STK11 Mutated Lung Adenocarcinoma: A Molecular and Clinicopathologic Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burney, L.E. Smoking and lung cancer: A statement of the Public Health Service. J. Am. Med. Assoc. 1959, 171, 1829–1837. [Google Scholar] [CrossRef] [PubMed]
- Giaccone, G.; Rodriguez, J.A. EGFR inhibitors: What have we learned from the treatment of lung cancer? Nat. Clin. Pract. Oncol. 2005, 2, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Ladanyi, M.; Pao, W. Lung adenocarcinoma: Guiding EGFR-targeted therapy and beyond. Mod. Pathol. 2008, 21 (Suppl 2), S16–S22. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Zhao, Z.Y.; Li, B.R.; Yang, F.; Li, J.; Jin, X.W.; Wang, H.; Yu, E.D.; Sun, S.H.; Ning, S.B. The altered activity of P53 signaling pathway by STK11 gene mutations and its cancer phenotype in Peutz-Jeghers syndrome. BMC Med. Genet. 2018, 19, 141. [Google Scholar] [CrossRef]
- Zyla, R.E.; Hahn, E.; Hodgson, A. Gene of the month: STK11. J. Clin. Pathol. 2021, 74, 681–685. [Google Scholar] [CrossRef]
- Jenne, D.E.; Reomann, H.; Nezu, J.I.; Friedel, W.; Loff, S.; Jeschke, R.; Müller, O.; Back, W.; Zimmer, M. Peutz-Jeghers syndrome is caused by mutations in a novel serine threoninekinase. Nat. Genet. 1998, 18, 38–43. [Google Scholar] [CrossRef]
- Daniell, J.; Plazzer, J.P.; Perera, A.; Macrae, F. An exploration of genotype-phenotypelink between Peutz-Jeghers syndrome and STK11: A review. Fam. Cancer 2018, 17, 421–427. [Google Scholar] [CrossRef]
- Sanchez-Cespedes, M.; Parrella, P.; Esteller, M.; Nomoto, S.; Trink, B.; Engles, J.M.; Westra, W.H.; Herman, J.G.; Sidransky, D. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002, 62, 3659–3662. [Google Scholar]
- Collisson, E.A.; Campbell, J.D.; Brooks, A.N.; Berger, A.H.; Lee, W.; Chmielecki, J.; Beer, D.G.; Cope, L.; Creighton, C.J.; Danilova, L.; et al. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014, 511, 543–550. [Google Scholar] [CrossRef]
- Ji, H.; Ramsey, M.R.; Hayes, D.N.; Fan, C.; McNamara, K.; Kozlowski, P.; Torrice, C.; Wu, M.C.; Shimamura, T.; Perera, S.A.; et al. Lkb1 modulates lung cancer differentiation and metastasis. Nature 2007, 448, 807–810. [Google Scholar] [CrossRef]
- Jagirdar, J. Application of immunohistochemistry to the diagnosis of primary and metastatic carcinoma to the lung. Arch. Pathol. Lab. Med. 2008, 132, 384–396. [Google Scholar] [CrossRef]
- Vidarsdottir, H.; Tran, L.; Nodin, B.; Jirström, K.; Planck, M.; Mattsson, J. Comparison of Three Different TTF-1 Clones in Resected Primary Lung Cancer and Epithelial Pulmonary Metastases. Am. J. Clin. Pathol. 2018, 150, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Matoso, A.; Singh, K.; Jacob, R.; Greaves, W.O.; Tavares, R.; Noble, L.; Resnick, M.B.; DeLellis, R.A.; Wang, L.J. Comparison of thyroid transcription factor-1 expression by 2 monoclonal antibodies in pulmonary and nonpulmonary primary tumors. Appl. Immunohistochem. Mol. Morphol. 2010, 18, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, R.; Li, Y.; Pan, Y.; Hu, H.; Zhang, Y.; Li, H.; Shen, L.; Yu, Y.; Sun, Y.; et al. Negative thyroid transcription factor 1 expression defines an unfavorable subgroup of lung adenocarcinomas. J. Thorac. Oncol. 2015, 10, 1444–1450. [Google Scholar] [CrossRef]
- Caris Molecular Intelligence: Technical Information. Available online: https://www.carismolecularintelligence.com/wp-content/uploads/2017/03/Profile (accessed on 3 February 2025).
- Malhotra, J.; Ryan, B.; Patel, M.; Chan, N.; Guo, Y.; Aisner, J.; Jabbour, S.K.; Pine, S. Clinical outcomes and immune phenotypes associated with STK11 co-occurring mutations in non-small cell lung cancer. J. Thorac. Dis. 2022, 14, 1772–1783. [Google Scholar] [CrossRef]
- Maeda, Y.; Tsuchiya, T.; Hao, H.; Tompkins, D.H.; Xu, Y.; Mucenski, M.L.; Du, L.; Keiser, A.R.; Fukazawa, T.; Naomoto, Y.; et al. Kras(G12D) and NKX2.1 haploinsufficiency induce mucinous adenocarcinoma of the lung. J. Clin. Investig. 2012, 122, 4388–4400. [Google Scholar]
- Hwang, D.H.; Sholl, L.M.; Rojas-Rudilla, V.; Hall, D.L.; Shivdasani, P.; Garcia, E.P.; MacConaill, L.E.; Vivero, M.; Hornick, J.L.; Kuo, F.C.; et al. KRAS and NKX2-1 Mutations in Invasive Mucinous Adenocarcinoma of the Lung. J. Thorac. Oncol. 2016, 11, 496–503. [Google Scholar] [CrossRef]
- Longo, V.; Catino, A.; Montrone, M.; Montagna, E.S.; Pesola, F.; Marech, I. Treatment of Thoracic SMARCA4-Deficient Undifferentiated Tumors: Where We Are and Where We Will Go. Int. J. Mol. Sci. 2024, 25, 3237. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, G.; Han, W.; Jiang, H. The role of SMARCA4 in lung cancer. Sci. Rep. 2025, 15, 28605. [Google Scholar] [CrossRef]
- Le Loarer, F.; Watson, S.; Pierron, G.; de Montpreville, V.T.; Ballet, S.; Firmin, N.; Auguste, A.; Pissaloux, D.; Boyault, S.; Paindavoine, S.; et al. SMARCA4 inactivation defines a group of undifferentiated thoracic malignancies transcriptionally related to BAF-deficient sarcomas. Nat. Genet. 2015, 47, 1200–1205. [Google Scholar] [CrossRef]
- WHO Classification of Tumours, 5th ed.; Thoracic Tumors; World Health Organization: Geneva, Switzerland, 2021; Volume 5, pp. 111–114.
- Biton, J.; Mansuet-Lupo, A.; Pécuchet, N.; Alifano, M.; Ouakrim, H.; Arrondeau, J.; Boudou-Rouquette, P.; Goldwasser, F.; Leroy, K.; Goc, J.; et al. TP53, STK11, and EGFR Mutations Predict Tumor Immune Profile and the Response to Anti-PD-1 in Lung Adenocarcinoma. Clin. Cancer Res. 2018, 24, 5710–5723. [Google Scholar] [CrossRef]
- Mograbi, B.; Heeke, S.; Hofman, P. The importance of STK11/LKB1 assessment in non-small cell lung carcinomas. Diagnostics 2021, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Deng, Y.; Huang, B.; Chen, X. Prognostic implications of STK11 with different mutation status and its relationship with tumor-infiltrating immune cells in non-small cell lung cancer. Front. Immunol. 2024, 15, 1387896. [Google Scholar] [CrossRef] [PubMed]
- Sumbly, V.; Landry, I. Unraveling the Role of STK11/LKB1 in Non-small Cell Lung Cancer. Cureus 2022, 14, e21078. [Google Scholar] [CrossRef] [PubMed]
- Pons-Tostivint, E.; Lugat, A.; Fontenau, J.F.; Denis, M.G.; Bennouna, J. STK11/LKB1 Modulation of the Immune Response in Lung Cancer: From Biology to Therapeutic Impact. Cells 2021, 10, 3129. [Google Scholar] [CrossRef]
- Gutiérrez-Babativa, L.; Wagner-Gutiérrez, N.; Rojas, L.; Zuluaga, J.; Arrieta, O.; Cardona, A.F. Overcoming immunotherapy resistance in non-small cell lung cancer: A narrative review of related factors. Immunotherapy 2025, 17, 823–833. [Google Scholar] [CrossRef]
- Knetki-Wróblewska, M.; Wojas-Krawczyk, K.; Krawczyk, P.; Krzakowski, M. Emerging insights into STK11, KEAP1 and KRAS mutations: Implications for immunotherapy in patients with advanced non-small cell lung cancer. Transl. Lung Cancer Res. 2024, 13, 3718–3730. [Google Scholar] [CrossRef]
- Boeschen, M.; Kuhn, C.K.; Wirtz, H.; Seyfarth, H.J.; Frille, A.; Lordick, F.; Hacker, U.T.; Obeck, U.; Stiller, M.; Bläker, H.; et al. Comparative bioinformatic analysis of KRAS, STK11 and KEAP1 (co-)mutations in non-small cell lung cancer with a special focus on KRAS G12C. Lung Cancer 2023, 184, 107361. [Google Scholar] [CrossRef]
- Skoulidis, F.; Goldberg, M.E.; Greenawalt, D.M.; Hellmann, M.D.; Awad, M.M.; Gainor, J.F.; Schrock, A.B.; Hartmaier, R.J.; Trabucco, S.E.; Gay, L.; et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer Discov. 2018, 8, 822–835. [Google Scholar] [CrossRef]
- Manolakos, P.; Ward, L.D. A Critical Review of the Prognostic and Predictive Implications of KRAS and STK11 Mutations and Co-Mutations in Metastatic Non-Small Lung Cancer. J. Pers. Med. 2023, 13, 1010–1031. [Google Scholar] [CrossRef]
- Dziadziuszko, R. STK11 and KEAP1 Mutations in Lung Adenocarcinoma: Solving the Puzzle Continues. J. Thorac. Oncol. 2022, 17, 351–352. [Google Scholar] [CrossRef]
- Fang, Y.; Kong, Y.; Rong, G.; Luo, Q.; Liao, W.; Zeng, D. Systematic investigation of tumor microenvironment and antitumor immunity with IOBR. Med. Res. 2025, 1, 136–140. [Google Scholar] [CrossRef]
- Saliba, M.; Febres-Aldana, C.; Baine, M.; Yang, S.R.; Sauter, J.; Travis, W.; Rudin, C.; Ladanyi, M.; Rekhtman, N.; Chang, J. Immunohistochemistry for the Detection of STK11 (LKB1) Genomic Alterations in Lung Adenocarcinoma. Lab. Investig. 2025, 105 (Suppl 3), 103971. [Google Scholar] [CrossRef]
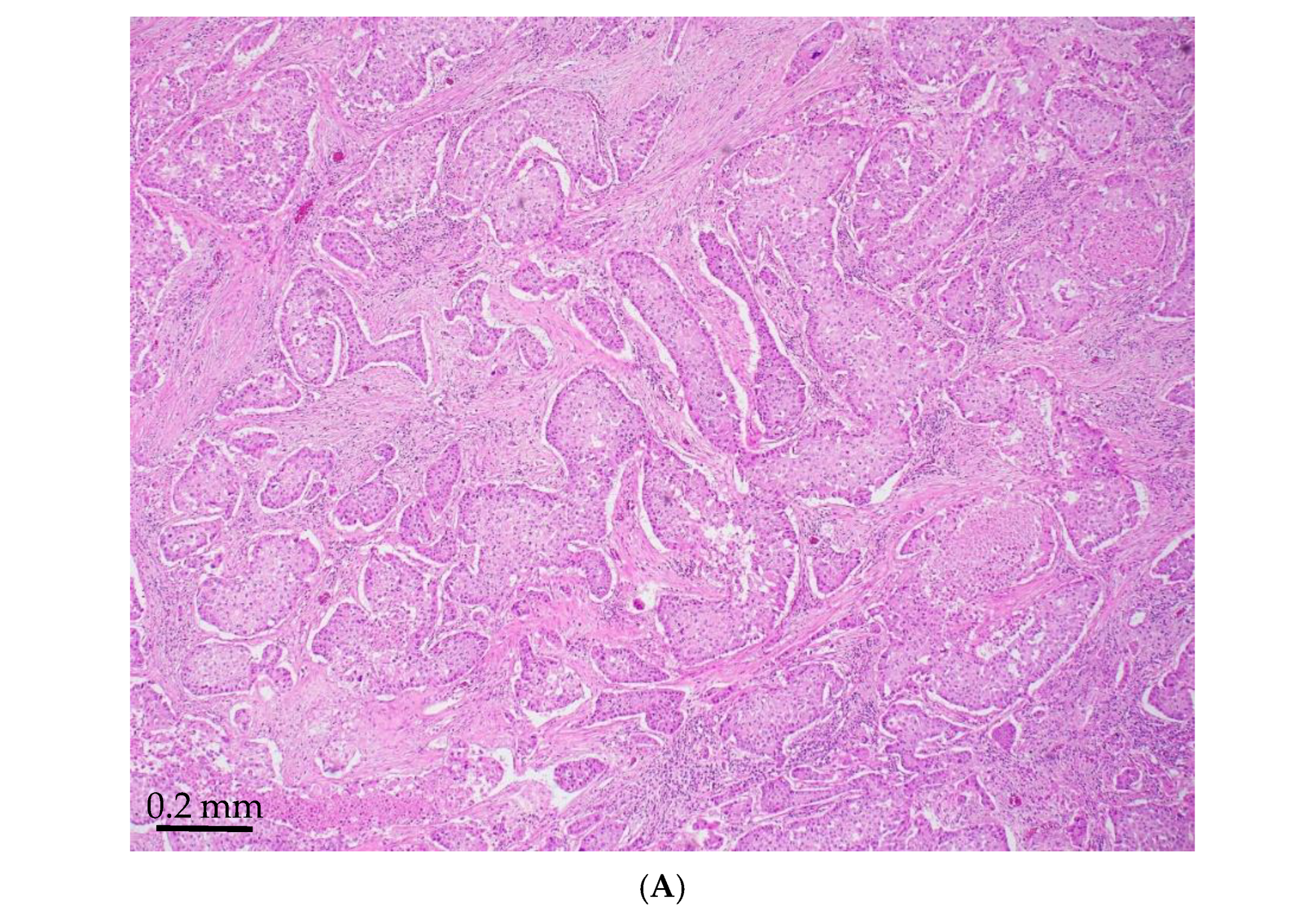
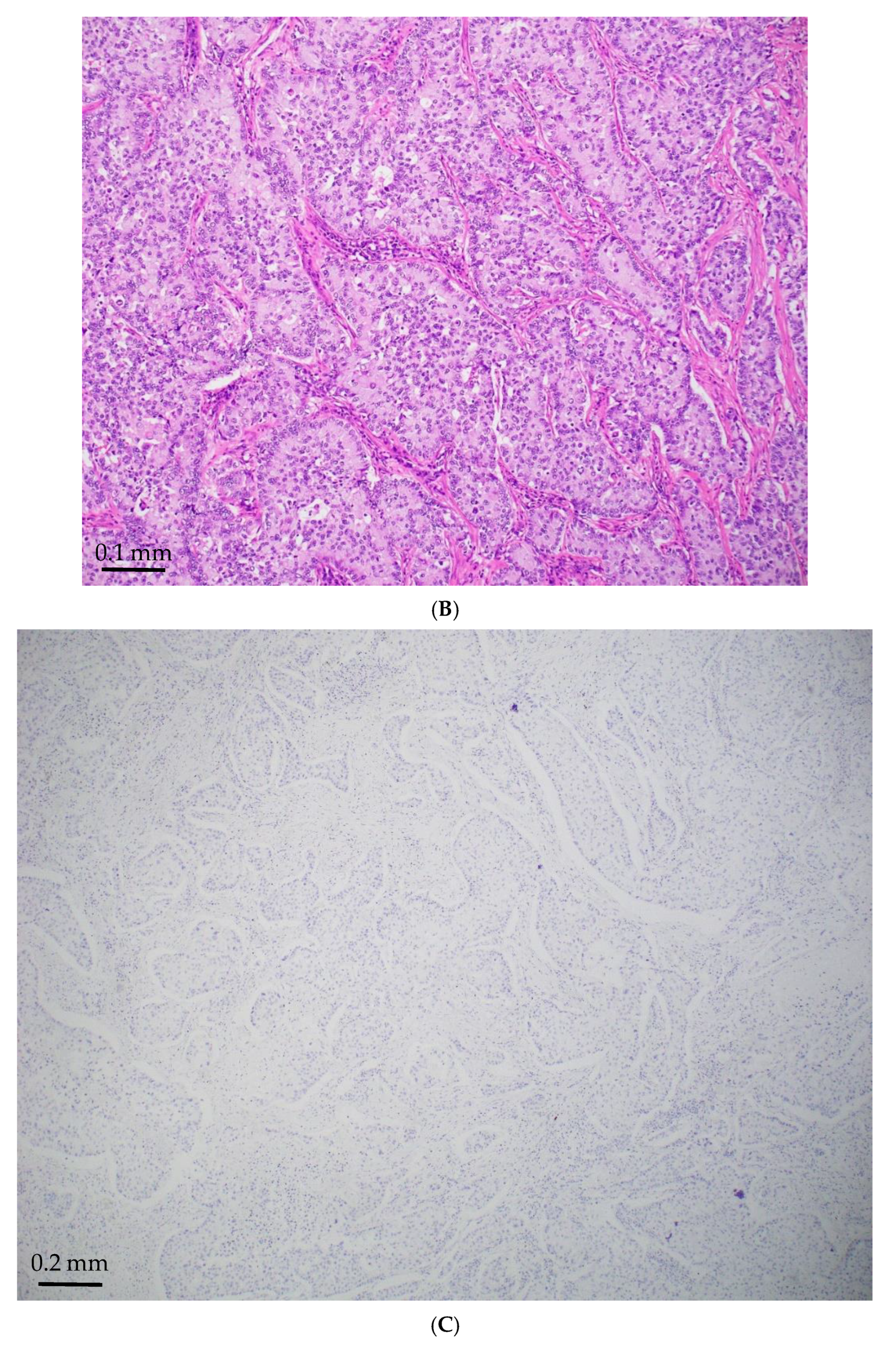
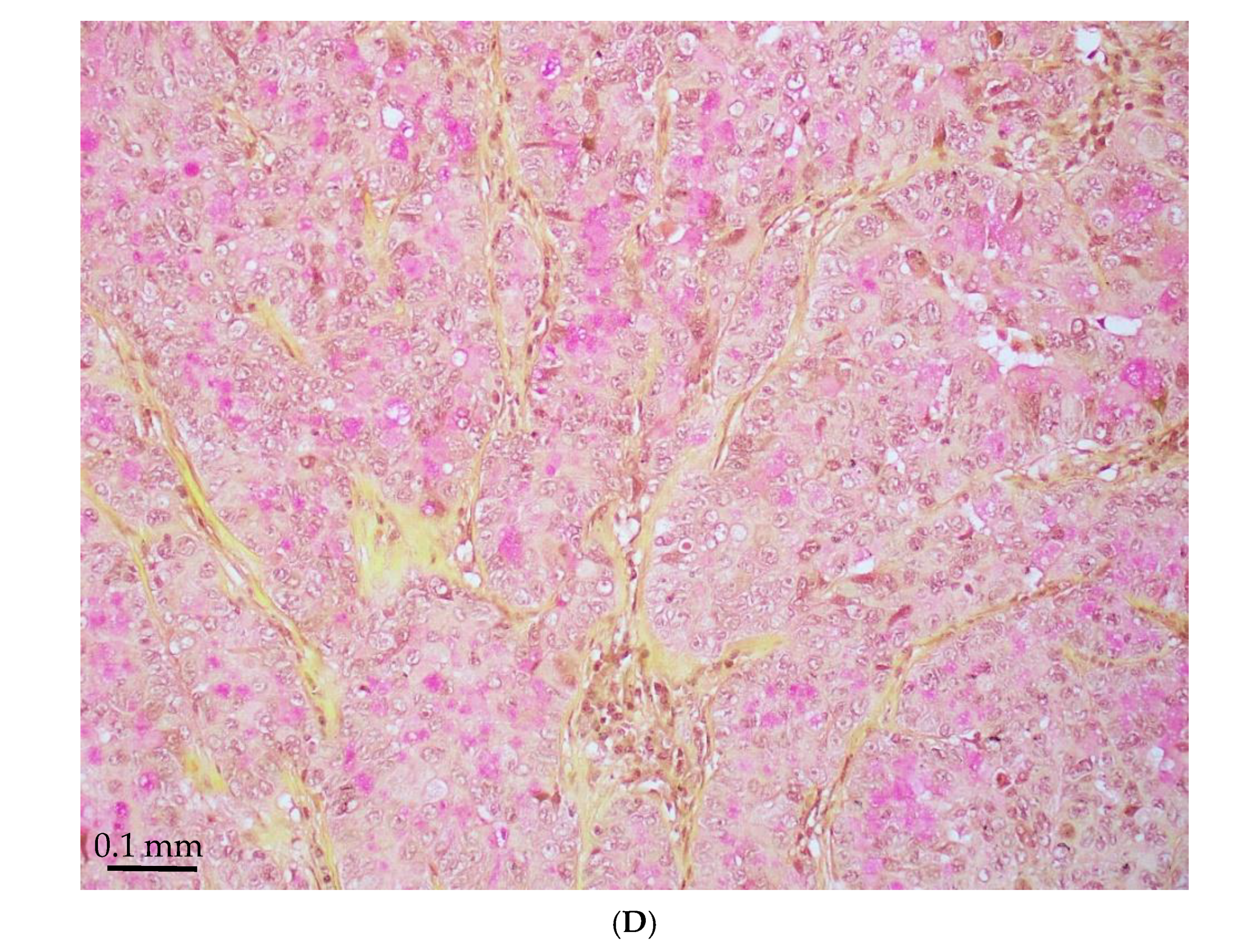
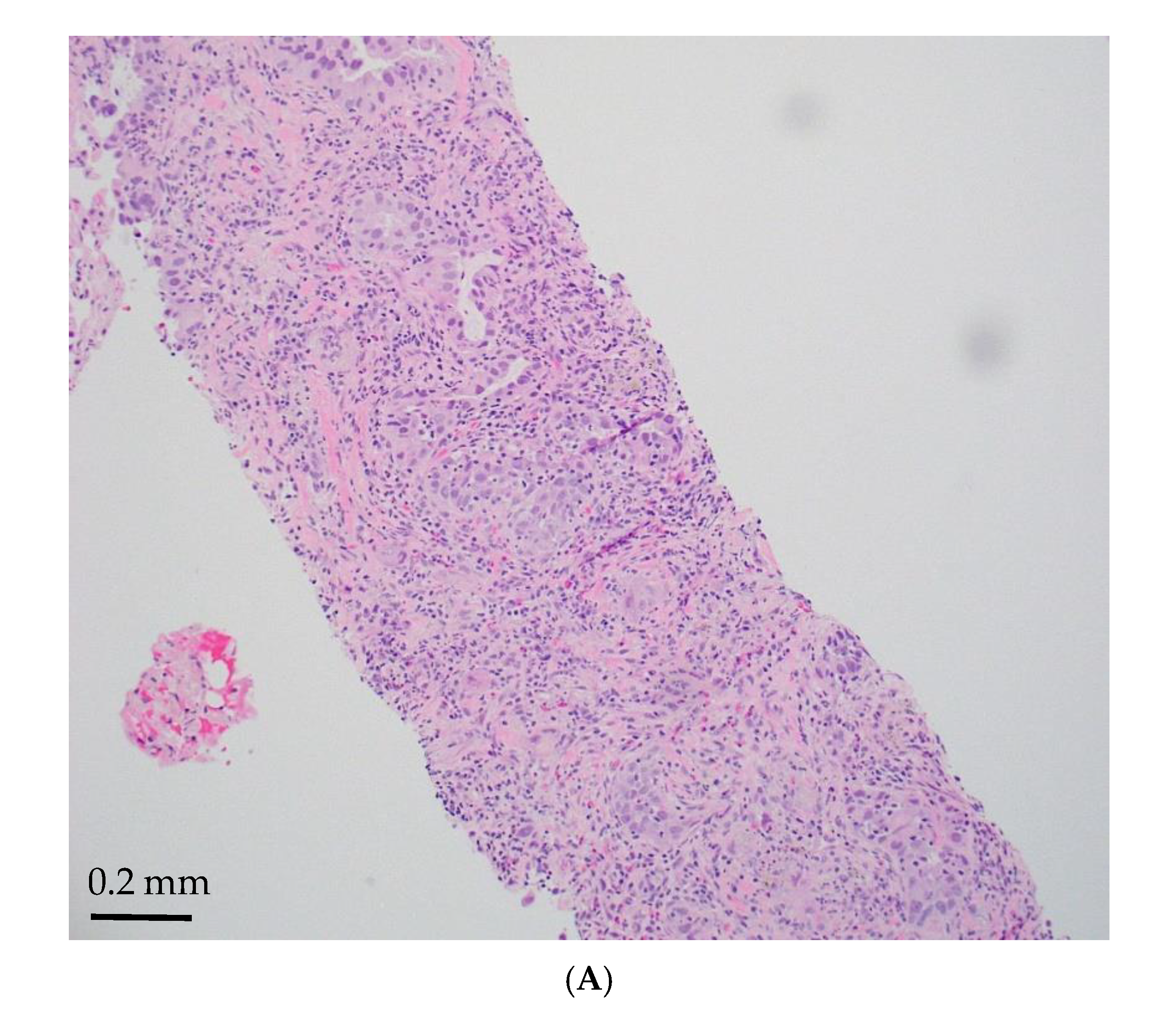
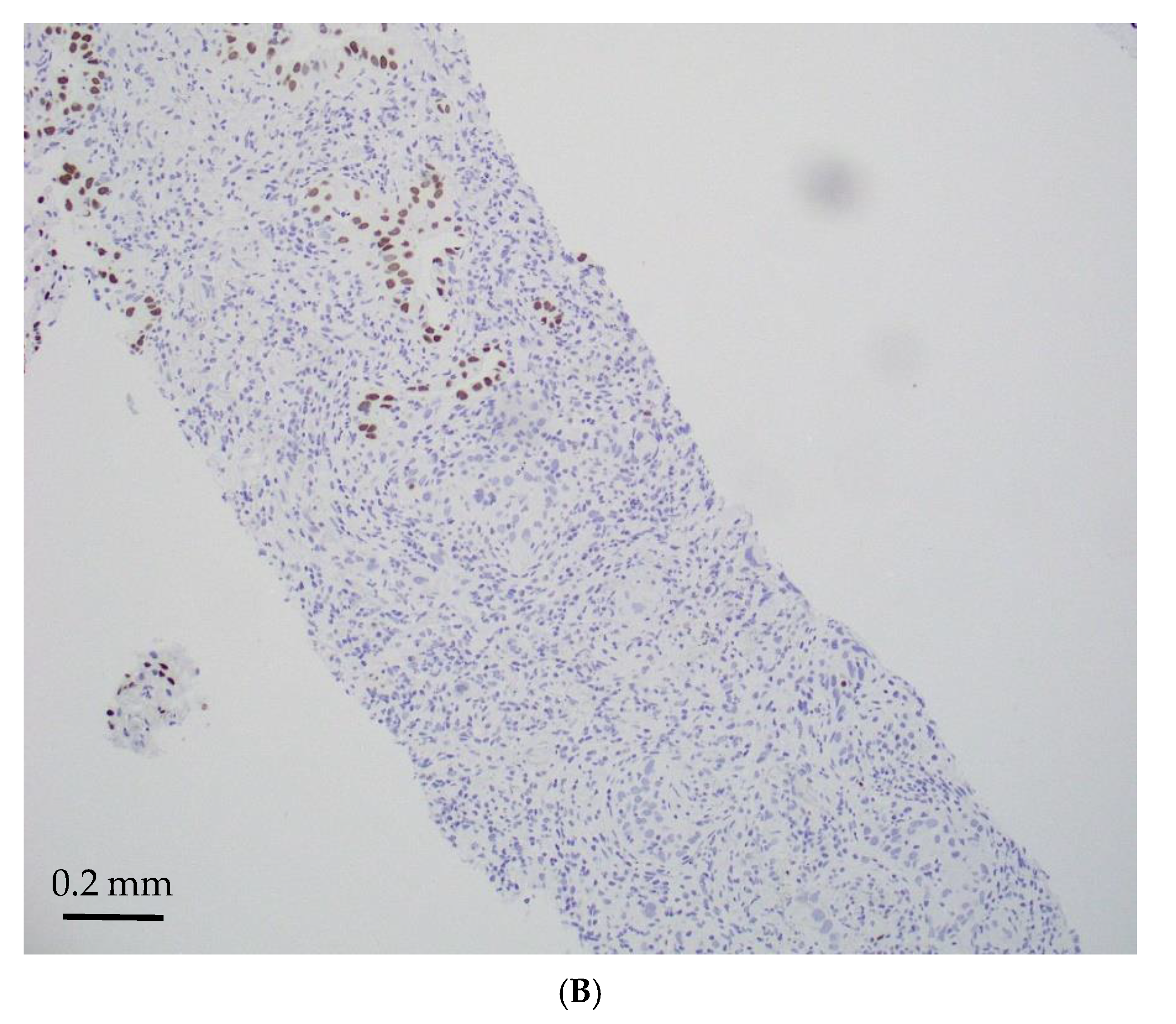
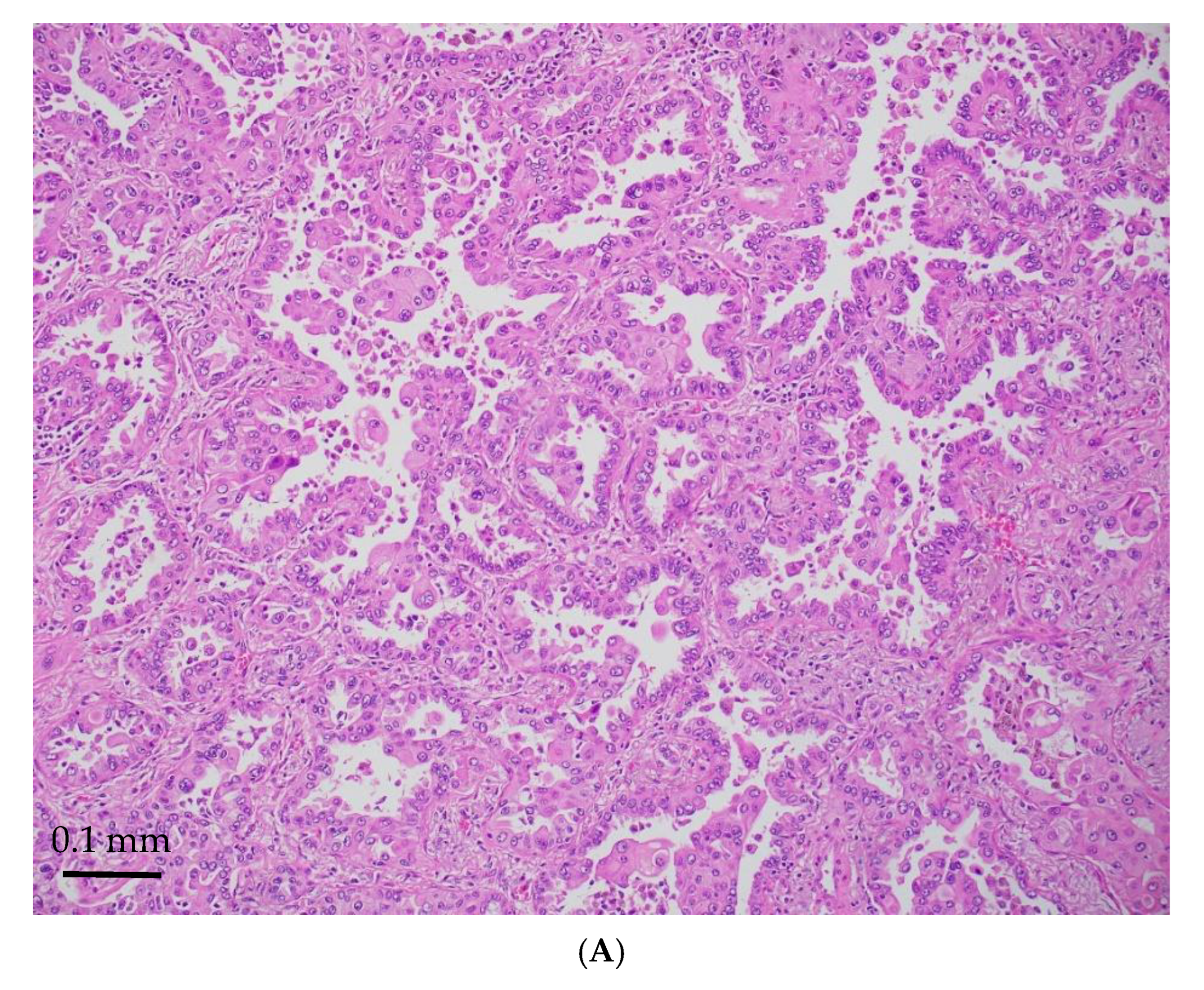
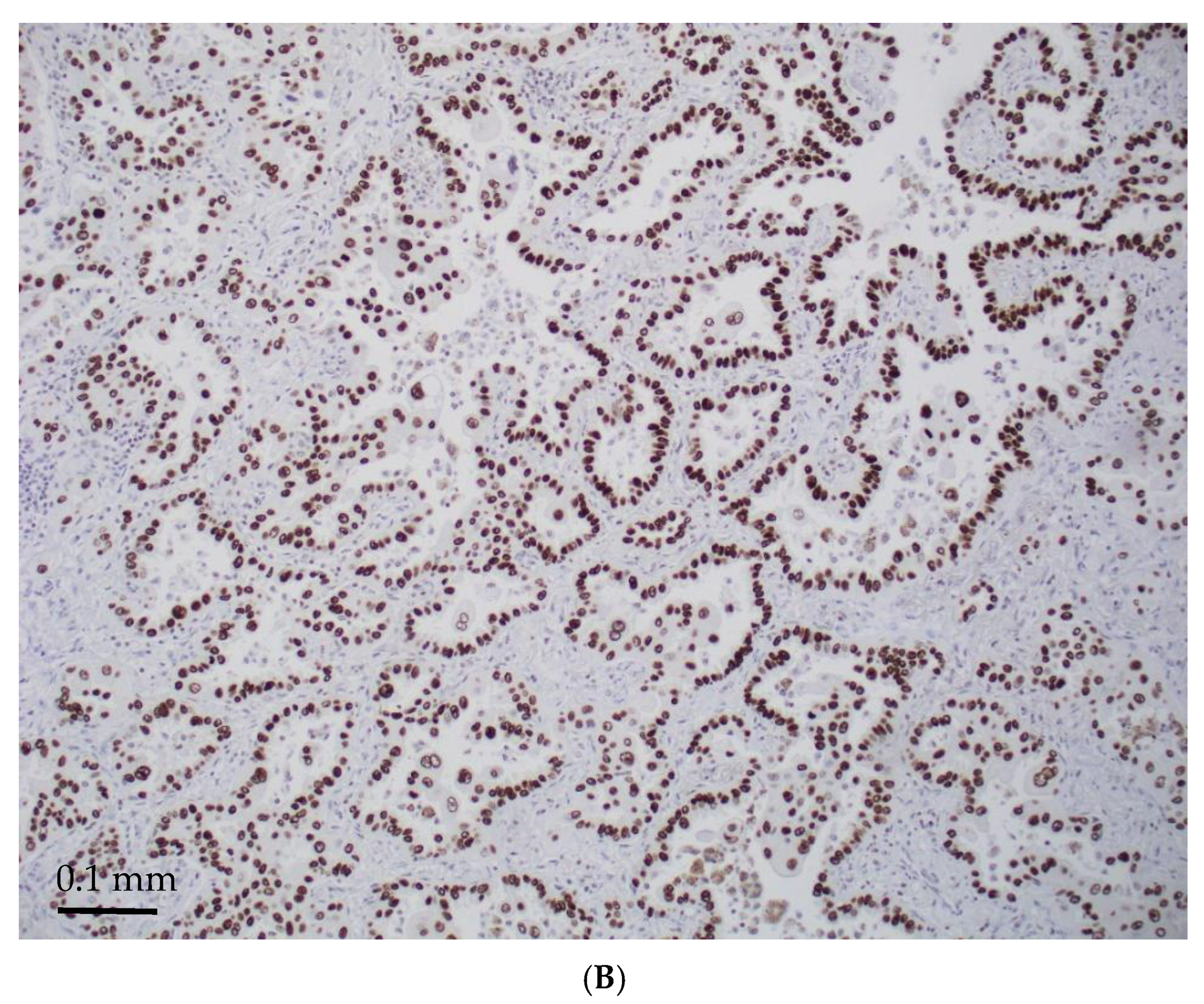
| Age/ Sex | Morphology | Smoking History | IHC (TTF-1/Napsin A, CK7 and p40) | Other Associated Gene Alterations in Addition to STK11 | PD-L1/TPS (%) | TMB (mut/Mb) | MSI | Follow Up (Recurrence and Metastasis) | Stage |
|---|---|---|---|---|---|---|---|---|---|
| 92/M | Mucinous | Yes | TTF-1+, CK7+ | BRCA2, KRAS, RNF43, SMARCA4 | 0 | 11 | MSS | Lost to follow-up | NA |
| 69/M | Mucinous | Yes | TTF-1+, Napsin A+, p40- | KRAS, POT1, TP53 | 23 | 9 | MSS | No recurrence or metastasis | T2aN0 |
| 79/M | Mucinous | Yes | NA | ATM, KRAS | 0 | 8 | MSS | Lost to follow-up | T1aN0 |
| 78M | Mucinous | Yes | TTF-1-, Napsin A- | EGFR, NFKBIA, KRAS, RBM10 SMARCA4 | 0 | 4 | MSS | Metastasis to bone, pleura and lymph nodes | NA |
| 86M | Mucinous | Yes | NA | KRAS, NKX2.1 | 0 | 4 | MSS | Recurrence in lung | NA |
| 83/F | Mucinous | Yes | NA | KRAS, U2AF1 | 0 | 8 | MSS | Metastasis to brain | NA |
| 80/M | Acinar, papillary | Yes | NA | KEAP1 | 0 | 5 | MSS | No recurrence or metastasis | T2aN0 |
| 81/M | N/A | Yes | TTF-1+, Napsin A+, CK7+, P40- | TP53, BCOR, MYC, NF1, NFE2L2 | 0 | 17 | MSS | Metastasis to lymph nodes | NA |
| 53/M | Small solid nests | Yes | TTF-1-, Napsin A-, p40-, CK7+ | TP53, ARID2 | 0 | 7 | MSS | Metastasis to lymph nodes | NA |
| 82/M | Acinar | Yes | TTF-1+, Napsin A+, P40- | CCNE1, KRAS | 0 | 7 | MSS | Metastasis to lymph nodes | NA |
| 73/M | N/A | Yes | TTF-1+, Napsin A+, CK7+ | KRAS, TP53 | 0 | 15 | MSS | Metastasis to lymph nodes, bone and brain | NA |
| 65/F | Cribriform, solid | Yes | TTF-1,+ Napsin A+, CK7+ | CCNE1, MAP2K1 (MEK1), TP53 | 0 | 13 | MSS | Metastasis to brain | NA |
| 78/F | Basaloid, adenoid cyst-like | Yes | TTF-1-, Napsin A-, p40-, CK7+ | KRAS, STK11, SMARCA4, APC, KEAP1 | 0 | 10 | MSS | Metastasis to brain and bone | T1cN0 |
| 71M | N/A | No | TTF-1+, Napsin A+ | ATM, KRAS | 3 | 3 | MSS | Lost to follow up | NA |
| 64M | N/A | Yes | TTF-1+, Napsin A+, Ck7+ | DNMT3A, KEAP1, TP53 | 2 | 12 | MSS | Metastasis to liver, deceased | NA |
| 82F | Small nests, trabecular | No | NA | MDM2 | 0 | 2 | MSS | Metastasis to bone | NA |
| 82M | Solid, acinar | Yes | Solid: TTF-1-, Napsin A-; acinar: TTF-1+, Napsin A+ | MGA, PRKDC, TP53 | 1 | 13 | MSS | Lost to follow-up | T2aN0 |
| 62M | Trabecular, solid | Yes | TTF-1-, Napsin A-, p40-, CK7+ | ATM, KEAP1, KRAS | 100 | 11 | MSS | Metastasis to lymph nodes | T1cN2 |
| 70M | Acinar, complex glandular pattern | Yes | acini: TTF-1+; complex glands: TTF-1- | TP53, ARID2, PDGFRA | 0 | 9 | MSS | No recurrence or metastasis | T1cN0 |
| 77F | Solid, basaloid | Yes | TTF-1-, p40- | KEAP1, KRAS, RBM10 | 1 | 10 | MSS | Recurrence in lung | T2bN0 |
| 69M | Acinar, solid nest | Yes | TTF-1+, Napsin A+, CK7+ | CTNNB1, TP53 | 0 | 13 | MSS | No recurrence or metastasis | yT3N2 |
| 59M | Basaloid, solid nest | Yes | TTF-1-, Napsin A-, p40-, CK7+ | TP53, KEAP1 | 7 | 4 | MSS | Metastasis to scalp | NA |
| 73M | Acinar, papillary | No | TTF-1+, CK7+ | BRCA2, KEAP1 | 0 | 4 | MSS | Metastasis to pleura | NA |
| 70M | Lepidic, acinar, papillary | Yes | NA | ARID2, TP53 | 15 | 10 | MSS | No recurrence or metastasis | pT1cN0 |
| 84F | Lepidic, acinar, papillary | No | NA | KEAP1, MYC, RAF1, TET2 | 0 | 5 | MSS | No recurrence or metastasis | pT1bN0 |
| 72M | Lepidic, acinar, papillary | No | NA | KRAS, NFE2L2 | 0 | 6 | MSS | No recurrence or metastasis | pT1cN0 |
| 83M | Acinar, papillary | Yes | TTF-1+, Napsin A+, p40-, CK7+ | KRAS, RUNX1 | 0 | 11 | MSS | Lost to follow-up | NA |
| 62M | Acinar, solid, nested | No | solid: TTF-1-, CK7+; acinar: TTF-1+, CK7+ | ATM, KEAP1, KRAS, TGFBR2 | 90 | 11 | MSS | Metastasis to lymph nodes | T1cN2 |
| 64F | Lepidic, acinar, papillary | Yes | NA | BRAF, FAT1, KEAP1, MET, U2AF | 0 | 8 | MSS | Lost to follow-up | pT1bN0 |
| 63M | LCNEC | Yes | TTF-1-, Napsin A-, p40-, CK7+, NE markers+ | RB1, TP53, EED, JAK2, KRAS | 3 | 47 | MSS | Metastasis to brain | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jean, J.; Wallace, W.D.; Xiao, G.-Q. STK11 Mutated Lung Adenocarcinoma: A Molecular and Clinicopathologic Study. J. Mol. Pathol. 2025, 6, 28. https://doi.org/10.3390/jmp6040028
Jean J, Wallace WD, Xiao G-Q. STK11 Mutated Lung Adenocarcinoma: A Molecular and Clinicopathologic Study. Journal of Molecular Pathology. 2025; 6(4):28. https://doi.org/10.3390/jmp6040028
Chicago/Turabian StyleJean, Jeffrey, William D. Wallace, and Guang-Qian Xiao. 2025. "STK11 Mutated Lung Adenocarcinoma: A Molecular and Clinicopathologic Study" Journal of Molecular Pathology 6, no. 4: 28. https://doi.org/10.3390/jmp6040028
APA StyleJean, J., Wallace, W. D., & Xiao, G.-Q. (2025). STK11 Mutated Lung Adenocarcinoma: A Molecular and Clinicopathologic Study. Journal of Molecular Pathology, 6(4), 28. https://doi.org/10.3390/jmp6040028






