1. Introduction
Breast cancer (BC) remains one of the most heterogeneous malignancies, characterized by marked molecular, biological, and clinical diversity. Classification based on molecular markers such as the human epidermal growth factor receptor 2 (HER2), estrogen receptor (ER), and progesterone receptor (PR) has been instrumental in defining subtypes—luminal A, luminal B, HER2-enriched, and triple-negative breast cancer (TNBC)—each with unique prognostic and therapeutic implications [
1]. These molecular distinctions have guided the era of precision oncology, enabling targeted treatments and improved patient outcomes. Yet, even within defined subtypes, variability in clinical behavior and therapeutic response persists, underscoring the existence of additional molecular factors that influence disease progression and treatment resistance. Identifying such factors is critical for refining prognostic tools and expanding therapeutic options, especially in the face of adaptive tumor metabolism and evolving resistance mechanisms.
One of the emerging molecular regulators at this intersection of metabolism and oncogenesis is solute carrier family 7 member 5 (
SLC7A5), which encodes the L-type amino acid transporter 1 (LAT1). LAT1 is a sodium-independent transmembrane transporter responsible for the uptake of large neutral amino acids—particularly leucine, phenylalanine, and valine—required for protein synthesis and cellular growth [
2]. Beyond its primary transport function, LAT1 plays a pivotal role in cancer cell metabolism by modulating the mammalian target of rapamycin complex 1 (mTORC1) signaling pathway. Through leucine-mediated activation of mTORC1, LAT1 contributes to nutrient sensing, metabolic adaptation, and survival under nutrient stress conditions—hallmarks of aggressive tumors [
3]. This coupling of amino acid transport to growth signaling places LAT1 at a strategic metabolic checkpoint linking nutrient availability to oncogenic signaling, proliferation, and therapy resistance.
Recent advances in cancer therapeutics increasingly recognize metabolic reprogramming as a defining feature of tumor evolution. As described in reviews such as “Cancer Treatments: Past, Present, and Future”, metabolic and transport-related mechanisms have progressively shifted from being ancillary processes to becoming central targets of therapeutic intervention. Inhibitors of metabolic pathways—including amino acid transporters like LAT1—are now viewed as potential adjuncts to overcome resistance to conventional therapies and to enhance biomarker-driven patient selection. Within this paradigm, LAT1’s dual function—as both a metabolic enabler and a signaling modulator—renders it a compelling biomarker and therapeutic target, especially in tumors that thrive under metabolic stress, such as TNBC.
In breast cancer, elevated LAT1 expression has been linked to enhanced proliferation, angiogenesis, and poor differentiation, all of which correlate with aggressive clinical behavior and adverse outcomes [
4]. LAT1 facilitates sustained amino acid influx, ensuring the biosynthetic and energetic demands of rapidly dividing tumor cells are met. By maintaining intracellular leucine levels, LAT1 not only drives protein synthesis but also activates nutrient-sensing pathways that promote anabolic metabolism and suppress apoptosis [
5]. Moreover, the interplay between LAT1 and the AKT/mTORC1 axis has been observed in hormone receptor-positive cell lines such as MCF-7, where its upregulation augments proliferative signaling [
6,
7]. Collectively, these findings suggest that
SLC7A5 is not merely a metabolic transporter but a dynamic participant in oncogenic signaling and cellular adaptation—key processes that underpin therapeutic resistance and disease recurrence.
Clinically, high SLC7A5 expression has been correlated with poor histological differentiation, higher tumor grade, and advanced stage, indicating its potential as a prognostic indicator. However, despite extensive research across diverse populations, there remains a paucity of data from South Asian cohorts, where distinct genetic, environmental, and dietary factors may shape tumor metabolism differently. Understanding LAT1 expression in this regional context is crucial for building a more globally inclusive molecular landscape of breast cancer biology.
Therefore, this study examined the LAT1 (SLC7A5) expression in a cohort of Pakistani patients with invasive breast carcinoma, aiming to determine its prevalence and its association with clinicopathological parameters such as tumor grade, size, nodal status, molecular subtype, and the Nottingham Prognostic Index. By correlating LAT1 expression with established prognostic markers, this research sought to clarify its role in breast tumor biology and assessed its potential as a biomarker for disease aggressiveness and therapeutic targeting. Grounding this investigation within the broader continuum of cancer metabolism and therapeutic evolution highlights how understanding nutrient transporters like LAT1 can inform the next generation of precision oncology strategies—where targeting cellular metabolism may complement and enhance traditional receptor- and gene-driven treatments.
2. Materials and Methods
This was a prospective study. The samples were collected from two tertiary care hospitals in Lahore, Pakistan: Mayo Hospital and Shaikh Zayed Hospital.
Ethical considerations
The study was conducted as part of a Ph.D. research project and was approved by the Advanced Studies and Research Board of the University of Health Sciences (UHS), Pakistan, with ethical approval number: UHS/Education/126-16/215.
Inclusion and Exclusion criteria
Included samples were taken from mastectomy or modified radical mastectomy specimens, from patients with microscopically confirmed primary invasive carcinoma of no special type (ductal).
Patients who had received neoadjuvant therapy were excluded. Neoadjuvant therapy in cancer is primarily administered before the surgery to downstage the tumor, making previously inoperable or borderline resectable tumors amenable to surgical removal. To avoid treatment-induced alterations in histological and molecular features, these specimens were excluded.
Histopathological Assessment
All tissue samples underwent a standard fixation and paraffin embedding, followed by sectioning and staining with hematoxylin and eosin (H&E). Tumors were graded histologically using the Nottingham Histological Grading System, in accordance with the College of American Pathologists (CAP) Cancer Protocol of resection specimens from patients with invasive carcinoma of the breast (June 2024).
The Nottingham Prognostic Index (NPI) was calculated for each case using the following formula: NPI = (0.2 × tumor diameter in cm) + lymph node stage + tumor grade. Lymph node stage and tumor grade were categorized as shown in the
Table 1 below:
The NPI-based prognostic groups were classified as follows:
Good outcome: NPI < 3.4, Moderate risk: NPI 3.4–5.3, Poor outcome: NPI ≥ 5.4.
Immunohistochemistry (IHC)
For biomarker analysis, immunohistochemical staining was performed on 4 µm sections from two representative paraffin blocks of each tumor. Expressions of estrogen receptor (ER), progesterone receptor (PR), HER2/neu, and Ki-67 were assessed based on College of American Pathologists (CAP) Guidelines (March 2023).
SLC7A5 protein expression was evaluated using a recombinant rabbit monoclonal antibody (Anti-
SLC7A5/LAT1, Abcam, Code: ab208776, dilution 1:300). Testis tissue was used as a positive control, following manufacturer recommendations. Expression was considered positive when strong membranous and/or cytoplasmic staining was observed in >10% of tumor cells; negative if staining was seen in <10% of cells [
8].
Three independent consultant histopathologists evaluated the IHC slides. In cases of interpretational variance, the majority consensus (2 out of 3 pathologists) was considered final. In order to assess scoring reproducibility, inter-observer variability was analyzed using Cohen’s kappa (κ) statistic, which demonstrated substantial agreement (κ = 0.78) among pathologists, confirming consistency of LAT1 scoring. The localization of LAT1 staining (membranous versus cytoplasmic) was specifically documented, as LAT1 can exhibit mixed localization depending on the quality of fixation and the performance of the antibody batch. Representative photomicrographs (
Figure 1) have been relabeled to clearly indicate staining intensity and localization, ensuring the distinction between membranous and cytoplasmic expression is visually evident.
Statistical Analysis
Data were compiled and analyzed using IBM SPSS Statistics, version 27. Frequencies and percentages were calculated for categorical variables like age of the patient, side of the tumor (left/right breast), nodal stage (stages N0, N1, N2, N3), tumor stage (T2, T3, T4), Nottingham grade (I, II, III), and the molecular classifications. The Pearson chi-square test was applied to evaluate associations between clinicopathological variables. A p-value < 0.05 was considered statistically significant.
3. Results
A total of 83 patients with invasive ductal carcinoma meeting the criteria were included in this study. The expression of SLC7A5 was evaluated, and a comparative analysis was conducted between positive and negative cases. Among these patients, 63 (75.9%) were SLC7A5-negative, while 20 patients (24.1%) were SLC7A5-positive. This difference in expression was statistically significant (p < 0.001), indicating a relatively lower rate of SLC7A5 positivity in the study cohort.
The mean age of SLC7A5-positive patients was 48.4 ± 10.8 years. When grouped by age, patients ≤ 50 years showed a non-significantly higher frequency of SLC7A5 positivity compared to those > 50 years, suggesting a potential trend without statistical support. Regarding tumor laterality, 14 of the 20 SLC7A5-positive tumors (70%) were located on the right side, while 6 (30%) were on the left. Although SLC7A5 positivity appeared more common in right-sided tumors, this difference was not statistically significant.
The association of
SLC7A5 expression with lymph node status showed that most positive cases were in the N1–N2 category (4–9 metastatic nodes). Specifically, 9 patients (45%) were N1 and 7 (35%) were N2, whereas only 4 (20%) had no nodal involvement (N0). This pattern suggests a tendency for
SLC7A5 positivity in patients with lymph node involvement, although statistical significance was not reached (
Table 2).
Table 3 summarizes the key outcomes.
SLC7A5 expression was more frequently observed in tumors with higher histological grade (Grade III) compared to Grade I/II, indicating a trend toward association with aggressive tumor phenotype, though the difference was not statistically significant (
p = 0.129). Similarly, patients with poor Nottingham Prognostic Index (NPI) scores showed a higher frequency of
SLC7A5 positivity (
p = 0.14), suggesting a potential link to worse clinical outcomes. However, no significant association with age was found (
p = 0.567), indicating that
SLC7A5 expression is independent of patient age.
Furthermore, associations between
SLC7A5 expression and common breast cancer biomarkers—estrogen receptor (ER), progesterone receptor (PR), HER2, and Ki67—were assessed. As summarized in
Table 4, no statistically significant associations were observed between
SLC7A5 and any of these biomarkers.
Overall, the reactivity of SLC7A5 does not significantly correlate with age, tumor laterality, lymph node stage, tumor grade, molecular subtype, or biomarker profile. These findings suggest that SLC7A5 may function independently of conventional clinicopathological parameters and could potentially serve as an independent prognostic or diagnostic marker in invasive ductal breast carcinoma.
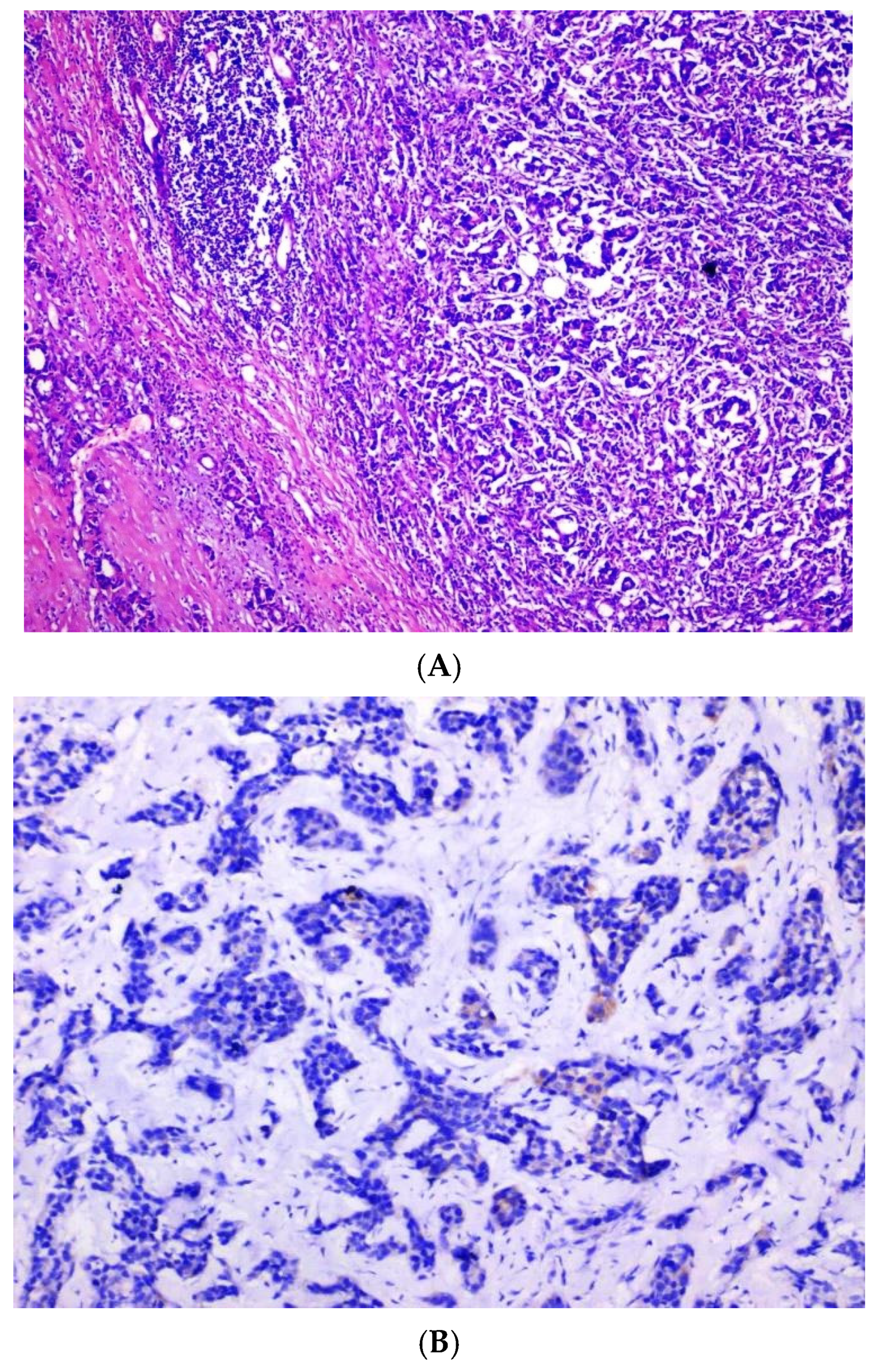
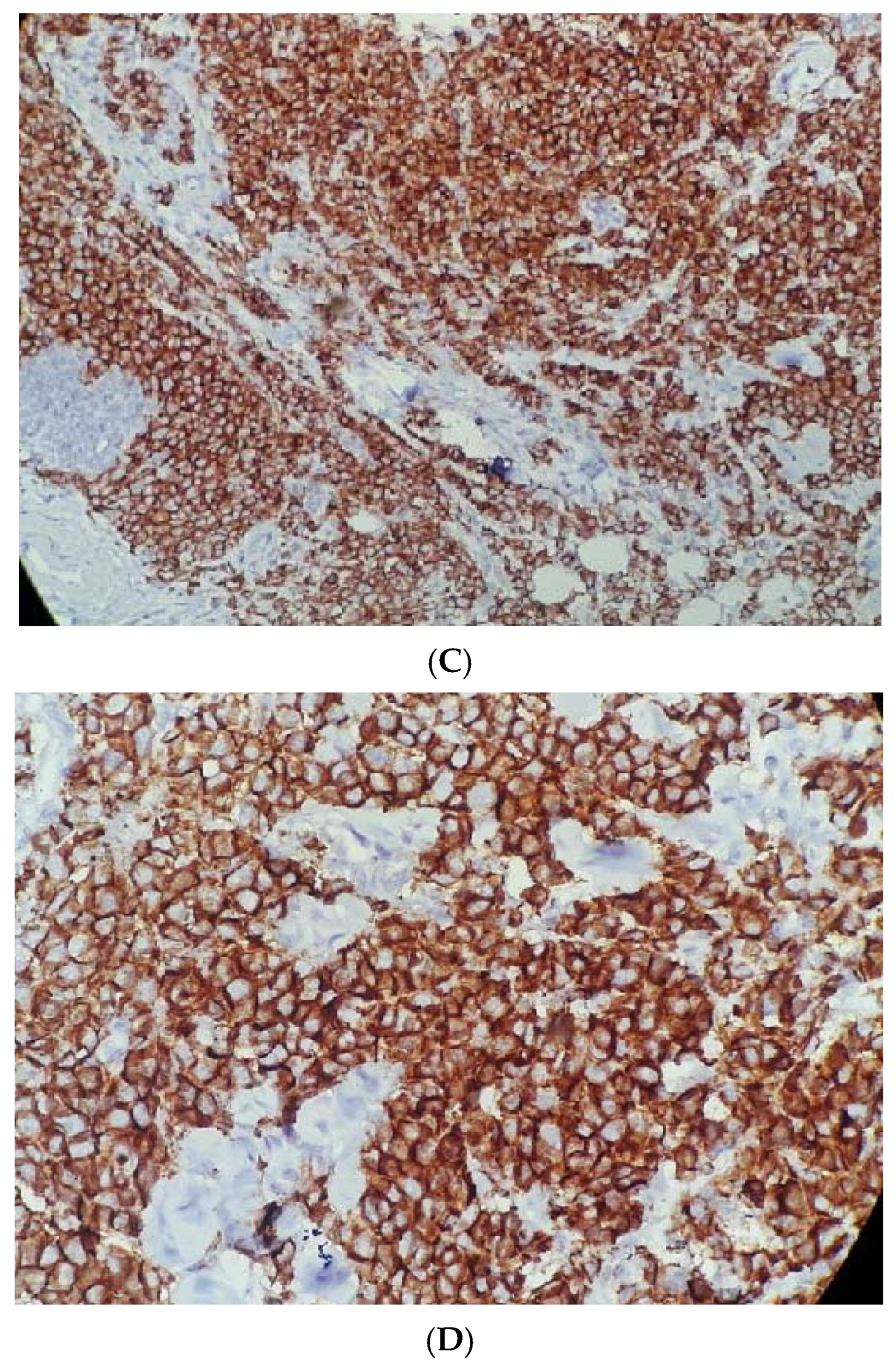
The following
Figure 1A–D show standard Nottingham grading system.
Figure 1A shows tubule formation of 10–75%, with mitosis of 10–19/HPF and moderate nuclear pleomorphism, graded as moderately differentiated invasive ductal carcinoma, NST based on standard Nottingham grading system.
Figure 1B shows complete negative staining of
SLC7A5, whereas
Figure 1C,D show complete and strong membranous staining of
SLC7A5 in more than 10% tumor cells in invasive ductal carcinoma, NST.
Figure 1.
(A) Moderately differentiated invasive carcinoma, NST (H&E, 40×), showing tubule formation (10–75%), mitoses (10–19/HPF), and moderate nuclear pleomorphism. (B) Complete negative staining for SLC7A5 (100×). (C) Strong membranous and cytoplasmic SLC7A5 positivity in >10% of tumor cells (100×). (D) Intense membranous and cytoplasmic staining for SLC7A5 (400×).
Figure 1.
(A) Moderately differentiated invasive carcinoma, NST (H&E, 40×), showing tubule formation (10–75%), mitoses (10–19/HPF), and moderate nuclear pleomorphism. (B) Complete negative staining for SLC7A5 (100×). (C) Strong membranous and cytoplasmic SLC7A5 positivity in >10% of tumor cells (100×). (D) Intense membranous and cytoplasmic staining for SLC7A5 (400×).
4. Discussion
Breast cancer is a highly heterogeneous malignancy that exhibits considerable molecular, cellular, and biological variability, influencing treatment responses and clinical outcomes. The molecular subtypes of BC—luminal A, luminal B, HER2-enriched, and triple-negative breast cancer (TNBC)—each exhibit distinct biological behaviors, prognoses, and therapeutic responses. This inherent diversity emphasizes the urgent need for novel biomarkers that can better inform prognosis, aid in risk stratification, and identify potential therapeutic targets in BC patients [
7,
8].
In this study, we investigated the expression of the solute carrier family 7, member 5 (
SLC7A5), which encodes the L-type amino acid transporter 1 (LAT1), in invasive ductal carcinoma (IDC) samples from Pakistani breast cancer patients. Our findings did not reveal statistically significant correlations between
SLC7A5 expression and key clinicopathological parameters such as tumor grade, stage, lymph node involvement, or molecular subtype. While these observations may initially suggest that
SLC7A5 expression is regulated independently of these clinical variables, this interpretation must be made cautiously. Several of the observed associations were descriptive trends with non-significant
p-values (0.1–0.5), indicating insufficient strong evidence of definitive biological relevance. The relatively small sample size (
n = 83) limits the statistical power of the study, reducing the ability to detect weak-to-moderate associations. Therefore, the absence of significant correlations should not be viewed as proof of independence but rather as a reflection of limited sample resolution or potential random variation [
9]. Future studies with larger, multi-institutional cohorts are warranted to clarify whether these trends represent true biological effects or statistical noise.
Interestingly, previous studies have reported significant associations between
SLC7A5 expression and adverse clinical outcomes, such as poor survival and metastatic progression, in various cancers, including breast cancer. Elevated levels of
SLC7A5 have been linked to more aggressive tumor phenotypes—higher tumor grade, advanced stage, and hormone receptor negativity—suggesting a role in disease aggressiveness and poor prognosis [
10,
11]. Mechanistically,
SLC7A5 facilitates amino acid transport and activates the mTORC1 signaling pathway, thereby promoting tumor cell proliferation and metabolic adaptation under stress conditions. In contrast, the absence of statistically significant associations in our cohort may reflect the study’s preliminary nature, sample size limitations, or population-specific variability rather than a genuine lack of biological significance [
12,
13]. Hence, the potential of
SLC7A5 as an independent prognostic biomarker requires validation through studies with greater statistical power and extended clinical follow-up.
In this cohort,
SLC7A5 was expressed in 24.1% of patients, with relatively higher expression levels observed in certain molecular subtypes such as luminal, HER2-positive, and TNBC, though these differences did not reach statistical significance. This aligns with studies conducted in other populations, such as a Japanese cohort that reported higher
SLC7A5 expression in HER2-positive and TNBC subtypes [
14]. The variability in expression across ethnic and demographic groups emphasizes the importance of considering regional, genetic, and environmental factors when evaluating biomarker utility. Conducting region-specific studies can therefore provide critical insight into population-based tumor biology and improve the applicability of molecular markers in diverse clinical settings.
Our findings also indicated that
SLC7A5 expression tended to be higher among patients in the moderate and poor prognostic categories based on the Nottingham Prognostic Index (NPI), although this trend did not reach statistical significance. These observations are consistent with regional studies reporting associations between high
SLC7A5 expression and worse outcomes, particularly in advanced-stage disease [
15,
16]. Given that the NPI integrates tumor size, lymph node status, and histological grade, incorporating molecular markers like
SLC7A5 into this framework may improve predictive accuracy—especially in populations where traditional prognostic models may have reduced discriminative power. Nevertheless, such integration requires validation through studies with larger cohorts to confirm the consistency and magnitude of these effects.
The biological role of
SLC7A5 in tumor metabolism further highlights its potential as a therapeutic target. By transporting essential amino acids, particularly leucine,
SLC7A5 supports protein synthesis and activates the mTORC1 pathway, fostering anabolic metabolism, cell proliferation, and survival—all critical processes in cancer progression. Inhibition of LAT1 has emerged as a promising therapeutic approach, especially in aggressive subtypes like TNBC, where metabolic dependency on amino acid transport is high [
17,
18]. Current clinical trials on LAT1 inhibitors aim to exploit these vulnerabilities by restricting nutrient supply to tumor cells, thereby impeding their growth and survival [
19,
20]. Such approaches could complement existing targeted and immunotherapeutic strategies, contributing to a broader shift toward metabolism-directed cancer therapy.
While survival data and Kaplan–Meier analyses were not included in this study due to the limited sample size, their inclusion in future work would significantly strengthen the evaluation of
SLC7A5’s prognostic impact. Longitudinal studies with larger and more diverse cohorts will be essential to determine whether
SLC7A5 expression correlates with overall or disease-free survival outcomes. Future research should also consider integrating transcriptomic and proteomic analyses to better understand
SLC7A5’s regulatory mechanisms and its interactions with other oncogenic pathways [
21].
Although SLC7A5 expression was detected in 24% of cases, the current data only describe its distribution and clinicopathological correlations rather than its prognostic or predictive impact. As this study did not include survival outcomes, any suggestion of SLC7A5 functioning as an independent prognostic factor would be premature. Its potential prognostic value should instead be explored in longitudinal studies that incorporate survival endpoints to establish its true predictive relevance.
Finally, it is important to acknowledge that this study’s conclusions are limited by the relatively small sample size and single-region design. While several descriptive patterns were observed, these should not be over interpreted as biologically meaningful until verified in larger, multi-institutional cohorts. The observed non-significant trends highlight the inherent complexity of biomarker interpretation in small studies and underscore the need for collaborative, statistically powered research to produce clinically translatable evidence.
5. Conclusions
SLC7A5 (LAT1) expression was detected in approximately one-quarter of invasive ductal carcinoma samples from Pakistani breast cancer patients. Although minor trends toward higher expression were observed in certain molecular subtypes and prognostic categories, these did not reach statistical significance. The findings suggest that SLC7A5 participates in tumor metabolic processes but, based on current data, cannot yet be considered a prognostic biomarker.
This study provides foundational insight into LAT1 expression patterns within an underrepresented population and underscores the importance of conducting larger, longitudinal studies that integrate clinical outcomes, molecular profiling, and therapeutic response. Such efforts will be essential to clarify whether SLC7A5 serves as a meaningful prognostic indicator or a viable target for metabolic intervention in breast cancer.
Author Contributions
The following authors contributed to the manuscript as stated—N.H., B.K.M.G., R.D., S.R., A.S.C. and A.H.N. Conceptualization, N.H., R.D., A.S.C. and A.H.N.; methodology, N.H., A.S.C. and A.H.N.; software, B.K.M.G. and R.D.; validation, B.K.M.G., R.D. and S.R.; formal analysis, N.H., B.K.M.G. and S.R.; investigation, N.H., S.R., A.S.C. and A.H.N.; resources, N.H., S.R. and A.S.C.; data curation, N.H., B.K.M.G., A.S.C. and A.H.N.; writing—original draft preparation, N.H. and B.K.M.G.; writing—review and editing, B.K.M.G. and R.D.; visualization, B.K.M.G., R.D., A.S.C. and A.H.N.; supervision, B.K.M.G., S.R. and A.H.N.; project administration, N.H., B.K.M.G. and A.H.N.; funding acquisition, N.H. and A.H.N. All authors have read and agreed to the published version of the manuscript.
Funding
“This research was partly funded by the University of Health Sciences, Lahore, Pakistan, as per Ph.D Research regulations,” but “The APC was NOT funded by any agency”.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the (Institutional Review Board) Advanced Studies and Research Board of the University of Health Sciences (UHS), Pakistan, with ethical approval number: UHS/Education/126-16/215.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data generated during this study are part of this manuscript. The detailed data is unavailable due to privacy or ethical restrictions, but can be obtained from the corresponding author with a reasonable request. Data is not deposited in any registry.
Acknowledgments
We would like to acknowledge Nadia Naseem, Head of pathology department, for her invaluable guidance and facilitation of this project. We would like to acknowledge Sameer Anjum and Late Ghulam Rasool for helping in the laboratory technical work, the administrative staff of hospitals for their assistance and cooperation in sample collection.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| LAT 1 | L-type amino acid transporter |
| SLC7A5 | solute carrier family 7, member 5 |
| AA | Amino acids |
| BC | Breast Cancer |
| NST | No Specific Type |
References
- Kunstič, T.T.; Debeljak, N.; Tacer, K.F. Heterogeneity in hormone-dependent breast cancer and therapy: Steroid hormones, HER2, melanoma antigens, and cannabinoid receptors. Adv. Cancer Biol. Metastasis 2023, 7, 100086. [Google Scholar]
- Kurozumi, S.; Kaira, K.; Matsumoto, H.; Kurosumi, M.; Yokobori, T.; Kanai, Y.; Sekine, C.; Honda, C.; Katayama, A.; Furuya, M.; et al. Association of L-type amino acid transporter 1 (LAT1) with the immune system and prognosis in invasive breast cancer. Sci. Rep. 2022, 12, 2742. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Wu, X.; Ling, S.; Ma, Y.; Huang, P. SLC7A5 serves as a prognostic factor of breast cancer and promotes cell proliferation through activating AKT/mTORC1 signaling pathway. Ann. Transl. Med. 2021, 9, 892. [Google Scholar] [CrossRef] [PubMed]
- Lieu, E.L.; Nguyen, T.; Rhyne, S.; Kim, J. Amino acids in cancer. Exp. Mol. Med. 2020, 52, 15–30. [Google Scholar] [CrossRef]
- Jewell, J.L.; Kim, Y.C.; Russell, R.C.; Yu, F.X.; Park, H.W.; Plouffe, S.W.; Tagliabracci, V.S.; Guan, K.-L. Differential regulation of mTORC1 by leucine and glutamine. Science 2015, 347, 194–198. [Google Scholar] [CrossRef]
- El Ansari, R.; Craze, M.L.; Miligy, I.; Diez-Rodriguez, M.; Nolan, C.C.; Ellis, I.O.; Rakha, E.A.; Green, A.R. The amino acid transporter SLC7A5 confers a poor prognosis in the highly proliferative breast cancer subtypes and is a key therapeutic target in luminal B tumors. Breast Cancer Res. 2018, 20, 21. [Google Scholar] [CrossRef]
- Singh, N.; Scalise, M.; Galluccio, M.; Wieder, M.; Seidel, T.; Langer, T.; Indiveri, C.; Ecker, G.F. Discovery of Potent Inhibitors for the Large Neutral Amino Acid Transporter 1 (LAT1) by Structure-Based Methods. Int. J. Mol. Sci. 2018, 20, 27. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.M.; Thomas, J.; Ross, D.T.; Seitz, R.S.; Ring, B.Z.; Beck, R.A.; Pedersen, H.C.; Munro, A.; Kunkler, I.H.; Campbell, F.M.; et al. Mammostrat as a tool to stratify breast cancer patients at risk of recurrence during endocrine therapy. Breast Cancer Res. 2010, 12, R47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Furuya, M.; Horiguchi, J.; Nakajima, H.; Kanai, Y.; Oyama, T. Correlation of L-type amino acid transporter 1 and CD98 expression with triple negative breast cancer prognosis. Cancer Sci. 2012, 103, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Ichinoe, M.; Mikami, T.; Yanagisawa, N.; Yoshida, T.; Hana, K.; Endou, H.; Okayasu, I.; Sengoku, N.; Ogata, H.; Saegusa, M.; et al. Prognostic values of L-type amino acid transporter 1 and CD98hc expression in breast cancer. J. Clin. Pathol. 2021, 74, 589–595. [Google Scholar] [CrossRef]
- Bodoor, K.; Almomani, R.; Alqudah, M.; Haddad, Y.; Samouri, W. LAT1 (SLC7A5) overexpression in negative Her2/neu group of breast cancer: A potential therapy target. Asian Pac. J. Cancer Prev. 2020, 21, 1453–1458. [Google Scholar] [CrossRef]
- Sato, M.; Harada-Shoji, N.; Toyohara, T.; Soga, T.; Itoh, M.; Miyashita, M.; Tada, H.; Amari, M.; Anzai, N.; Furumoto, S.; et al. L-type amino acid transporter 1 is associated with chemoresistance in breast cancer via the promotion of amino acid metabolism. Sci. Rep. 2021, 11, 589. [Google Scholar] [CrossRef]
- Zhou, L.; Rueda, M.; Alkhateeb, A. Classification of Breast Cancer Nottingham Prognostic Index Using High-Dimensional Embedding and Residual Neural Network. Cancers 2022, 13, 934. [Google Scholar] [CrossRef] [PubMed]
- Kerin, E.P.; Davey, M.G.; McLaughlin, R.P.; Sweeney, K.J.; Barry, M.K.; Malone, C.M.; Elwahab, S.A.; Lowery, A.J.; Kerin, M.J. Comparison of the Nottingham Prognostic Index and OncotypeDX© recurrence score in predicting outcome in estrogen receptor positive breast cancer. Breast 2022, 66, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, Z.M.; Altaf, R.; Amin, M.S.; Butt, S. Relationship of Nottingham Prognostic Index with prognostic parameters of breast carcinoma. PJMHS 2022, 15, 26–28. [Google Scholar] [CrossRef]
- Muir, A.; Vander Heiden, M.G. The nutrient environment affects therapy. Science 2018, 360, 962–963. [Google Scholar] [CrossRef] [PubMed]
- Törnroos, R.; Tina, E.; Göthlin Eremo, A. SLC7A5 is linked to increased expression of genes related to proliferation and hypoxia in estrogen-receptor-positive breast cancer. Oncol. Rep. 2022, 47, 17. [Google Scholar] [CrossRef]
- Fong, Y.; Evans, J.; Brook, D.; Kenkre, J.; Jarvis, P.; Gower-Thomas, K. The Nottingham Prognostic Index: Five- and ten-year data for all-cause survival within a screened population. Ann. R. Coll. Surg. Engl. 2015, 97, 137–139. [Google Scholar] [CrossRef]
- Sidoni, A.; Bellezza, G.; Cavaliere, A.; Del Sordo, R.; Scheibel, M.; Bucciarelli, E. Prognostic indexes in breast cancer: Comparison of the Nottingham and Adelaide indexes. Breast 2004, 13, 23–27. [Google Scholar] [CrossRef]
- Shindo, H.; Harada-Shoji, N.; Ebata, A.; Sato, M.; Soga, T.; Miyashita, M.; Tada, H.; Kawai, M.; Kosaka, S.; Onuki, K.; et al. Targeting Amino Acid Metabolic Reprogramming via L-Type Amino Acid Transporter 1 (LAT1) for Endocrine-Resistant Breast Cancer. Cancers 2021, 13, 4375. [Google Scholar] [CrossRef]
- Alfarsi, L.H.; El-Ansari, R.; Craze, M.L.; Masisi, B.K.; Mohammed, O.J.; Ellis, I.O.; Rakha, E.A.; Green, A.R. Co-Expression Effect of SLC7A5/SLC3A2 to Predict Response to Endocrine Therapy in Oestrogen-Receptor-Positive Breast Cancer. Int. J. Mol. Sci. 2020, 21, 1407. [Google Scholar] [CrossRef] [PubMed]
Table 1.
Lymph node stage and tumor stage used to calculate the Nottingham Prognostic index.
Table 1.
Lymph node stage and tumor stage used to calculate the Nottingham Prognostic index.
| Lymph Node Stage | Tumor Grade |
|---|
| 1: No node metastasis | 1: Grade 1 |
| 2: 1–3 node metastasis | 2: Grade 2 |
| 3: >3 nodes metastasis | 3: Grade 3 |
Table 2.
Association of SLC7A5 expression with clinicopathological parameters in women with invasive carcinoma of no-special type.
Table 2.
Association of SLC7A5 expression with clinicopathological parameters in women with invasive carcinoma of no-special type.
| Parameters | | Negative, n (%) | Positive, n (%) | Total, n (%) | Chi Square
(p-Value) |
|---|
| Total | 83 | 63 (75.9) | 20 (24.1) | 83 | <0.001 * |
| Age | <50 | 43 (51.8) | 15 (18) | 58 (69.9) | χ2 = 0.33, p = 0.57 |
| >50 | 20 (24.1) | 5 (6) | 25 (30.1) |
| Laterality | Right | 37 (44.6) | 14 (16.9) | 51 (61.4) | χ2 = 0.81, p = 0.37 |
| Left | 26 (31.3) | 6 (7.2) | 32 (38.6) |
| Nodal stage | No | 20 (24.1) | 4 (4.8) | 24 (28.9) | χ2 = 4.74, p = 0.19 |
| N1 | 20 (24.1) | 9 (10.8) | 29 (34.9) |
| N2 | 15 (18.1) | 7 (8.4) | 22 (26.5) |
| N3 | 8 (9.6) | 0 | 8 (9.6) |
| Tumor stage | T2 | 30 (36.1) | 10 (12) | 40 (48.2) | χ2 = 4.97, p = 0.08 |
| T3 | 23 (27.7) | 3 (3.6) | 26 (31.3) |
| T4 | 10 (12) | 7 (8.4) | 17 (20.5) |
| Nottingham grade | I | 4 (4.8) | 0 | 4 (4.8) | χ2= 4.096, p = 0.129 |
| II | 27 (32.5) | 5 (6) | 32 (38.6) |
| III | 32 (38.6) | 15 (18.1) | 47 (56.6) |
| Molecular classification | Luminal A | 25 (30.1) | 6 (7.2) | 31 (37.3) | χ2= 1.638, p = 0.651 |
| Luminal B | 8 (9.6) | 2 (2.4) | 10 (12) |
| HER2 | 11 (13.3) | 6 (7.2) | 17 (20.5) |
| Triple negative (TN) | 19 (22.9) | 6 (7.2) | 25 (30.1) |
| NPI | Good | 5 (6) | 0 | 5 (6) | χ2= 3.932, p = 0.140 |
| Moderate | 22 (26.5) | 4 (4.8) | 26 (31.3) |
| Poor | 36 (43.4) | 16 (19.3) | 52 (62.7) |
Table 3.
Summary table with effect sizes and interpretation.
Table 3.
Summary table with effect sizes and interpretation.
| Variable | Key Association | Direction | Strength | p-Value | Clinical
Interpretation |
|---|
| Tumor Grade III vs. I/II | Higher SLC7A5 positivity | ↑ | Moderate | 0.129 | Suggests aggressive phenotype |
| NPI Poor | More positive cases | ↑ | Moderate | 0.14 | May predict poor outcome |
| Age | Not significant | – | Weak | 0.567 | No age association |
Table 4.
Association of SLC7A5 with biochemical parameters of invasive carcinoma of no special type.
Table 4.
Association of SLC7A5 with biochemical parameters of invasive carcinoma of no special type.
| | | SLC7A5 Negative, n (%) | SLC7A5
Positive, n (%) | Total,
n (%) | Chi Square
(p Value) |
|---|
| ER | Negative | 28 (33.7) | 10 (12) | 38 (45.8) | 0.189, 0.664 |
| | Positive | 35 (42.2) | 10 (12) | 45 (54.2) |
| PR | Negative | 29 (34.9) | 11 (13.3) | 40 (48.2) | 0.489, 0.484 |
| | Positive | 34 (41) | 9 (10.8) | 43 (51.8) |
| HER2 | Negative | 44 (53) | 12 (14.5) | 56 (67.5) | 0.670, 0.413 |
| | Positive | 19 (22.9) | 8 (9.6) | 27 (32.5) |
| Ki67 | <15% | 17 (20.5) | 5 (6) | 22 (26.5) | 0.031, 0.861 |
| | >15% | 46 (55.4) | 15 (18.1) | 61 (73.5) |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).