, Q.; Liao, W.; Zeng, D. Systematic investigation of tumor microenvironment and antitumor immunity with IOBR. Med. Res. 2025, 1, 136–140. [Google Scholar] [CrossRef]
- Saliba, M.; Febres-Aldana, C.; Baine, M.; Yang, S.R.; Sauter, J.; Travis, W.; Rudin, C.; Ladanyi, M.; Rekhtman, N.; Chang, J. Immunohistochemistry for the Detection of STK11 (LKB1) Genomic Alterations in Lung Adenocarcinoma. Lab. Investig. 2025, 105 (Suppl 3), 103971. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).