Influence of Diabetes on Expression of Ezrin and MMP-2 in Gingival Tissue of Patients with Periodontal Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Informed Consent
2.2. Inclusion and Exclusion Criteria
- type 2 diabetes for at least three years (according to criteria established by the American Diabetes Association);
- glycated hemoglobin levels between 6.5% to 11%.
- patients using antibiotics or anti-inflammatory medications 3 months prior to testing; any periodontal or orthodontic procedures 6 months prior to inclusion in the study;
- stage 1 and 4 periodontal disease; BMI > 30;
- mental disorders;
- pregnancy and breastfeeding;
- smoker;
- systemic conditions which could affect immune function or bone metabolism (such as osteoporosis, rheumatoid arthritis, or endocrine disorders), with exclusion of type 2 diabetes.
- Test group (DM + PD) (n = 27): type 2 diabetes mellitus Group + periodontal disease;
- Control group (PD) (n = 26): periodontal disease, without diabetes mellitus.
2.3. Tissue Collection and Processing
2.4. Immunohistochemical Staining
2.5. Microscopy
2.6. Statistical Analysis
3. Results
3.1. Sociodemographic, Clinical, and Paraclinical Parameters of Study and Test Groups
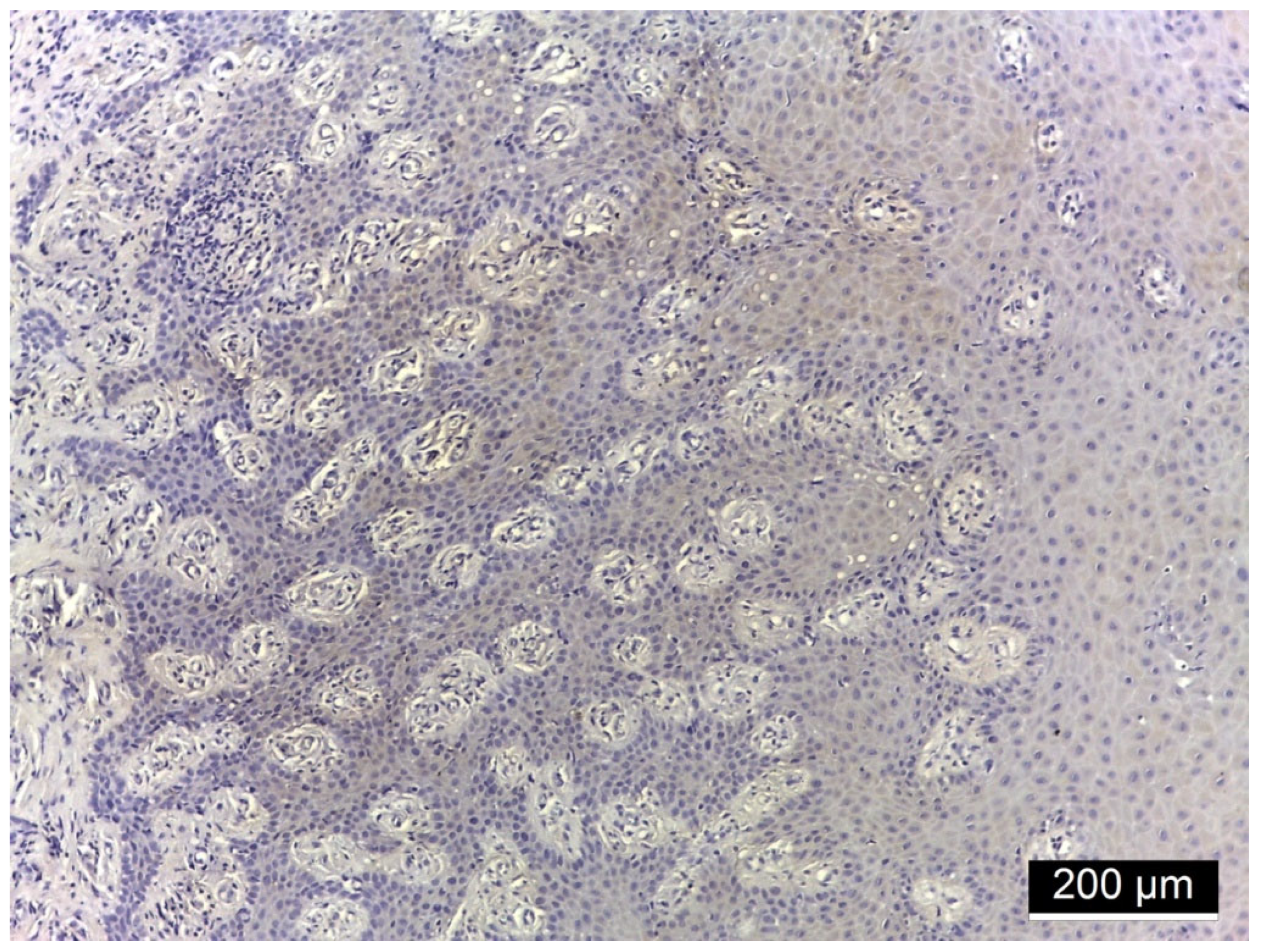
3.2. Ezrin Immunoreactivity
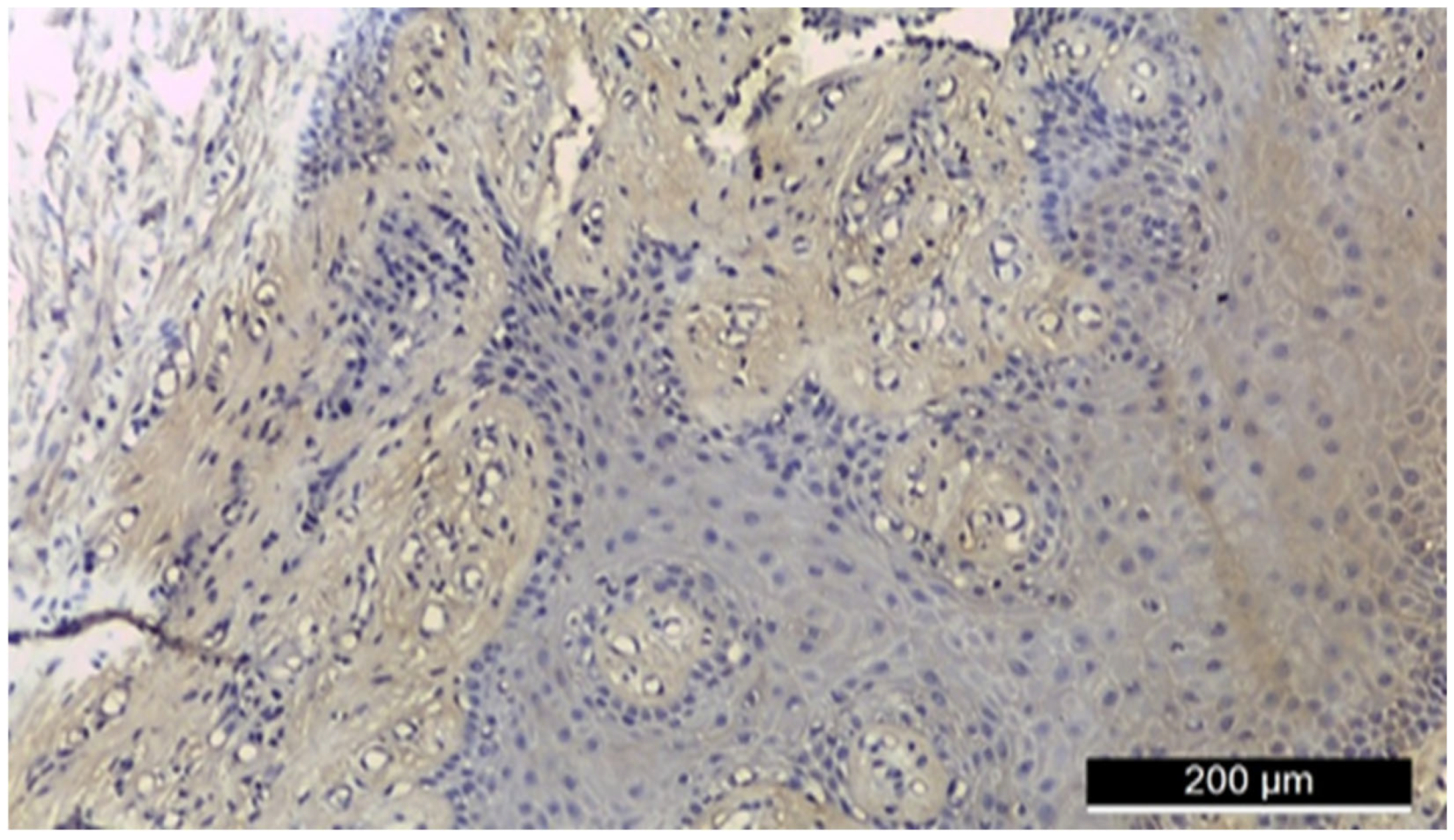
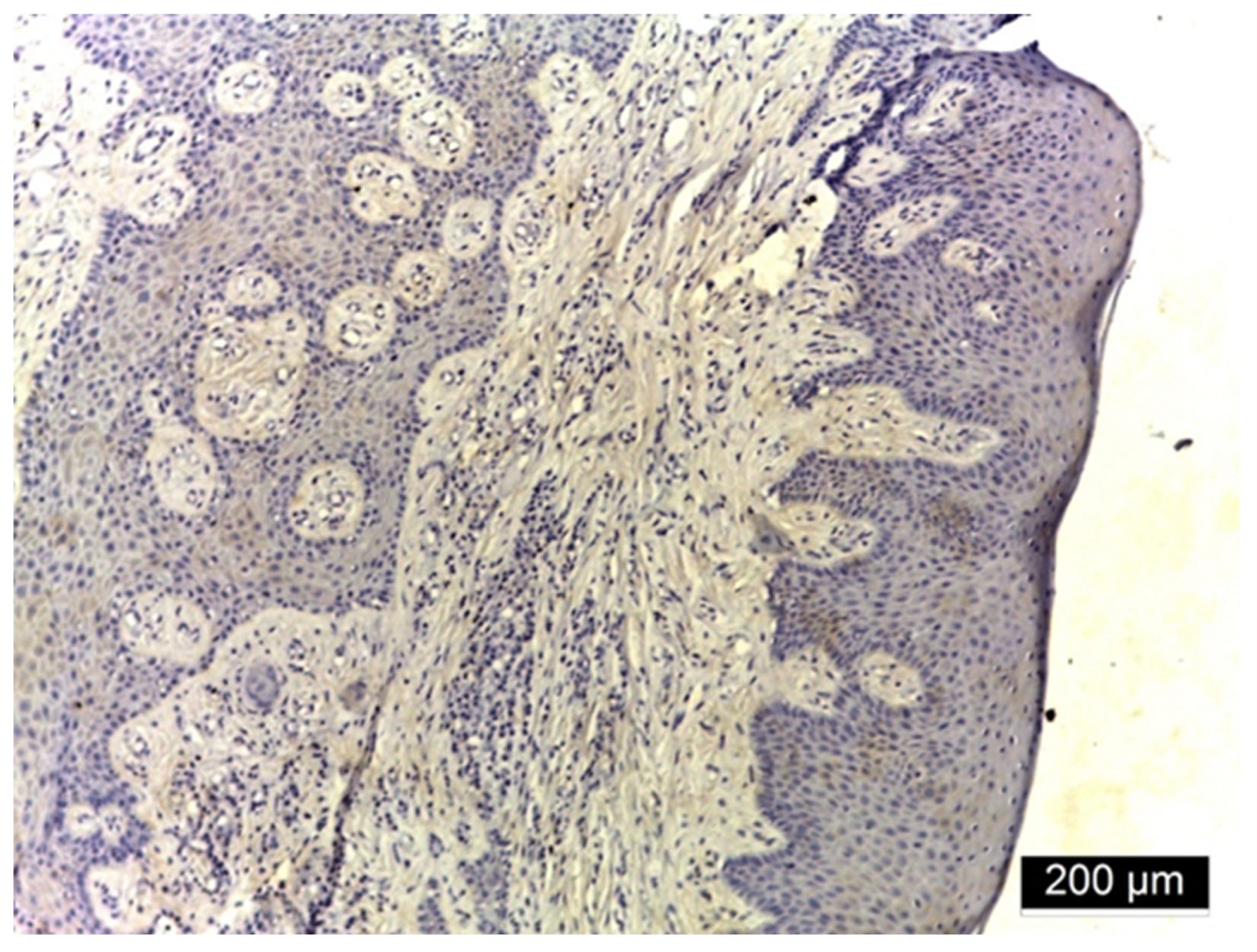

3.3. MMP-2 Immunoreactivity
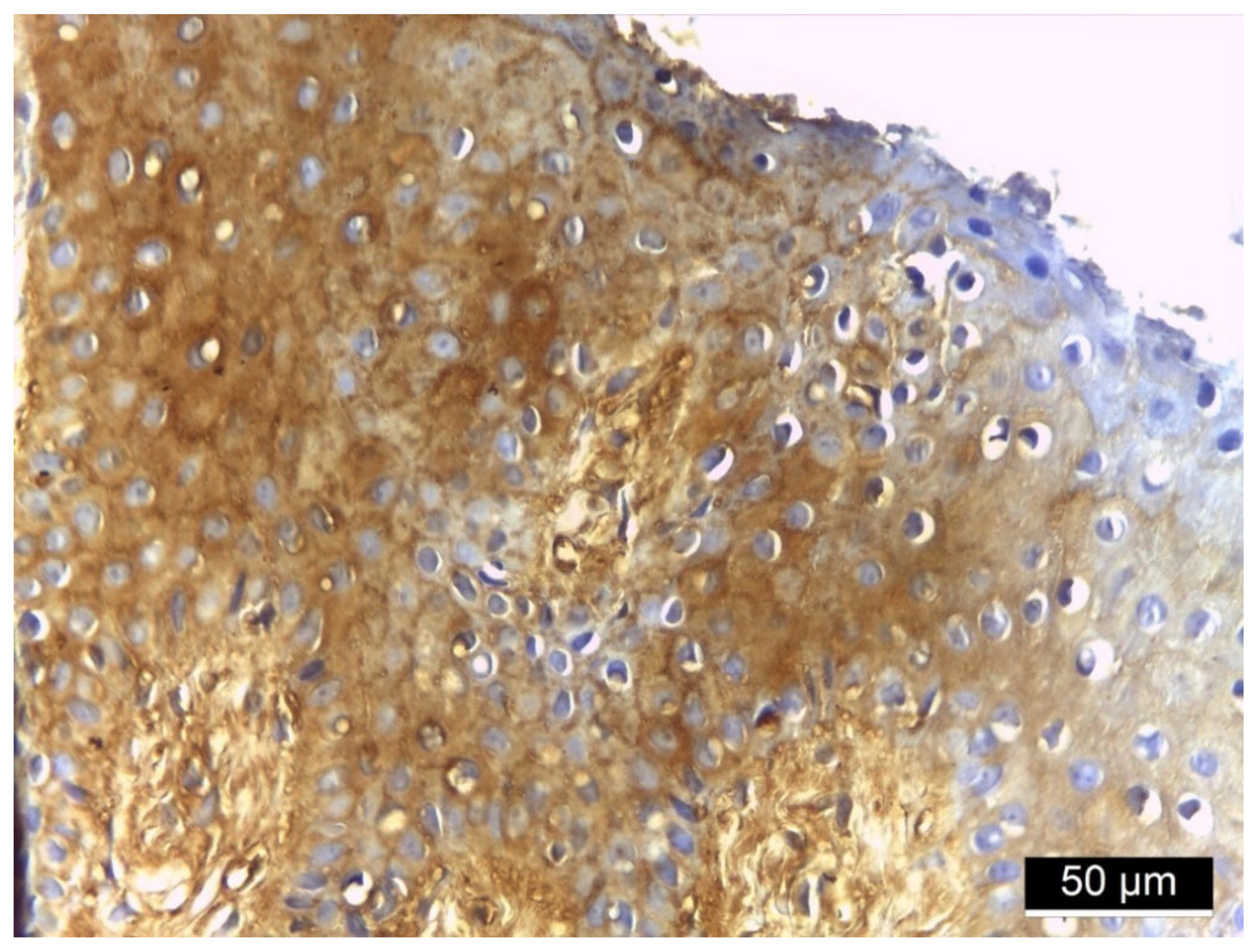
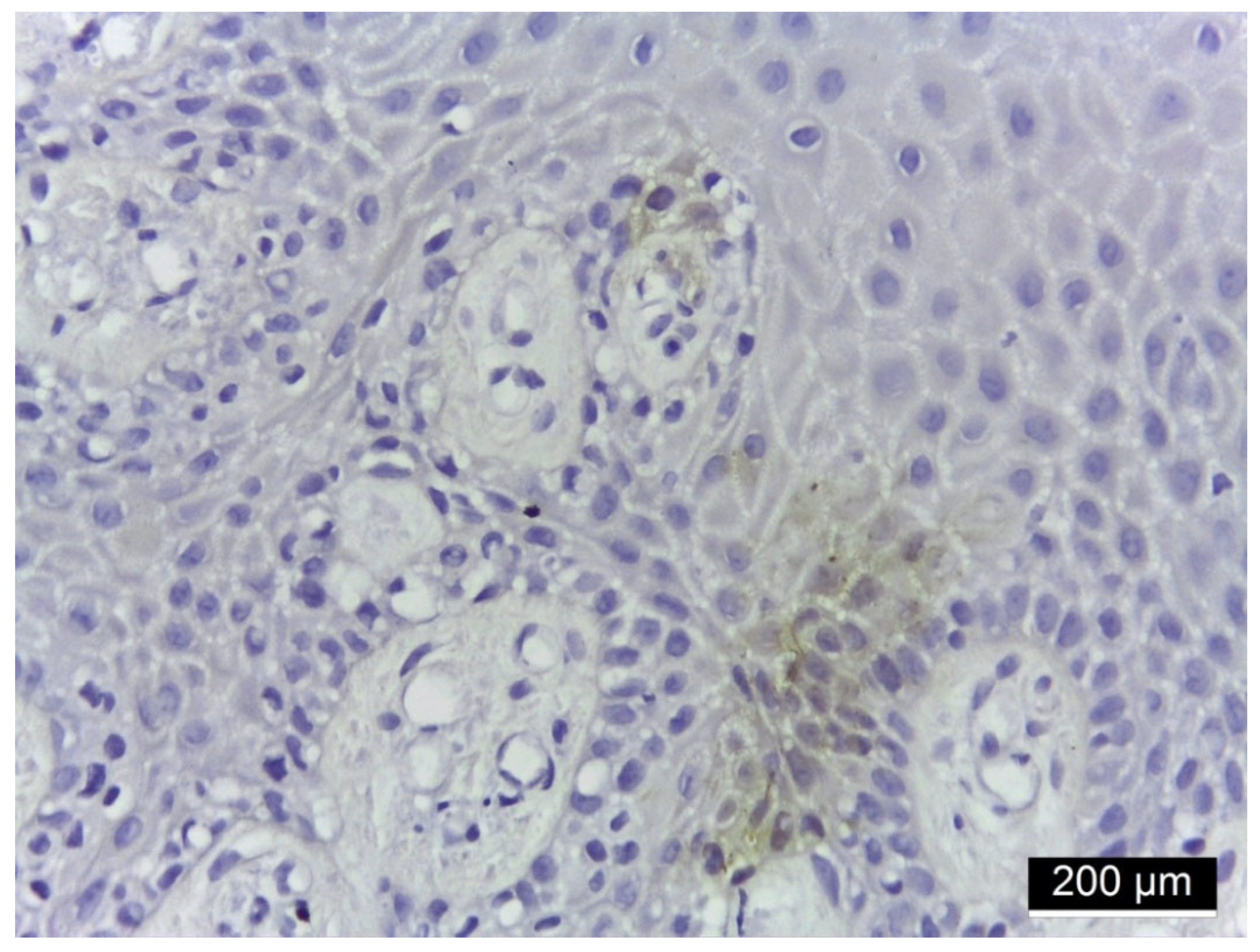
4. Discussion
4.1. Ezrin Immunoreactivity
4.2. MMP-2 Immunoreactivity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, R.; Haque, M. Oral Health Messiers: Diabetes Mellitus Relevance. Diabetes Metab. Syndr. Obes. 2021, 14, 3001–3015. [Google Scholar] [CrossRef]
- Alqadi, S.F. Diabetes mellitus and its influence on oral health. Diabetes Metab. Syndr. Obes. 2024, 31, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Sonar, D.P.R.; Panchbhai, D.A.S.; Mishra, D.S.; Dangore, D.S.B. Oral Manifestations in Diabetes Mellitus and Management Considerations: A Review. Int. J. Life Sci. Pharma Res. 2023, 13, L349–L357. [Google Scholar] [CrossRef]
- Slowik, J.; Kaczynski, L.; Kaczor, M.; Wnuk, M. Oral health-related quality of life in patients with type II diabetes mellitus: A systematic review and meta-analysis. BMC Oral Health 2025, 25, 485. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.I.; Sculean, A.; Graves, D.T. Diabetic wound healing in soft and hard oral tissues. Transl. Res. 2021, 236, 72–86. [Google Scholar] [CrossRef]
- Shinjo, T.; Nishimura, F. The bidirectional association between diabetes and periodontitis, from basic to clinical. Jpn. Dental Sci. Rev. 2024, 60, 15–21. [Google Scholar] [CrossRef]
- Sato, M.; Ono, S.; Yamana, H.; Okada, A.; Ishimaru, M. Effect of periodontal therapy on glycaemic control in type 2 diabetes. J. Clin. Periodontol. 2024, 51, 380–389. [Google Scholar] [CrossRef]
- Genco, R.J.; Borgnakke, W.S. Diabetes as a potential risk for periodontitis: Association studies. Periodontol. 2000 2020, 83, 40–45. [Google Scholar] [CrossRef]
- Singhal, S.; Pradeep, A.R.; Kanoriya, D.; Garg, V. Human soluble receptor for advanced glycation end products and tumor necrosis factor-α as gingival crevicular fluid and serum markers of inflammation in chronic periodontitis and type 2 diabetes. J. Oral Sci. 2016, 58, 547–553. [Google Scholar] [CrossRef]
- Bui, F.Q.; Almeida-da Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Yoshida, S.; Hatano, R.; Asano, S. Pathophysiological roles of Ezrin/Radixin/Moesin proteins. Biol. Pharm. Bull. 2017, 40, 381–390. [Google Scholar] [CrossRef]
- Pore, D.; Matsui, K.; Parameswaran, N.; Gupta, N. Cutting edge: Ezrin regulates inflammation by limiting B cell IL-10 production. J. Immunol. 2016, 196, 558–562. [Google Scholar] [CrossRef]
- Pore, D.; Gupta, N. The ezrin-radixin-moesin family of proteins in the regulation of B-cell immune response. Crit. Rev. Immunol. 2015, 35, 15–31. [Google Scholar] [CrossRef]
- Jiang, L.; Phang, J.M.; Yu, J.; Harrop, S.J.; Sokolova, A.V.; Duff, A.P.; Wilk, K.E.; Alkhamici, H.; Breit, S.N.; Valenzuela, S.M.; et al. CLIC proteins, ezrin, radixin, moesin and the coupling of membranes to the actin cytoskeleton: A smoking gun? Biochim. Biophys. Acta 2014, 1838, 643–657. [Google Scholar] [CrossRef]
- Pore, D.; Parameswaran, N.; Matsui, K.; Stone, M.B.; Saotome, I.; McClatchey, A.I.; Veatch, S.L.; Gupta, N. Ezrin tunes the magnitude of humoral immunity. J. Immunol. 2013, 191, 4048–4058. [Google Scholar] [CrossRef]
- Li, M.; Li, S.; Zhou, L.; Yang, L.; Wu, X.; Tang, B.; Hong, T. Immune infiltration of MMP14 in pan cancer and its prognostic effect on tumors. Front. Oncol. 2021, 11, 717606. [Google Scholar] [CrossRef]
- Kümper, M.; Zamek, J.; Steinkamp, J.; Pach, E.; Mauch, C.; Zigrino, P. Role of MMP3 and fibroblast-MMP14 in skin homeostasis and repair. Eur. J. Cell Biol. 2022, 101, 151276. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q. Ezrin/Radixin/Moesin Proteins in the Development of Diabetes and its Cardiovascular Complications. J. Diabetes Metab. 2011, 1, S4:005. [Google Scholar] [CrossRef]
- Cecil, A.; Sambashivaiah, S.; Bilichodmath, S.; John, R.S. mRNA Expression of Ezrin in Gingival Crevicular Fluid and Whole Blood of Gingivitis and Chronic Periodontitis Patients—A Polymerase Chain Reaction Study. Contemp. Clin. Dent. 2022, 13, 267–273. [Google Scholar] [CrossRef]
- Luchian, I.; Goriuc, A.; Sandu, D.; Covasa, M. The Role of Matrix Metalloproteinases (MMP-8, MMP-9, MMP-13) in Periodontal and Peri-Implant Pathological Processes. Int. J. Mol. Sci. 2022, 23, 1806. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, E.A.; Ławicka, R.; Grygorczuk, P.; Nowosielska, M.; Kicman, A.; Ławicki, S. Importance of Metalloproteinase 8 (MMP-8) in the Diagnosis of Periodontitis. Int. J. Mol. Sci. 2024, 25, 2721. [Google Scholar] [CrossRef]
- Noack, B.; Kipping, T.; Tervahartiala, T.; Sorsa, T.; Hoffmann, T.; Lorenz, K. Association between serum and oral matrix metalloproteinase-8 levels and periodontal health status. J. Periodontal Res. 2017, 52, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, S.; Satoh, M.; Takiwaki, M.; Nomura, F. Current Status of Proteomic Technologies for Discovering and Identifying Gingival Crevicular Fluid Biomarkers for Periodontal Disease. Int. J. Mol. Sci. 2018, 20, 86. [Google Scholar] [CrossRef]
- Preianò, M.; Savino, R.; Villella, C.; Pelaia, C.; Terracciano, R. Gingival Crevicular Fluid Peptidome Profiling in Healthy and in Periodontal Diseases. Int. J. Mol. Sci. 2020, 21, 5270. [Google Scholar] [CrossRef] [PubMed]
- Preianò, M.; Maggisano, G.; Murfuni, M.S.; Villella, C.; Pelaia, C.; Montalcini, T.; Lombardo, N.; Pelaia, G.; Savino, R.; Terracciano, R. An Analytical Method for Assessing Optimal Storage Conditions of Gingival Crevicular Fluid and Disclosing a Peptide Biomarker Signature of Gingivitis by MALDI-TOF MS. Proteom. Clin. Appl. 2018, 12, e1800005. [Google Scholar] [CrossRef] [PubMed]
- Bellei, E.; Bertoldi, C.; Monari, E.; Bergamini, S. Proteomics Disclose the Potential of Gingival Crevicular Fluid (GCF) as a Source of Biomarkers for Severe Periodontitis. Materials 2022, 15, 2161. [Google Scholar] [CrossRef]
- Agrafioti, P.; Morin-Baxter, J.; Tanagala, K.K.K.; Dubey, S.; Sims, P.; Lalla, E.; Momen-Heravi, F. Decoding the role of macrophages in periodontitis and type 2 diabetes using single-cell RNA-sequencing. FASEB J. 2022, 36, e22136. [Google Scholar] [CrossRef]
- Mashhadiabbas, F.; Neamatzadeh, H.; Foroughi, E.; Dastgheib, S.A.; Farahnak, S.; Nasiri, R.; Ahmadi, S. Association of MMP-2-753C>T and MMP-9-1562C>T Polymorphisms with Chronic/Aggressive Periodontitis Risk: A Systematic Review and Meta-Analysis. Iran. J. Public Health 2019, 48, 1227–1238. [Google Scholar] [CrossRef]
- Feng, P.; Zhang, H.; Zhang, Z.; Dai, X.; Mao, T.; Fan, Y.; Xie, X.; Wen, H.; Yu, P.; Hu, Y.; et al. The interaction of MMP-2/B7-H3 in human osteoporosis. Clin. Immunol. 2016, 162, 118–124. [Google Scholar] [CrossRef]
- Choubaya, C.; Chahine, N.; Aoun, G.; Anil, S.; Zalloua, P.; Salameh, Z. Expression of inflammatory mediators in periodontitis over established diabetes: An experimental study in rats. Med. Arch. 2021, 75, 436. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Yan, L.; Du, W.; Zhang, M.; Chen, H.; Zhang, L.; Li, G.; Li, J.; Dong, Y.; et al. MMP-2 and MMP-9 contribute to the angiogenic effect produced by hypoxia/15-HETE in pulmonary endothelial cells. J. Mol. Cell. Cardiol. 2018, 121, 36–50. [Google Scholar] [CrossRef]
- Botezatu, I.C.; Martu, M.-A.; Stoica, L.; Botez, A.E.; Onofrei, P.; Dimitriu, C.D.; Grecu, B.V.; Grigoriu, I.D.G.; Ciurcanu, O.; Solcan, C. Expression of MMP-14 and CD147 in Gingival Tissue of Patients With and Without Diabetes Mellitus Type II. Diagnostics 2025, 15, 609. [Google Scholar] [CrossRef]
- Idris, T.; Chanson, M.; Badaoui, M. Biology of the CF Airway Epithelium. In Hodson and Geddes’ Cystic Fibrosis; CRC Press: Boca Raton, FL, USA, 2023; pp. 48–58. [Google Scholar]
- Aqeel, H.; Ghani, M.U.; Naeem, Z.; Awan, F.I.; Khan, M.U.; Tanveer, S.; Shaikh, R.S. Potential modifier genes for cystic fibrosis disease. Hum. Gene 2025, 43, 201377. [Google Scholar] [CrossRef]
- Niu, J.; Hu, J.; Wang, Z. Scutellaria barbata D. Don extract regulates Ezrin-mediated triple negative breast cancer progress via suppressing the RhoA/ROCK1 signaling. Toxicol. Res. 2024, 13, tfae033. [Google Scholar] [CrossRef] [PubMed]
- Buenaventura, R.G.M.; Merlino, G.; Yu, Y. Ez-metastasizing: The crucial roles of Ezrin in metastasis. Cells 2023, 12, 1620. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, K.; Asano, S. Pathophysiological roles of actin-binding scaffold protein, ezrin. Int. J. Mol. Sci. 2022, 23, 3246. [Google Scholar] [CrossRef] [PubMed]
- Nethe, M.; Hordijk, P.L. The role of ubiquitylation and degradation in RhoGTPase signalling. J. Cell Sci. 2010, 123, 4011–4018. [Google Scholar] [CrossRef]
- Chiappetta, C.; Leopizzi, M.; Censi, F.; Puggioni, C.; Petrozza, V.; Rocca, C.D.; Di Cristofano, C. Correlation of the Rac1/RhoA pathway with ezrin expression in osteosarcoma. Appl. Immunohistochem. Mol. Morphol. 2014, 22, 162–170. [Google Scholar] [CrossRef]
- Zhou, Q.; Jiang, J.; Chen, G.; Qian, C.; Sun, G. Inflammatory immune cytokine TNF-α modulates Ezrin protein activation via FAK/RhoA signaling pathway in PMVECs hyperpermeability. Front. Pharmacol. 2021, 12, 676817. [Google Scholar] [CrossRef]
- Fukasawa, H.; Obayashi, H.; Schmieder, S.; Lee, J.; Ghosh, P.; Farquhar, M.G. Phosphorylation of podocalyxin (Ser415) prevents RhoA and ezrin activation and disrupts its interaction with the actin cytoskeleton. Am. J. Pathol. 2011, 179, 2254–2265. [Google Scholar] [CrossRef]
- Totsukawa, G.; Yamakita, Y.; Yamashiro, S.; Hartshorne, D.J.; Sasaki, Y.; Matsumura, F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J. Cell Biol. 2000, 150, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Tran Quang, C.; Gautreau, A.; Arpin, M.; Treisman, R. Ezrin function is required for ROCK-mediated fibroblast transformation by the Net and Dbl oncogenes. EMBO J. 2000, 19, 4565–4576. [Google Scholar] [CrossRef] [PubMed]
- Kosako, H.; Yoshida, T.; Matsumura, F.; Ishizaki, T.; Narumiya, S.; Inagaki, M. Rho-kinase/ROCK is involved in cytokinesis through the phosphorylation of myosin light chain and not ezrin/radixin/moesin proteins at the cleavage furrow. Oncogene 2000, 19, 6059–6064. [Google Scholar] [CrossRef] [PubMed]
- Verdys, P.; Rey Barroso, J.; Girel, A.; Vermeil, J.; Bergert, M.; Sanchez, T.; Poincloux, R. Ezrin, radixin, and moesin are dispensable for macrophage migration and cellular cortex mechanics. EMBO J. 2024, 43, 4822–4845. [Google Scholar] [CrossRef]
- Yu, H.; Luo, C.; Linghu, R.; Yang, J.; Wu, H. Ezrin contributes to the damage of airway epithelial barrier related to diabetes mellitus. J. Inflamm. Res. 2024, 17, 2609–2621. [Google Scholar] [CrossRef]
- Mastrogiovanni, M.; Di Bartolo, V.; Alcover, A. Cell polarity regulators, multifunctional organizers of lymphocyte activation and function. Biomed. J. 2022, 45, 299–309. [Google Scholar] [CrossRef]
- Neurath, N.; Kesting, M. Cytokines in gingivitis and periodontitis: From pathogenesis to therapeutic targets. Front. Immunol. 2024, 15, 1435054. [Google Scholar] [CrossRef]
- Popa, C.G.; Luchian, I.; Ioanid, N.; Goriuc, A.; Martu, I.; Bosinceanu, D.; Martu, M.A.; Tirca, T.; Martu, S. ELISA Evaluation of RANKL Levels in Gingival Fluid in Patients with Periodontitis and Occlusal Trauma. Rev. Chim. 2018, 69, 1578–1580. [Google Scholar] [CrossRef]
- Łasica, A.; Golec, P.; Laskus, A.; Zalewska, M.; Gędaj, M.; Popowska, M. Periodontitis: Etiology, conventional treatments, and emerging bacteriophage and predatory bacteria therapies. Front. Microbiol. 2024, 15, 1469414. [Google Scholar] [CrossRef]
- Hurjui, I.; Delianu, C.; Hurjui, L.L.; Jipu, R. Platelet derivatives with dental medicine applications. Rom. J. Oral Rehabil. 2020, 12, 142–152. [Google Scholar]
- Maybee, D.V.; Ink, N.L.; Ali, M.A.M. Novel Roles of MT1-MMP and MMP-2: Beyond the Extracellular Milieu. Int. J. Mol. Sci. 2022, 23, 9513. [Google Scholar] [CrossRef]
- Martu, M.A.; Maftei, G.A.; Sufaru, I.G.; Jelihovschi, I.; Luchian, I.; Hurjui, L.; Martu, I.; Pasarin, L. COVID-19 and periodontal disease—Ethiopathogenic and clinical implications. Rom. J. Oral Rehabil. 2020, 12, 116–124. [Google Scholar]
- Vo, H.V.T.; Nguyen, Y.T.; Kim, N.; Lee, H.J. Vitamin A, D, E, and K as Matrix Metalloproteinase-2/9 Regulators That Affect Expression and Enzymatic Activity. Int. J. Mol. Sci. 2023, 24, 17038. [Google Scholar] [CrossRef]
- Wolosowicz, M.; Prokopiuk, S.; Kaminski, T.W. The Complex Role of Matrix Metalloproteinase-2 (MMP-2) in Health and Disease. Int. J. Mol. Sci. 2024, 25, 13691. [Google Scholar] [CrossRef]
- Maftei, G.A.; Martu, M.A.; Martu, M.C.; Popescu, D.; Surlin, P.; Tatarciuc, D.; Popa, C.; Foia, L.G. Correlations between salivary immuno-biochemical markers and HbA1c in type 2 diabetes subjects before and after dental extraction. Antioxidants 2021, 10, 1741. [Google Scholar] [CrossRef]
- Dai, J.; Shen, J.; Chai, Y.; Chen, H. IL-1β impaired diabetic wound healing by regulating MMP-2 and MMP-9 through the p38 pathway. Mediat. Inflamm. 2021, 1, 6645766. [Google Scholar] [CrossRef]
- Sufaru, I.-G.; Martu, M.-A.; Luchian, I.; Stoleriu, S.; Diaconu-Popa, D.; Martu, C.; Teslaru, S.; Pasarin, L.; Solomon, S.M. The Effects of 810 nm Diode Laser and Indocyanine Green on Periodontal Parameters and HbA1c in Patients with Periodontitis and Type II Diabetes Mellitus: A Randomized Controlled Study. Diagnostics 2022, 12, 1614. [Google Scholar] [CrossRef] [PubMed]
- Buca, B.R.; Mititelu-Tartau, L.; Lupusoru, R.V.; Popa, G.E.; Rezus, C.; Lupusoru, C.E. New nitric oxide donors with therapeutic potential. Med. Surg. J. 2016, 120, 942–946. [Google Scholar]
- Chiriac, A.P.; Diaconu, A.; Nita, L.E.; Tudorachi, N.; Mititelu-Tartau, L.; Creteanu, A.; Dragostin, O.; Rusu, D.; Popa, G. The influence of excipients on physical and pharmaceutical properties of oral lyophilisates containing a pregabalin-acetaminophen combination. Expert. Opin. Drug Deliv. 2016, 14, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Anton, I.C.; Mititelu-Tartau, L.; Popa, E.G.; Poroch, M.; Poroch, V.; Pelin, A.-M.; Pavel, L.L.; Drochioi, I.C.; Botnariu, G.E. Zinc Chloride Enhances the Antioxidant Status, Improving the Functional and Structural Organic Disturbances in Streptozotocin-Induced Diabetes in Rats. Medicina 2022, 58, 1620. [Google Scholar] [CrossRef]
- Panainte, A.D.; Popa, G.; Pamfil, D.; Butnaru, E.; Vasile, C.; Tartau, L.M.; Gafitanu, C. In vitro characterization of polyvinyl alcohol/chitosan hydrogels as modified release systems for bisoprolol. Farmacia 2018, 66, 44–48. [Google Scholar]
| Location | Intensity | D + P n = 27 | P n = 26 | Chi-Square Test p |
|---|---|---|---|---|
| EZRIN | ||||
| Membranous | (−) | 26 (100%) | 0.001 | |
| (+) | nc | |||
| (++) | 12 (44.4%) | 0.001 | ||
| (+++) | 15 (55.6%) | 0.001 | ||
| Cytoplasmic | (−) | 26 (100%) | 0.001 | |
| (+) | nc | |||
| (++) | 12 (44.4%) | 0.001 | ||
| (+++) | 15 (55.6%) | 0.001 | ||
| Nuclear | (−) | 26 (100%) | 0.001 | |
| (+) | nc | |||
| (++) | 12 (44.4%) | 0.001 | ||
| (+++) | 15 (55.6%) | 0.001 | ||
| Interstitial | (−) | 26 (100%) | 0.001 | |
| (+) | nc | |||
| (++) | 12 (44.4%) | 0.001 | ||
| (+++) | 15 (55.6%) | 0.001 |
| Location | Intensity | D + P n = 27 | P n = 26 | Chi-Square Test p |
|---|---|---|---|---|
| MMP-2 | ||||
| Membranous | (−) | 26 (100%) | 0.001 | |
| (+) | 3 (11.1%) | nc | ||
| (++) | 9 (33.3%) | 0.001 | ||
| (+++) | 15 (55.6%) | 0.001 | ||
| Cytoplasmic | (−) | 26 (100%) | 0.001 | |
| (+) | 9 (11.1%) | nc | ||
| (++) | 3 (33.3%) | 0.001 | ||
| (+++) | 15 (55.6%) | 0.001 | ||
| Nuclear | (−) | 26 (100%) | 0.001 | |
| (+) | 5 (18.5%) | nc | ||
| (++) | 7 (25.9%) | 0.001 | ||
| (+++) | 15 (55.6%) | 0.001 | ||
| Interstitial | (−) | 26 (100%) | 0.001 | |
| (+) | 2 (7.4%) | nc | ||
| (++) | 10 (37%) | 0.001 | ||
| (+++) | 15 (55.6%) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botezatu, I.C.; Baean, M.L.; Martu, M.-A.; Botez, A.E.; Dimitriu, C.D.; Solcan, C.; Sin, A.I.; Topoliceanu, C.; Cotrutz, E.-C.; Ciurcanu, O.E. Influence of Diabetes on Expression of Ezrin and MMP-2 in Gingival Tissue of Patients with Periodontal Disease. J. Mol. Pathol. 2025, 6, 26. https://doi.org/10.3390/jmp6040026
Botezatu IC, Baean ML, Martu M-A, Botez AE, Dimitriu CD, Solcan C, Sin AI, Topoliceanu C, Cotrutz E-C, Ciurcanu OE. Influence of Diabetes on Expression of Ezrin and MMP-2 in Gingival Tissue of Patients with Periodontal Disease. Journal of Molecular Pathology. 2025; 6(4):26. https://doi.org/10.3390/jmp6040026
Chicago/Turabian StyleBotezatu, Ionut Catalin, Maria Luiza Baean, Maria-Alexandra Martu, Ana Emanuela Botez, Cristina Daniela Dimitriu, Carmen Solcan, Anca Ileana Sin, Claudiu Topoliceanu, Elena-Carmen Cotrutz, and Oana Elena Ciurcanu. 2025. "Influence of Diabetes on Expression of Ezrin and MMP-2 in Gingival Tissue of Patients with Periodontal Disease" Journal of Molecular Pathology 6, no. 4: 26. https://doi.org/10.3390/jmp6040026
APA StyleBotezatu, I. C., Baean, M. L., Martu, M.-A., Botez, A. E., Dimitriu, C. D., Solcan, C., Sin, A. I., Topoliceanu, C., Cotrutz, E.-C., & Ciurcanu, O. E. (2025). Influence of Diabetes on Expression of Ezrin and MMP-2 in Gingival Tissue of Patients with Periodontal Disease. Journal of Molecular Pathology, 6(4), 26. https://doi.org/10.3390/jmp6040026







