Methylene Blue Increases Active Mitochondria and Cellular Survival Through Modulation of miR16–UPR Signaling Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture Treatments
2.2. Cell Viability Assay
2.3. RNA Isolation
2.4. Primary Cardiomyocyte Isolation
2.5. cDNA Synthesis and qPCR Analysis
2.6. Mitotracker Labeling
2.7. Immunochemestry Analysis
2.8. Statistical Analyses
3. Results
3.1. Methylene Blue Increases the Number of Active Mitochondria and Modulates the Expression of Stress Oxidative Genes in HL1 and AC16 Cell Lines
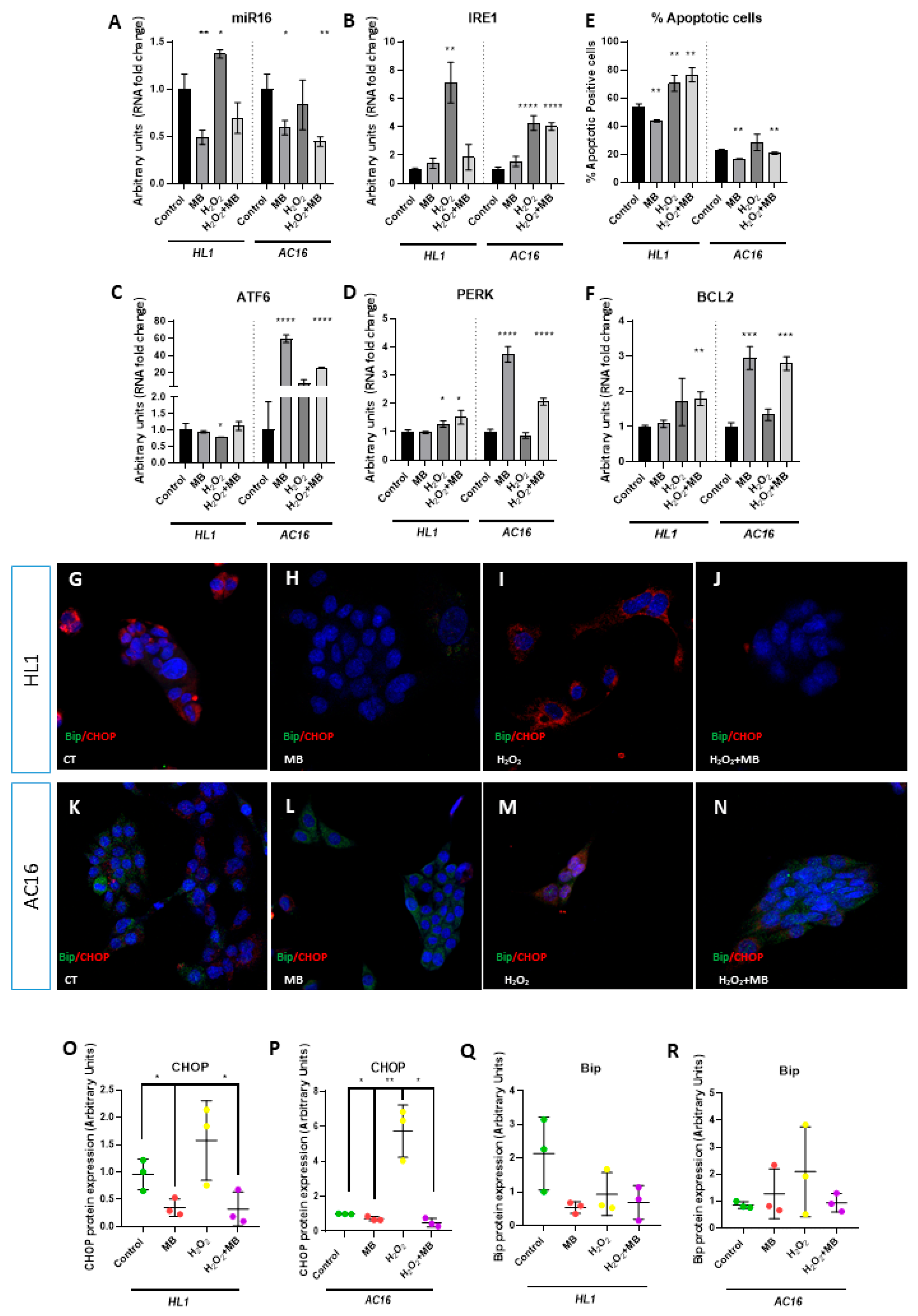
3.2. Methylene Blue Modulates miR16–UPR Signalling Axis, Increasing the Cellular Supervivence of Cardiomyocytes and Cardiomyoblast
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schirmer, R.H.; Adler, H.; Pickhardt, M.; Mandelkow, E. “Lest we forget you—methylene blue...”. Neurobiol. Aging 2011, 32, 2325.e7–2325.e16. [Google Scholar] [CrossRef] [PubMed]
- Oz, M.; Lorke, D.E.; Hasan, M.; Petroianu, G.A. Cellular and molecular actions of Methylene Blue in the nervous system. Med. Res. Rev. 2011, 31, 93–117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akbar, M.; Essa, M.M.; Daradkeh, G.; Abdelmegeed, M.A.; Choi, Y.; Mahmood, L.; Song, B.J. Mitochondrial dysfunction and cell death in neurodegenerative diseases through nitroxidative stress. Brain Res. 2016, 1637, 34–55. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rambani, V.; Hromnikova, D.; Gasperikova, D.; Skopkova, M. Mitochondria and mitochondrial disorders: An overview update. Endocr. Regul. 2022, 56, 232–248. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jin, L.; Qin, Y.; Ouyang, Z.; Zhong, J.; Zeng, Y. Harnessing Mitochondrial Stress for Health and Disease: Opportunities and Challenges. Biology 2024, 13, 394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sváb, G.; Kokas, M.; Sipos, I.; Ambrus, A.; Tretter, L. Methylene Blue Bridges the Inhibition and Produces Unusual Respiratory Changes in Complex III-Inhibited Mitochondria. Studies on Rats, Mice and Guinea Pigs. Antioxidants 2021, 10, 305. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Atamna, H.; Nguyen, A.; Schultz, C.; Boyle, K.; Newberry, J.; Kato, H.; Ames, B.N. Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways. FASEB J. 2008, 22, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Atamna, H.; Kumar, R. Protective role of methylene blue in Alzheimer’s disease via mitochondria and cytochrome c oxidase. J. Alzheimers Dis. 2010, 20 (Suppl. S2), S439–S452. [Google Scholar] [CrossRef] [PubMed]
- Roy Choudhury, G.; Winters, A.; Rich, R.M.; Ryou, M.G.; Gryczynski, Z.; Yuan, F.; Yang, S.H.; Liu, R. Methylene blue protects astrocytes against glucose oxygen deprivation by improving cellular respiration. PLoS ONE 2015, 10, e0123096. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haouzi, P.; Gueguinou, M.; Sonobe, T.; Judenherc-Haouzi, A.; Tubbs, N.; Trebak, M.; Cheung, J.; Bouillaud, F. Revisiting the physiological effects of methylene blue as a treatment of cyanide intoxication. Clin. Toxicol. 2018, 56, 828–840. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duicu, O.M.; Privistirescu, A.; Wolf, A.; Petruş, A.; Dănilă, M.D.; Raţiu, C.D.; Muntean, D.M.; Sturza, A. Methylene blue improves mitochondrial respiration and decreases oxidative stress in a substrate-dependent manner in diabetic rat hearts. Can. J. Physiol. Pharmacol. 2017, 95, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.M.; Choi, J.Y.; Wang, K.; Zhang, H.; Tariq, Z.; Wu, D.; Ko, E.; LaDana, C.; Sesaki, H.; Cao, K. Methylene blue alleviates nuclear and mitochondrial abnormalities in progeria. Aging Cell 2016, 15, 279–290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gureev, A.P.; Syromyatnikov, M.Y.; Gorbacheva, T.M.; Starkov, A.A.; Popov, V.N. Methylene blue improves sensorimotor phenotype and decreases anxiety in parallel with activating brain mitochondria biogenesis in mid-age mice. Neurosci. Res. 2016, 113, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Poudel, S.B.; Frikha-Benayed, D.; Ruff, R.R.; Yildirim, G.; Dixit, M.; Korstanje, R.; Robinson, L.; Miller, R.A.; Harrison, D.E.; Strong, J.R.; et al. Targeting mitochondrial dysfunction using methylene blue or mitoquinone to improve skeletal aging. Aging 2024, 16, 4948–4964. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krutskikh, E.P.; Potanina, D.V.; Samoylova, N.A.; Gryaznova, M.V.; Sadovnikova, I.S.; Gureev, A.P.; Popov, V.N. Brain Protection by Methylene Blue and Its Derivative, Azur B, via Activation of the Nrf2/ARE Pathway in Cisplatin-Induced Cognitive Impairment. Pharmaceuticals 2022, 15, 815. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mayer, B.; Brunner, F.; Schmidt, K. Inhibition of nitric oxide synthesis by methylene blue. Biochem. Pharmacol. 1993, 45, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.K.; Burns, S.L. The Story of Nitric Oxide, Sepsis and Methylene Blue: A Comprehensive Pathophysiologic Review. Am. J. Med. Sci. 2020, 360, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, M.U.; Ahmed, R.; Mahmoud, S.; Ahmed, K.; Bushra, N.M.; Ahmed, A.; Elwadie, B.; Madni, A.; Saad, A.B.; Abdelrahman, N. Exploring Methylene Blue and Its Derivatives in Alzheimer’s Treatment: A Comprehensive Review of Randomized Control Trials. Cureus 2023, 15, e46732. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, L.; Li, W.; Winters, A.; Yuan, F.; Jin, K.; Yang, S. Methylene blue induces macroautophagy through 5′ adenosine monophosphate-activated protein kinase pathway to protect neurons from serum deprivation. Front. Cell Neurosci. 2013, 7, 56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Smith, E.S.; Clark, M.E.; Hardy, G.A.; Kraan, D.J.; Biondo, E.; Gonzalez-Lima, F.; Cormack, L.K.; Monfils, M.; Lee, H.J. Daily consumption of methylene blue reduces attentional deficits and dopamine reduction in a 6-OHDA model of Parkinson’s disease. Neuroscience 2017, 359, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Bariotto-Dos-Santos, K.; Padovan-Neto, F.E.; Bortolanza, M.; Dos-Santos-Pereira, M.; Raisman-Vozari, R.; Tumas, V.; Del Bel, E. Repurposing an established drug: An emerging role for methylene blue in L-DOPA-induced dyskinesia. Eur. J. Neurosci. 2019, 49, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Fernandes Junior, H.J.; de Araújo, E.A.; Machado Junior, J.A.; Lutz Motta, F.M.; Guarize, G.F.; Cheng, L.C.; Tantray, J.; Medeiros, J.V.R.; Nicolau, L.A.D.; Barbosa, A.H.P.; et al. Cardiotoxic and Cardioprotective Effects of Methylene Blue in the Animal Model of Cardiac Ischemia and Reperfusion. Biomedicines 2024, 12, 2575. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miclescu, A.; Basu, S.; Wiklund, L. Cardio-cerebral and metabolic effects of methylene blue in hypertonic sodium lactate during experimental cardiopulmonary resuscitation. Resuscitation 2007, 75, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Xavier, M.S.; Vane, M.F.; Vieira, R.F.; Oliveira, C.C.; Maia, D.R.R.; de Castro, L.U.C.; Carmona, M.J.C.; Costa Auler JOJr Otsuki, D.A. Methylene blue as an adjuvant during cardiopulmonary resuscitation: An experimental study in rats. Braz. J. Anesthesiol. 2024, 74, 744470. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, M.; Lv, J.; Pan, Z.; Wang, D.; Zhao, L.; Guo, X. Mitochondrial dysfunction in heart failure and its therapeutic implications. Front. Cardiovasc. Med. 2022, 9, 945142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bongiovanni, C.; Miano, C.; Sacchi, F.; Da Pra, S.; Del Bono, I.; Boriati, S.; D’Uva, G. Protocol for isolating and culturing neonatal murine cardiomyocytes. STAR Protoc. 2024, 5, 103461. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Toro, R.; Pérez-Serra, A.; Mangas, A.; Campuzano, O.; Sarquella-Brugada, G.; Quezada-Feijoo, M.; Ramos, M.; Alcalá, M.; Carrera, E.; García-Padilla, C.; et al. miR-16-5p Suppression Protects Human Cardiomyocytes against Endoplasmic Reticulum and Oxidative Stress-Induced Injury. Int. J. Mol. Sci. 2022, 23, 1036. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siasos, G.; Tsigkou, V.; Kosmopoulos, M.; Theodosiadis, D.; Simantiris, S.; Tagkou, N.M.; Tsimpiktsioglou, A.; Stampouloglou, P.K.; Oikonomou, E.; Mourouzis, K.; et al. Mitochondria and cardiovascular diseases-from pathophysiology to treatment. Ann. Transl. Med. 2018, 6, 256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poznyak, A.V.; Ivanova, E.A.; Sobenin, I.A.; Yet, S.F.; Orekhov, A.N. The Role of Mitochondria in Cardiovascular Diseases. Biology 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wen, Y.; Li, W.; Poteet, E.C.; Xie, L.; Tan, C.; Yan, L.J.; Ju, X.; Liu, R.; Qian, H.; Marvin, M.A.; et al. Alternative mitochondrial electron transfer as a novel strategy for neuroprotection. J. Biol. Chem. 2011, 286, 16504–16515. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rojas, J.C.; Bruchey, A.K.; Gonzalez-Lima, F. Neurometabolic mechanisms for memory enhancement and neuroprotection of methylene blue. Prog. Neurobiol. 2012, 96, 32–45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shen, J.; Xin, W.; Li, Q.; Gao, Y.; Yuan, L.; Zhang, J. Methylene Blue Reduces Neuronal Apoptosis and Improves Blood-Brain Barrier Integrity After Traumatic Brain Injury. Front. Neurol. 2019, 10, 1133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Handy, D.E.; Loscalzo, J. Responses to reductive stress in the cardiovascular system. Free Radic. Biol. Med. 2017, 109, 114–124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Oria, R.; Schipani, R.; Leonardini, A.; Natalicchio, A.; Perrini, S.; Cignarelli, A.; Laviola, L.; Giorgino, F. The Role of Oxidative Stress in Cardiac Disease: From Physiological Response to Injury Factor. Oxid. Med. Cell Longev. 2020, 2020, 5732956. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive Oxygen Species (ROS) Regulates Different Types of Cell Death by Acting as a Rheostat. Oxid. Med. Cell Longev. 2021, 2021, 9912436. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuznetsov, A.V.; Javadov, S.; Sickinger, S.; Frotschnig, S.; Grimm, M. H9c2 and HL-1 cells demonstrate distinct features of energy metabolism, mitochondrial function and sensitivity to hypoxia-reoxygenation. Biochim. Biophys. Acta 2015, 1853, 276–284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eimre, M.; Paju, K.; Pelloux, S.; Beraud, N.; Roosimaa, M.; Kadaja, L.; Gruno, M.; Peet, N.; Orlova, E.; Remmelkoor, R.; et al. Distinct organization of energy metabolism in HL-1 cardiac cell line and cardiomyocytes. Biochim. Biophys. Acta. 2008, 1777, 514–524. [Google Scholar] [CrossRef] [PubMed]
- ENCODE Project Consortium; Moore, J.E.; Purcaro, M.J.; Pratt, H.E.; Epstein, C.B.; Shoresh, N.; Adrian, J.; Kawli, T.; Davis, C.A.; Dobin, A.; et al. Expanded encyclopaedias of DNA elements in the human and mouse genomes. Nature 2020, 583, 699–710, Erratum in Nature 2022, 605, E3.. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anmann, T.; Guzun, R.; Beraud, N.; Pelloux, S.; Kuznetsov, A.V.; Kogerman, L.; Kaambre, T.; Sikk, P.; Paju, K.; Peet, N.; et al. Different kinetics of the regulation of respiration in permeabilized cardiomyocytes and in HL-1 cardiac cells. Importance of cell structure/organization for respiration regulation. Biochim. Biophys. Acta 2006, 1757, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Alan, L.; Opletalova, B.; Hayat, H.; Markovic, A.; Hlavackova, M.; Vrbacky, M.; Mracek, T.; Alanova, P. Mitochondrial metabolism and hypoxic signaling in differentiated human cardiomyocyte AC16 cell line. Am. J. Physiol.-Cell Physiol. 2025, 328, C1571–C1585. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wang, X.; Li, F.; Wang, Y.; Yang, L.; Zhen, X.; Tan, W. Mitochondrial activity and oxidative stress functions are influenced by the activation of AhR-induced CYP1A1 overexpression in cardiomyocytes. Mol. Med. Rep. 2017, 16, 174–180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, Y.; Ji, J.; Zhao, Q.; Song, J. Editorial: Regulation of endoplasmic reticulum and mitochondria in cellular homeostasis. Front. Cell Dev. Biol. 2022, 10, 1004376. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caballano-Infantes, E.; Terron-Bautista, J.; Beltrán-Povea, A.; Cahuana, G.M.; Soria, B.; Nabil, H.; Bedoya, F.J.; Tejedo, J.R. Regulation of mitochondrial function and endoplasmic reticulum stress by nitric oxide in pluripotent stem cells. World J. Stem Cells 2017, 9, 26–36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, P.; Konja, D.; Zhang, Y.; Wang, Y. Communications between Mitochondria and Endoplasmic Reticulum in the Regulation of Metabolic Homeostasis. Cells 2021, 10, 2195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martinez-Amaro, F.J.; Garcia-Padilla, C.; Franco, D.; Daimi, H. LncRNAs and CircRNAs in Endoplasmic Reticulum Stress: A Promising Target for Cardiovascular Disease? Int. J. Mol. Sci. 2023, 24, 9888. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burkewitz, K.; Feng, G.; Dutta, S.; Kelley, C.A.; Steinbaugh, M.; Cram, E.J.; Mair, W.B. Atf-6 Regulates Lifespan through ER-Mitochondrial Calcium Homeostasis. Cell Rep. 2020, 32, 108125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, S.; Hu, B.; Ding, Z.; Dang, Y.; Wu, J.; Li, D.; Liu, X.; Xiao, B.; Zhang, W.; Ren, R.; et al. ATF6 safeguards organelle homeostasis and cellular aging in human mesenchymal stem cells. Cell Discov. 2018, 4, 2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garcia-Padilla, C.; Dueñas, A.; Franco, D.; Garcia-Lopez, V.; Aranega, A.; Garcia-Martinez, V.; Lopez-Sanchez, C. Dynamic MicroRNA Expression Profiles During Embryonic Development Provide Novel Insights into Cardiac Sinus Venosus/Inflow Tract Differentiation. Front. Cell Dev. Biol. 2022, 9, 767954. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garcia-Padilla, C.; Garcia-Lopez, V.; Aranega, A.; Franco, D.; Garcia-Martinez, V.; Lopez-Sanchez, C. Inhibition of RhoA and Cdc42 by miR-133a Modulates Retinoic Acid Signalling during Early Development of Posterior Cardiac Tube Segment. Int. J. Mol. Sci. 2022, 23, 4179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Guo, Y.; Tang, J.; Jiang, J.; Chen, Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim. Biophys. Sin. 2014, 46, 629–640, Erratum in Acta Biochim. Biophys. Sin. 2015, 47, 146–147. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front. Immunol. 2019, 9, 3083. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, R.; Hui, Z.; Wei, S.; Li, D.; Li, W.; Daping, W.; Alahdal, M. IRE1 signaling regulates chondrocyte apoptosis and death fate in the osteoarthritis. J. Cell Physiol. 2022, 237, 118–127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Padilla, C.; García-Serrano, D.; Franco, D. Methylene Blue Increases Active Mitochondria and Cellular Survival Through Modulation of miR16–UPR Signaling Axis. J. Mol. Pathol. 2025, 6, 16. https://doi.org/10.3390/jmp6030016
Garcia-Padilla C, García-Serrano D, Franco D. Methylene Blue Increases Active Mitochondria and Cellular Survival Through Modulation of miR16–UPR Signaling Axis. Journal of Molecular Pathology. 2025; 6(3):16. https://doi.org/10.3390/jmp6030016
Chicago/Turabian StyleGarcia-Padilla, Carlos, David García-Serrano, and Diego Franco. 2025. "Methylene Blue Increases Active Mitochondria and Cellular Survival Through Modulation of miR16–UPR Signaling Axis" Journal of Molecular Pathology 6, no. 3: 16. https://doi.org/10.3390/jmp6030016
APA StyleGarcia-Padilla, C., García-Serrano, D., & Franco, D. (2025). Methylene Blue Increases Active Mitochondria and Cellular Survival Through Modulation of miR16–UPR Signaling Axis. Journal of Molecular Pathology, 6(3), 16. https://doi.org/10.3390/jmp6030016






