Abstract
The extracellular matrix is an organized three-dimensional network of protein-based molecules and other macromolecules that provide structural and biochemical support to tissues. Depending on its biochemical and structural properties, the extracellular matrix influences cell adhesion and signal transduction and, in general, can influence cell differentiation and proliferation through specific mechanisms of chemical and mechanical sensing. The development of body tissues during ontogenesis is accompanied by changes not only in cells but also in the composition and properties of the extracellular matrix. Similarly, tumor development in carcinogenesis is accompanied by a continuous change in the properties of the extracellular matrix of tumor cells, called ‘oncomatrix’, as the tumor matures, from the development of the primary focus to the stage of metastasis. In this paper, the characteristics of the composition and properties of the extracellular matrix of tumor tissues are considered, as well as changes to the composition and properties of the matrix during the evolution of the tumor and metastasis. The extracellular matrix patterns of tumor tissues can be used as biomarkers of oncological diseases as well as potential targets for promising anti-tumor therapies.
1. Introduction
The extracellular matrix (ECM) of tissues consists primarily of collagen, elastin, and hyaluronic acid, which are expressed by connective tissue cells through the release of structural and functional biomolecules. The structural patterns of the ECM are formed by the organization of scaffolding biomolecules, while the secretory products of the cells enrich the structural framework of such a matrix with various growth factors and cytokines, which determine the molecular pattern of the ECM [1,2].
Tumor cells differ significantly from normal cells in their regulation of gene expression. Growing tumors use ‘ECM’ remodeling to create a microenvironment conducive to tumor growth and metastasis. The identification of a specific ECM in tumor tissues necessitated the introduction of the term ‘oncomatrix’ in 2002 to describe the specific ECM of tumor tissues whose endogenous properties facilitate the migration, adhesion, and proliferation of tumor cells [3,4]. The oncomatrix exerts a direct influence on tumor development and aggressiveness [5,6,7]. The properties of the matrix directly influence the effectiveness of treatment by regulating the access of chemotherapeutic agents to tumor cells and the resistance of tumors to ionizing radiation [8]. Tumor growth and development is accompanied by matrix remodeling, with tumor-associated fibroblasts playing a major role in remodeling the normal tissue matrix and synthesizing the oncomatrix [9].
Currently, the molecular patterns of the ECM that are directly associated with tumor progression have been well studied. Numerous studies suggest that biologically active molecules (peptides, proteins, and RNA) and extracellular vesicles deposited in the matrix may serve as targets for promising therapies or diagnostic markers [10,11,12]. However, the structural patterns of the matrix also influence tumor development through specific cellular mechanoreception [5,13]. For example, morphological patterns and matrix stiffness epigenetically regulate oncogenic processes by activating Yes-associated protein and stimulating cellular proliferation [14,15]. As tumor tissue matures, the composition and structure of the oncomatrix changes [16], reflecting the stages of tumor evolution at the macrostructural level [17,18,19].
Decellularized oncomatrix is used as a model to study the dynamics of normal and tumor cell properties when interacting with the oncomatrix [20]. It has been shown that the physicomechanical properties of the oncomatrix (stiffness, permeability, and density) stimulate tumor cell proliferation in vitro compared to in normal ECM [13,21,22,23].
An important property of the ECM is its ability to absorb soluble molecules such as growth factors, cytokines, and other proteins. Receptors on cell membranes interact with the structural components of the ECM and associated factors, mediating cellular adhesion and signal transduction. Various post-translational modifications of ECM components influence the interactions of the matrix with other molecules and cells [24,25,26].
As in the cases of genomics, proteomics, and other ‘omics’, the analysis of large data sets also distinguishes the matrisome, also known as the matrixome. The matrisome represents the total collection of all ECM molecules and consists of approximately 300 different types of macromolecules, including collagens, proteoglycans, and glycoproteins. The conformations of the protein components of the ECM can change through post-translational modifications induced by secreted remodeling enzymes both inside and outside the cell, increasing their diversity and forming a collection of ECM protein proteoforms [27,28,29].
The mechanical and microstructural properties of the ECM can influence cell function through mechanisms of mechanoreception and mechanotransduction [19,30]. Mechanoreception and mechanotransduction are the primary pathways of mechanical interaction between cells and the ECM. Mechanoreception refers to the molecular mechanisms by which cells sense the structural patterns of the ECM. Mechanotransduction involves the intracellular mechanisms that transmit external mechanical signals to alter cellular regulation. Mechanical and chemical signals from ECM components influence various processes such as proliferation, differentiation, migration, and apoptosis. During ECM degradation, the release of bound molecules can lead to local inflammation and changes in cell homing parameters, i.e., the ability of cells to migrate directionally within the organism [31,32].
Components of the ECM can be expressed and deposited by any cell in the body, with a tissue specificity that determines the differences in ECM properties between tissues. Thus, cell phenotype and the associated regulatory features influence the properties of the ECM. Conversely, changes in cell phenotype and regulation are accompanied by the remodeling of the ECM. This occurs, for example, during aging when there is an increase in the proportion of type I and III collagens in tissues [33].
The composition of growth factors and other signaling molecules in tumors is relatively well studied, but the structural patterns associated with the organization of oncomatrix macromolecules are not well understood. Questions regarding the composition and properties of the tumor ECM could be of great importance for diagnostics based on new physical principles, patient management, and treatment personalization based on matrix signatures, and intraoperative diagnostics.
2. Characteristics of Tumor and Normal ECM
Two primary forms of the ECM are distinguished based on their functions, composition, and localization: (1) the interstitial matrix and (2) the basement membrane matrix [4,34,35]. The interstitial matrix forms porous three-dimensional networks that connect cells within the stroma and can interface with the basement membrane, which is another organizational form of the ECM. The interstitial matrix provides structural integrity to tissues and organs and influences cell differentiation and migration. It is composed primarily of type I, III, and V collagens, fibronectin, and elastin. The structure and composition of the interstitial matrix varies among tissues in the body and can change in response to mechanical stress, inflammation, or regenerative processes [36]. Tumor cells, for example, stimulate fibroblasts to express type I and type III collagens and ECM-modifying enzymes such as lysyl oxidases and LOX-like proteins [37,38]. However, in both healthy tissue and tumors, the primary producers of ECM in the interstitial matrix are activated fibroblasts and myofibroblasts, whereas in cartilage and bone tissues, chondrocytes and osteoblasts, respectively, are the major ECM producers [39].
Oncologic transformation involves a remodeling of the interstitial ECM, leading to a variety of biophysical and biochemical changes that affect cellular mechanotransduction, ECM stiffness, cell migration, and tumor progression [40,41]. Tumor cell proliferation has been shown to be associated with changes in the physical properties of the ECM [41]. In contrast, basement membranes are more stable, sheet-like, dense structures that compartmentalize epithelial, muscle, and endothelial tissues [42]. The basement membrane is composed primarily of type IV collagen and laminins interconnected by various bridging proteins [43]. Cell attachment to the basement membrane is critical for establishing epithelial cell polarity and is essential for many developmental processes and the maintenance of tissue homeostasis [44]. During tumor growth, basement membrane remodeling is required for the invasion of stromal tissue by epithelial phenotype tumor cells and the formation of malignant tumors [45].
Changes in general ECM properties, such as scaffold molecule ratios, density, electrical conductivity, thermal conductivity, and other physicochemical properties, are accompanied by changes in topological properties, referred to as the microarchitecture of the ECM. Microarchitectonics refers to the three-dimensional spatial topology of the matrix, which is determined by the orientation, density, and connectivity of the structural molecules [46]. During ECM remodeling, the overall concentration, structure, and organization of its individual components change, leading to alterations in the three-dimensional spatial topology of the matrix and its biochemical and biophysical properties. Consequently, the influence of the ECM on cell fate changes [47].
The extracellular microenvironment of tumor cells plays a critical role in the development of oncological diseases by stimulating cell migration, selection, and proliferation. It is hypothesized that the segregation of cellular functions may be related to the properties of the ECM [48]. Thus, ECM properties in tumor tissues may support tissue homeostasis by ensuring the competitiveness of tumor cells under immune responses and the migration of normal cells into the tumor [49,50].
The question of whether the tumor ECM itself has sufficient carcinogenic potential in the absence of tumor cells remains controversial. It appears that the molecular and structural patterns of the tumor matrix inhibit its colonization by normal cells, including the infiltration of immune cells [51,52].
3. Protein Composition of Structural Components of the Extracellular Matrix
3.1. Macromolecular Components: Collagens, Laminin, Fibronectin, and Others
The primary and consistent protein components of the ECM include several types of collagens, elastin, laminin, fibronectin, and vitronectin. These major glycoproteins interact with the cell surface and form fibrillar structures with intermolecular covalent cross-links [53,54]. Cell-ECM interactions are largely mediated by the specific binding of transmembrane receptors—integrins—to their ligand, the RGD sequence (Arg-Gly-Asp), present in the polypeptide chains of collagens, laminin, fibronectin, vitronectin, tenascins, and thrombospondin. This binding initiates intracellular signaling cascades of protein kinase reactions that block anoikis and affect gene expression, cell adhesion, motility, proliferation, and differentiation [55]. It is believed that the interaction of integrins in cancer cells with ECM proteins stimulates the mechanisms of tumor invasion, metastasis, and resistance to therapeutic intervention [56].
Compared to normal tissue, the matrix of a growing solid tumor exhibits the increased activity of enzymes involved in collagen maturation and modification processes, leading to an increase in the density of cross-links between collagens, elastin, and laminin, as well as the accumulation of fibronectin [57] or vitronectin [58]. This effect increases the matrix ‘rigidity’ and results in the fibrotic ‘stiffness’ of the tumor, which correlates with poor prognosis [59]. In addition, the densely cross-linked (rigid) tumor matrix increases mechanical stress and slows oxygen diffusion within the tumor microenvironment, leading to hypoxia. These processes initiate phenotypic modulation and the selection of cancer cells, providing material for the evolutionary progression of the tumor [60,61].
Less abundant but pathogenetically important minor protein components of the tumor matrix include growth factors (EGF, FGF, TGF-β, etc.), chaperones (HSP90, HSP70, clusterin, etc.), enzymes that cross-link or degrade the ECM (transglutaminases, metalloproteinases, and hyaluronidase), and regulators of adhesion and angiogenesis (tenascins and thrombospondin). These proteins are secreted by tumor and stromal cells either as individual macromolecules or as part of exosomes, and this secretion influences the structure and properties of the ECM and promotes malignant growth [62,63].
3.2. Hyaluronic Acid and Other Proteoglycans
Hyaluronic acid (HA) is a structural biomolecule that maintains the water balance in tissues. Depending on its molecular weight, HA has been shown to act either as a tumor suppressor or as a mediator [57,64]. Low molecular weight HA (LMW-HA) interacts with cell receptors that regulate pro-tumor signaling cascades, including glycolysis—the primary energy source in tumors—and promotes cell migration. High levels of LMW-HA are associated with poor prognosis in certain cancers such as colon, breast, and prostate. The dysregulation of HA synthase and HA-degrading hyaluronidases leads to the accumulation of LMW-HA. The mechanotransduction of LMW-HA through CD44 signaling also stimulates stress resistance in tumor cells, potentially contributing to tumorigenesis [65].
Conversely, high-molecular-weight hyaluronic acid (HMW-HA)-associated mechanotransduction activates the expression of key tumor suppressor genes by binding to the CD44 receptor, leading to cell cycle arrest—a common mechanism of tumor growth inhibition. For example, the tumor resistance observed in the longest-lived rodent, the naked mole rat, is associated with the expression of a unique high-molecular-weight hyaluronic acid (HMW-HA) as a major component of its ECM, with a molecular mass of approximately 15–20 MDa [66]. The presence of proteoglycans in tumor tissue, such as chondroitin sulfates, heparan sulfates, dermatan sulfates, and keratan sulfates, regulates the ECM density and the deposition of growth factors [67]. Thus, it can be hypothesized that large macromolecules such as HMW-HA influence the inhibition of inflammation through interactions with the cellular cytoskeleton and the regulation of growth factor concentrations.
4. Structural and Functional Properties of the Oncomatrix
4.1. Microarchitecture of the Oncomatrix
An increased concentration of type I collagen in tumor tissue leads to an increase in its specific density, potentially protecting against immune cell migration and contributing to the formation of a specific tumor cell niche [68]. In addition, collagen fibers in closeproximity to the tumor boundary align and form cross-links [69,70], thereby supporting invasive tumor growth.
4.2. Density, Stiffness, and Rheological Properties of the Oncomatrix
Compared to the normal ECM, the density of the oncomatrix is higher, primarily due to the increased deposition of type I collagen and increased density of cross-links between low molecular weight components [10,71]. The increase in ECM density leads to higher tissue osmolarity and promotes tumor mineralization through the adsorption of salts. The rigid and dense oncomatrix exerts excessive mechanical stress on the surrounding normal tissues, contributing to inflammation and the disruption of the natural ECM structure of normal tissues, thereby creating conditions favorable for tumor cell invasion [6,10,72].
In addition, the remodeling of the tumor ECM toward increased density and stiffness results in abnormal intercellular adhesion, the activation of integrin signaling, and the subsequent initiation of cellular stress resistance cascades, promoting tumor growth and progression [73,74,75,76,77]. Disease progression leads to the fragmentation of the tumor body; however, the specific stiffness and density of the oncomatrix fragments remain higher than those of the normal tissue ECM [13].
The physical properties of the oncomatrix facilitate cell migration and invasion into normal stromal tissue, a phenomenon known as ‘topotaxis’ [78]. The stiffness of the tumor matrix has been shown to influence malignant transformation, with a stiffer matrix promoting the migration of tumor cells beyond the tumor body [79]. Another phenomenon, ‘durotaxis’, is related to directed cell migration along gradients of substrate stiffness. Durotaxis plays an important role in processes such as tissue development, wound healing, and cancer metastasis. This process is often mediated by mechanotransduction pathways that translate mechanical signals into biochemical responses [80].
The another microarchitecture structure, glycocalyx, is a carbohydrate-rich layer that coats the surface of cells, playing a crucial role in cellular mechanics. The glycocalyx consists of glycoproteins, glycolipids, and proteoglycans, which contribute to cell-cell communication, adhesion, and protection against mechanical stress. Alterations in the composition and structure of the glycocalyx can influence tumor cell behavior, enhancing their ability to evade the immune system, migrate, and invade surrounding tissues [72]. The glycocalyx is a key regulator of cancer progression by modulating interactions with the extracellular matrix and other cells.
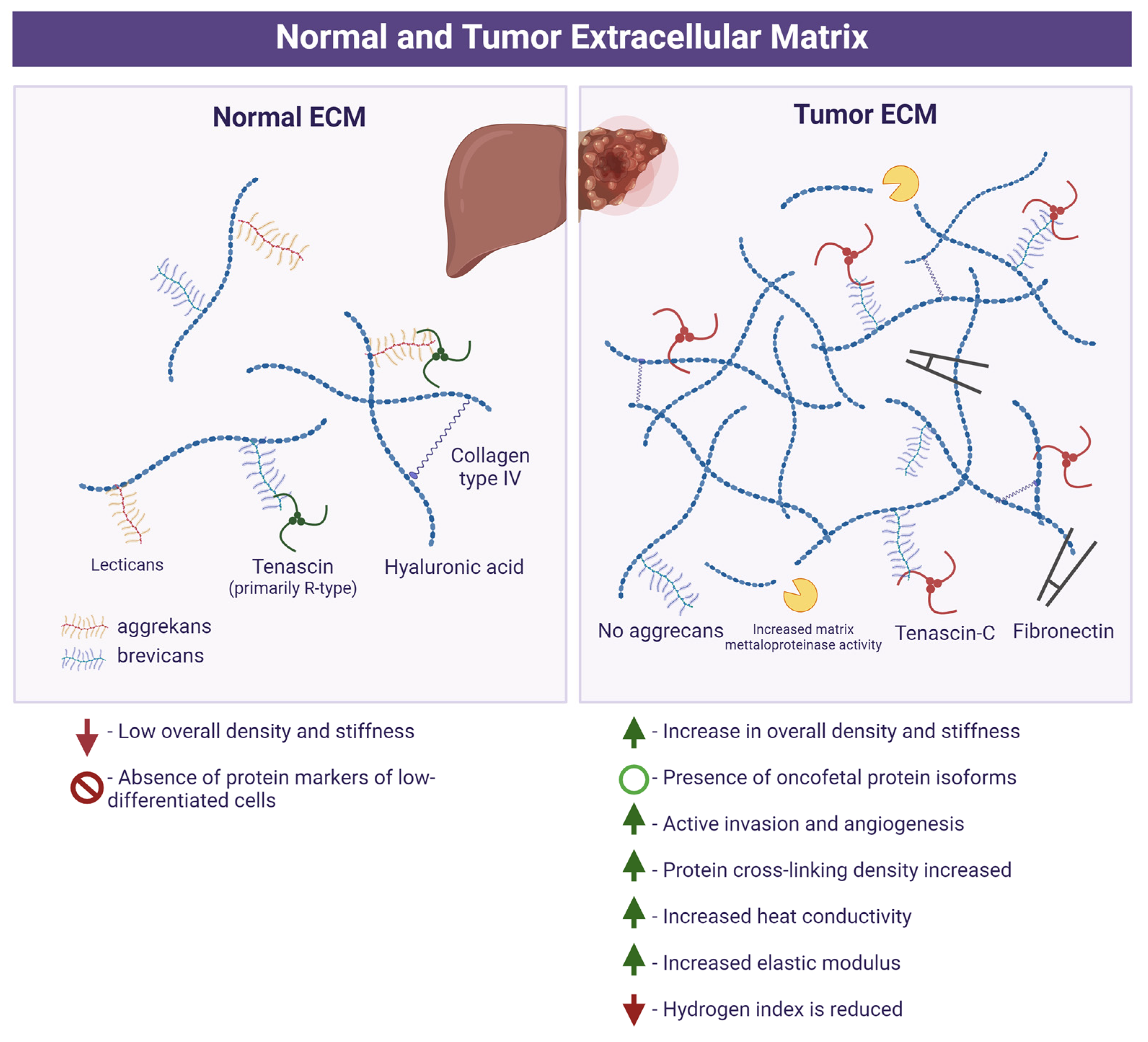
The properties of the ECM of the normal tissue and tumor are presented in Figure 1.
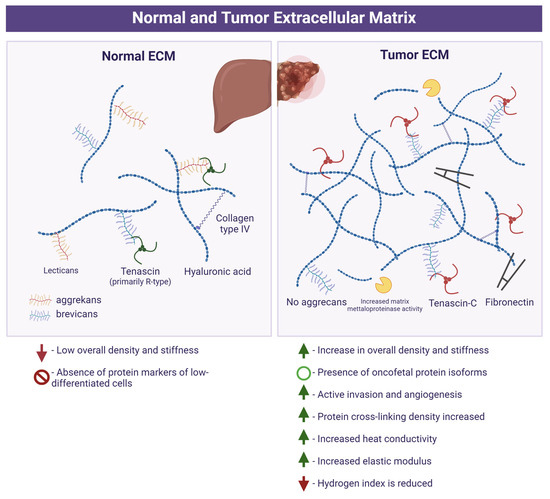
Figure 1.
Extracellular matrix in normal tissue and tumor (oncomatrix). Created with Biorender.com.
4.3. pH of the Extracellular Matrix
The pH of the oncomatrix is lower than that of the ECM in normal tissues (oncomatrix pH ∼6.8–7.0, normal ECM pH ∼7.4) for several reasons [81], including lactate released by tumor cells [82]. The reduced pH facilitates cell migration and invasion, in part by increasing the activity of acid-activated metalloproteinases that degrade cell-ECM contacts [83]. Interestingly, the intracellular pH of tumor cells is higher than that of normal cells [81]. Since pH is known to influence the rate of biochemical reactions, the acidification of the oncomatrix specifically activates enzymatic reactions and reduces the activity of immune cells.
4.4. Electrical Conductivity of the Extracellular Matrix
Tumor tissue is known to have a higher electrical conductivity than the surrounding healthy tissue [84]. Recent studies have shown that the electrical conductivity of tumor tissue is significantly higher over the entire frequency range (from 10 Hz to 1 MHz), with more pronounced differences at lower frequencies [85]. These differences may be due not only to variations in metabolite composition, but also to the composition and structure of the ECM in these tissues [86,87]. Differences in electrical conductivity have previously been used to screen for tumor disease.
4.5. Thermal Conductivity of the Extracellular Matrix
The thermal conductivity of the oncomatrix is higher than that of the ECM in normal tissue [88,89], which may be explained by the increased metabolic activity of cells and the need for increased heat dissipation to prevent overheating. This characteristic of tumors suggests that increased thermal conductivity could serve as a biomarker for tumors, which may be particularly useful in the diagnosis of malignant skin neoplasms [90].
4.6. Summarized Physical and Chemical Properties of Normal ECM and Oncomatrix
The summary of physical and chemical properties of normal ECM and oncomatrix are presented in Table 1.

Table 1.
Physical and chemical properties of normal ECM and oncomatrix.
5. Remodeling and Biodegradation of the Matrix during Oncogenesis
5.1. Matrix Remodeling and Biodegradation Processes
The natural degradation and renewal of the ECM is an integral part of its life cycle and is essential for facilitating cell migration and proliferation. Matrix metalloproteinases (MMPs), disintegrins, and other proteases are involved in matrix degradation, and their levels and activities within the ECM determine the intensity of remodeling [108,109].
Tumor cells express elevated levels of ECM-degrading proteases that serve multiple functions during tumor progression. First, the proteolytic degradation of the ECM components enables the progressive destruction of normal ECM in healthy tissue adjacent to the tumor, followed by its replacement by tumor-associated matrix (oncomatrix) [110,111]. Second, ECM degradation is a critical factor in facilitating cancer cell migration [112]. Third, the binding of soluble signaling molecules such as growth factors to the ECM renders them inactive, and remodeling releases these molecules from the ECM, triggering spontaneous cellular signaling [53,113,114].
5.2. Deposition of Growth Factors and Proteases in the Matrix
The ensemble of growth factors deposited in the ECM constitutes the molecular pattern of the matrix. Under natural conditions, the ECM accumulates these deposited factors; however, increased MMP activity in the oncomatrix leads to active biodegradation and the release of biologically active compounds, both soluble and those encapsulated in microvesicles. The active release of biologically active molecules results in the abnormal cellular regulation of resident cells [115].
5.3. Angiogenic Properties of the Matrix
Hypoxia is known to be one of the key physiological features of tumors that stimulates active angiogenesis [116]. Tumors are characterized not only by an increased blood vessel density, but also often by an increase in their diameter [117]. Tumor vessels differ from those in normal tissue by having a higher proportion of large vessels. The increase in vessel caliber leads to the so-called ‘steal syndrome’, which stimulates the development of hypoxia in the surrounding tissues. Thus, hypoxia also contributes to the evolutionary selection of tumor cells by promoting the survival of hypoxia-resistant cells through specific regulation that affects the presence of vascular growth factors in the oncomatrix and its structural microarchitecture [116].
5.4. Oncomatrix as a Driver of Epithelial-Mesenchymal Transition (EMT) in Cancer Cells
Epithelial-mesenchymal transition (EMT) is a biological process that allows epithelial cells, which are typically characterized by tight cell–cell adhesion and a polarized structure, to undergo a series of changes that enable them to adopt a mesenchymal cell phenotype. This transition is critical for embryonic development, wound healing, fibrosis, and cancer metastasis during the period of enhanced ability to degrade and remodel the ECM, often through the upregulation of MMPs [118]. Partial EMT refers to an intermediate state in which cells exhibit both epithelial and mesenchymal characteristics without fully transitioning to a mesenchymal phenotype. This intermediate state is increasingly recognized as important in cancer metastasis and tissue regeneration, allowing cells to maintain some degree of cell–cell adhesion while gaining motility [119].
In vivo, as a result of EMT, cancer cells phenotypically transform into malignant fibroblast-like cells that actively migrate through the basement membrane (BM) and infiltrate blood vessels, leading to tumor invasion of adjacent organs and metastasis. Importantly, after EMT, cancer cells acquire many characteristics of cancer stem cells (CSCs), including resistance to radiation and anticancer drugs; such consequences of EMT characterize this phenomenon as one of the major challenges in cancer therapy [120,121].
The oncomatrix has been shown to be a potent stimulator of EMT in solid tumors [122]. One of the major components of the BM, hyaluronic acid (HA), can act as an inducer of EMT in carcinomas of various origins [122,123,124]. The membrane receptor for HA, CD44, is known to be a marker of CSCs and CSC-like cells that have undergone EMT. The interaction of CD44 in cancer cells with HA in the oncomatrix triggers intracellular signaling pathways that activate mechanisms for EMT induction and the maintenance of the CSC-like phenotype [65,122]. Other components of the oncomatrix, such as proteoglycans, collagens, fibronectin, and metalloproteinases, are also involved in the initiation and execution of the EMT program in tumor cells [122]. It has been shown that even relatively minor matrix proteins such as thrombospondin [125], tenascin-C [126], and clusterin [127] can act as inducers or promoters of EMT in various types of malignant tumors.
It is not only the individual components of the oncomatrix, but also its structural properties and state that can mechanistically stimulate EMT in cancer cells. For example, increased matrix stiffness due to a high density of collagen cross-links and/or increased fibronectin and vitronectin content in the oncomatrix becomes an initiator and driver of EMT, triggering signaling responses in cancer cells that promote EMT in response to mechanical stress [59,128,129]. Such an EMT induction caused by oncomatrix stiffness and mechanical stress is often associated with increased metastasis [128,129]. It is suggested that blocking EMT by inhibiting the progressive stiffening of the oncomatrix or the signaling responses of cancer cells to mechanical stress may be a promising strategy for cancer therapy [59]. In addition to structural stiffness, other features of the oncomatrix, such as its pH, hypoxic conditions, and permeability to exosomes, may be stimulating factors or promoters of EMT in cancer cells [130,131].
6. Impact of Oncomatrix on Antitumor Therapy
6.1. Interaction of the Matrix with Chemotherapeutic Agents
The reduction in tumor sensitivity to drug therapy may be due, in part, to barriers to immune cell infiltration that depend on the properties of the tumor matrix (oncomatrix) [82]. For example, the increased density of the oncomatrix induced by chemotherapy compared to normal tissue may reduce the diffusion of drugs from the bloodstream, thereby decreasing tumor sensitivity to drug therapy. This phenomenon may serve as a compensatory mechanism to increase tumor resistance to treatment [132,133].
6.2. Interaction of the Oncomatrix with Ionizing Radiation
ECM properties can influence tissue sensitivity to ionizing radiation, with the ECM acting as a radiomodifier and facilitating cell recovery after exposure to ionizing radiation [134]. Regarding the oncomatrix, its specific properties may mediate the radioresistance of tumor cells [135]. In response to ionizing radiation, tumor cells produce an oncomatrix with less pronounced oncogenic properties [136]. Ionizing radiation induces increased structuring of paxillin-rich focal adhesions and cytoskeleton in resident tumor cells, resulting in increased tension at the level of actin filaments, causing cellular stiffness and consequently affecting cytoplasmic/nuclear morphology [137]. Thus, altering the composition and structure of the oncomatrix has the potential to increase tumor radiosensitivity during radiotherapy.
7. Discussion
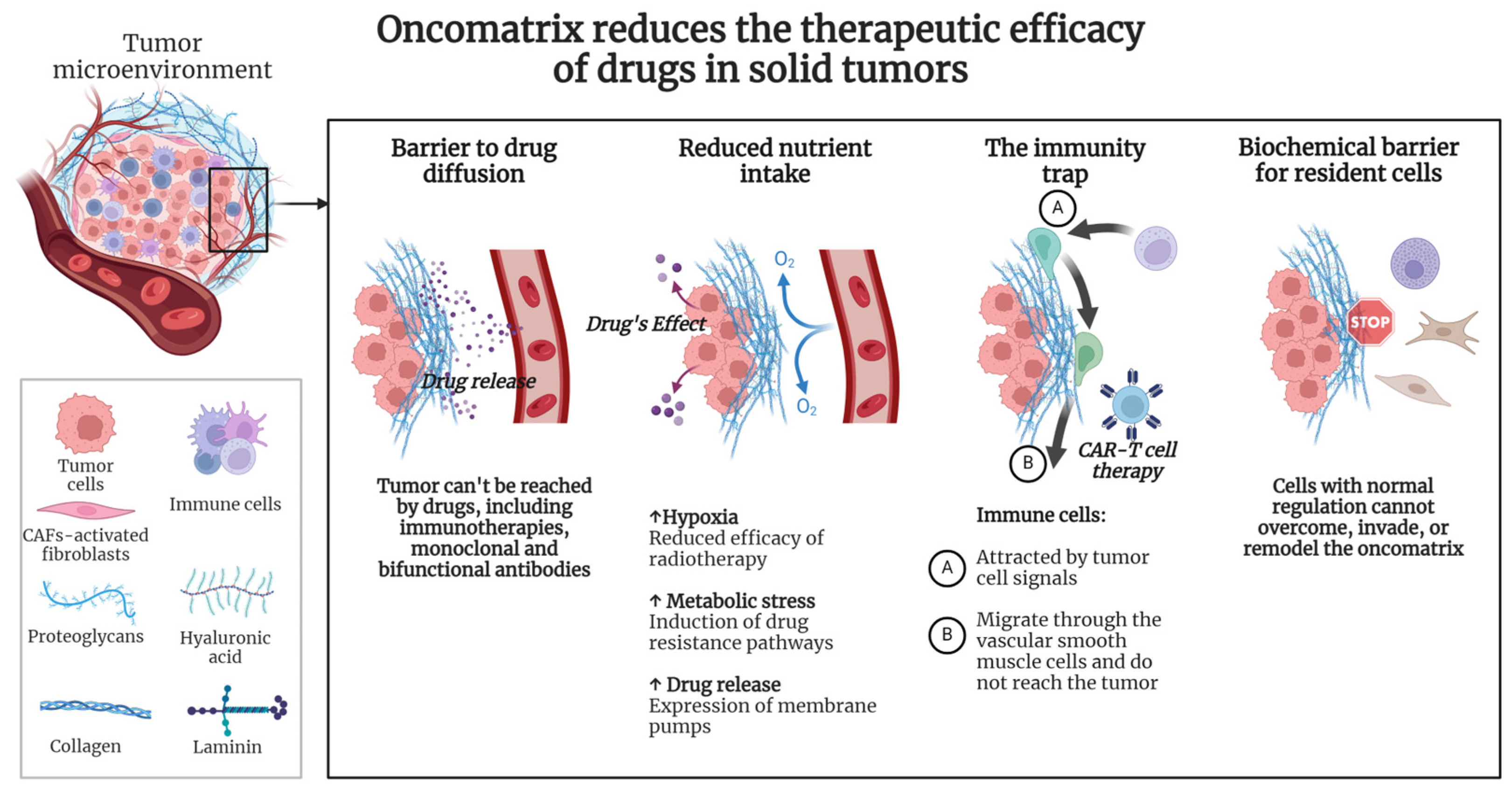
Recent studies have increasingly focused on deeply understanding the complex interactions between tumor cells and their surrounding ECM. It has been hypothesized that the primary driving forces behind the remodeling of the tumor matrix are both the need to provide a microenvironment for tumor cells and the creation of a milieu that prevents the invasion of normal cells from surrounding tissues. Tumor evolution is accompanied by changes in the composition and properties of the oncomatrix, with a radical remodeling of the ECM being associated with increased tumor aggressiveness and the suppression of the efficacy of the therapeutics (Figure 2).
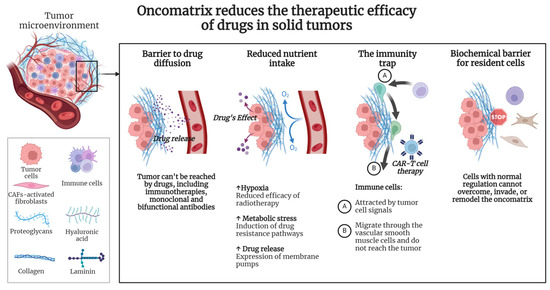
Figure 2.
The properties of the oncomatrix influence the efficacy of the therapeutics. Created with Biorender.com.
The hypoxic conditions in the oncomatrix force tumor cells to rely more on glycolysis rather than oxidative phosphorylation for energy production, a phenomenon known as the Warburg effect. This metabolic shift supports rapid cell proliferation, but also results in an acidic microenvironment that can promote further invasion and immune evasion. Hypoxia can enhance the invasive capabilities of tumor cells by upregulating MMPs, which degrade the ECM, allow cancer cells to invade surrounding tissues, and suppress the immune response by creating an immunosuppressive microenvironment [19,138]. For example, it can upregulate the expression of immune checkpoint molecules such as PD-L1 on tumor cells, leading to an inhibition of T-cell activity [139]. In addition, the hypoxic tumor cells are more resistant to radiation therapy because oxygen is a potent radiosensitizer [116]. Oxygen deprivation reduces the generation of reactive oxygen species (ROS), which cause DNA damage in cancer cells, and can induce the expression of drug efflux pumps and anti-apoptotic proteins, making tumor cells less susceptible to chemotherapeutic agents [140].
Another active immunomodulator, the resident microbiota, can modulate the immune response, either promoting or inhibiting tumor growth. Some bacteria can activate immune cells that attack the tumor, while others can create an immunosuppressive environment. The composition of the resident microbiota has been shown to influence the response to immune checkpoint inhibitors [141]. In fact, a diverse and balanced microbiota is generally associated with a better response to immunotherapy [142].
The composition of the oncomatrix may indicate tumor progression, as an oncomatrix associated with effective cellular selection indicates high aggressiveness. The prolonged process of pathologic ECM remodeling leads to the release of specific proteins into the bloodstream, which may also be of diagnostic significance, for example in lung, ovarian, breast, and colon tumors. In addition, pathological ECM remodeling itself contributes to carcinogenesis. For example, even before tumor development, the increased deposition of type I collagen and proteoglycans leads to increased tissue density, which is an independent risk factor for breast cancer [38,143,144]. Therefore, maintaining ECM homeostasis may be a novel mechanism to reduce the risk of carcinogenesis and prevent malignancy.
An analysis of changes in ECM composition and structure can be used for the early detection of oncologic diseases [4]. The characteristics of unique oncomatrix signatures of certain tumors change during progression, allowing for the prediction of clinical outcomes. Changes in matrix composition and structure allow the use of spectrophotometric methods to assess the ensemble of spectral patterns, especially in the far-IR region [145]. Based on the assessment of ECM properties, tests for tumor diagnosis have been developed; of particular note are those that utilize changes in matrix density, temperature, and electrical conductivity.
A spectral analysis of absorption and scattering parameters of tumor tissue can be used for optical biopsy. It has been shown that the absorption and scattering spectra of light on matrix components differ between normal ECM and oncomatrix, can be recorded by optical devices, and can potentially be used as spectral oncomarkers of tumors [146,147,148]. Currently, spectra of the ECM have been obtained under irradiation at various wavelengths ranging from the ultraviolet to the terahertz range [149]. The identification of resonant scattering and absorption frequencies allows the development of diagnostic and therapeutic tools based on new physical principles.
Specific features of the oncomatrix can also be exploited for adjuvant therapy aimed at the targeted destruction of the tumor microenvironment. It is known that adaptive phenotype modulations stimulated by hypoxia render tumor cells more radioresistant; thus, hypoxic tumors pose a significant challenge to radiotherapy [62,116]. Alkalizing agents for the tumor matrix to stimulate lymphoid cell migration, cross-linking agents to create a dense ECM that slows cancer cell migration, or conversely, enzymes to break down macromolecular cross-links, can collectively create conditions that reduce tumor cell resistance. The modification of matrix properties by various physical agents, such as laser irradiation, can alter surface properties and alter cellular chemotaxis [150].
In addition to resident cells, the tumor microenvironment can also alter the composition of the microbiota inhabiting the surface of human hollow epithelial organs [151,152]. Changes in the composition and properties of the ECM can stimulate the colonization and proliferation of exogenous bacteria, as well as create conditions for the contamination of tumor tissue with yeast and fungal cultures, as observed in clinical practice.
The oncogenic potential of a decellularized oncomatrix, including its implantation in laboratory animals to study its tumorigenic potential, is of particular interest [153]. However, an evaluation of the specific mechanical properties of such a matrix remains a complex methodological challenge in the context of the quantitative assessment of intertissue interaction parameters [154].
CAR-T cell therapy is an advanced form of immunotherapy in which a patient’s T cells are genetically engineered to express a chimeric antigen receptor (CAR) that specifically targets cancer cells. The ECM can act as both a physical barrier and a signaling environment that influences CAR-T cell migration, infiltration, and efficacy. Effective CAR-T cell therapy requires these cells to degrade and remodel the ECM to reach and eliminate tumor cells, often involving enzymes such as MMPs that degrade ECM components [155]. The targeted destruction of the oncomatrix is a key step in CAR-T cell therapy for solid tumors [11,156]. Therefore, the development of new adjuvant matrix-targeting drugs may be a promising direction for cancer therapy. Currently, clinical trials are underway for promising therapeutic modalities aimed at remodeling the tumor matrix under the influence of external factors, such as focal adhesion kinase inhibitors, renin-angiotensin system inhibitors, and hyaluronidase inhibitors [157,158,159]. These oncomatrix-targeted drugs can be considered to be potential adjuvants to existing chemo- and radiotherapies.
8. Conclusions
The ECM of normal and tumor tissue exhibits significant differences, including the amount and type of collagen synthesized and the secretion of specific signaling molecules. The ECM of tumor tissue changes its architecture and molecular composition, and the ECMs of different histologic types of cancer at the same site also show significant differences. The ECM is a structure that uniquely manifests itself at each step of the tumor maturation and evolution, and provides a distinct environment for a specific type of tumor cell. Thus, the composition of tumor tissue ECM is characterized not only by the presence of specific biomarkers, but also by structural features. The structural features of the oncomatrix can be used as promising targets and biomarkers. The remodeling of the oncomatrix corresponds to the stages of tumor progression and is associated with tumor evolution, with oncomatrix properties potentially stimulating tumor resistance or increasing tumor sensitivity to therapy. Maintaining ECM homeostasis may be a novel approach for cancer prevention.
Author Contributions
Conceptualization, I.K. and A.S.; methodology, I.K.; formal analysis, A.Y. and A.E.K.; investigation, D.A., D.P., V.A.S., Y.S., E.Y., M.I., E.E. and M.K.; writing—original draft preparation, I.K. and A.E.K.; writing—review and editing, A.E.K., D.S. and D.B.; visualization, I.K.; supervision, V.N.S., S.I., P.S. and A.D.K. All authors have read and agreed to the published version of the manuscript.
Funding
The present study was supported by the agreement of the Ministry of Science and Higher Education of the Russian Federation, Agreement No. 075-15-2021-1356 issued 7 October 2021 (15.CIN.21.0011, RF ID 0951.61321X0012).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data related to this article are available on demand by contacting the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pennesi, G.; Scaglione, S.; Giannoni, P.; Quarto, R. Regulatory influence of scaffolds on cell behavior: How cells decode biomaterials. Curr. Pharm. Biotechnol. 2011, 12, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Dandara, C. The extracellular matrix: Its composition, function, remodeling, and role in tumorigenesis. Biomimetics 2023, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Satpathy, M.; Cao, L.; Pincheira, R.; Emerson, R.; Bigsby, R.; Nakshatri, H.; Matei, D. Enhanced peritoneal ovarian tumor dissemination by tissue transglutaminase. Cancer Res. 2007, 67, 7194–7202. [Google Scholar] [CrossRef] [PubMed]
- Filipe, E.C.; Chitty, J.L.; Cox, T.R. Charting the unexplored extracellular matrix in cancer. Int. J. Exp. Pathol. 2018, 99, 58–76. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.R. The matrix in cancer. Nat. Rev. Cancer 2021, 21, 217–238. [Google Scholar] [CrossRef]
- Walker, C.; Mojares, E.; Del Río Hernández, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef]
- Stamenkovic, I. Extracellular matrix remodelling: The role of matrix metalloproteinases. J. Pathol. 2003, 200, 448–464. [Google Scholar] [CrossRef]
- Krisnawan, V.E.; Stanley, J.A.; Schwarz, J.K.; DeNardo, D.G. Tumor Microenvironment as a Regulator of Radiation Therapy: New Insights into Stromal-Mediated Radioresistance. Cancers 2020, 12, 2916. [Google Scholar] [CrossRef]
- Barbazán, J.; Matic Vignjevic, D. Cancer associated fibroblasts: Is the force the path to the dark side? Curr. Opin. Cell. Biol. 2019, 56, 71–79. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2020, 6, 160. [Google Scholar] [CrossRef]
- Gupta, R. Epigenetic regulation and targeting of ECM for cancer therapy. Am. J. Physiol. Physiol. 2022, 322, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Sükei, T.; Palma, E.; Urbani, L. Interplay between Cellular and Non-Cellular Components of the Tumour Microenvironment in Hepatocellular Carcinoma. Cancers 2021, 13, 5586. [Google Scholar] [CrossRef] [PubMed]
- Malandrino, A.; Mak, M.; Kamm, R.D.; Moeendarbary, E. Complex mechanics of the heterogeneous extracellular matrix in cancer. Extrem. Mech. Lett. 2018, 21, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; An, J.; Oh, S.W.; Lim, J.Y.; Kim, J.; Choi, J.K.; Cheong, J.-H.; Kim, P. Matrix stiffness epigenetically regulates the oncogenic activation of the Yes-associated protein in gastric cancer. Nat. Biomed. Eng. 2020, 5, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Abylkassov, R.; Xie, Y. Role of Yes-associated protein in cancer: An update. Oncol. Lett. 2016, 12, 2277–2282. [Google Scholar] [CrossRef]
- Li, Z.-L.; Wang, Z.-J.; Wei, G.-H.; Yang, Y.; Wang, X.-W. Changes in extracellular matrix in different stages of colorectal cancer and their effects on proliferation of cancer cells. World J. Gastrointest. Oncol. 2020, 12, 267–275. [Google Scholar] [CrossRef]
- Moreira, A.M.; Pereira, J.; Melo, S.; Fernandes, M.S.; Carneiro, P.; Seruca, R.; Figueiredo, J. The Extracellular Matrix: An Accomplice in Gastric Cancer Development and Progression. Cells 2020, 9, 394. [Google Scholar] [CrossRef]
- Rafaeva, M.; Erler, J.T. Framing cancer progression: Influence of the organ- and tumour-specific matrisome. FEBS J. 2020, 287, 1454–1477. [Google Scholar] [CrossRef]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Hoshiba, T. Decellularized Extracellular Matrix for Cancer Research. Materials 2019, 12, 1311. [Google Scholar] [CrossRef]
- Romero-López, M.; Trinh, A.L.; Sobrino, A.; Hatch, M.M.; Keating, M.T.; Fimbres, C.; Lewis, D.E.; Gershon, P.D.; Botvinick, E.L.; Digman, M.; et al. Recapitulating the human tumor microenvironment: Colon tumor-derived extracellular matrix promotes angiogenesis and tumor cell growth. Biomaterials 2017, 116, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Zanotelli, M.R.; Chada, N.C.; Johnson, C.A.; Reinhart-King, C.A. The physical microenvironment of tumors: Characterization and clinical impact. Biophys. Rev. Lett. 2020, 15, 51–82. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Zeltz, C.; Gullberg, D. Post-translational modifications of integrin ligands as pathogenic mechanisms in disease. Matrix Biol. 2014, 40, 5–9. [Google Scholar] [CrossRef]
- Leeming, D.J.; Bay-Jensen, A.C.; Vassiliadis, E.; Larsen, M.R.; Henriksen, K.; Karsdal, M.A. Post-translational modifications of the extracellular matrix are key events in cancer progression: Opportunities for biochemical marker development. Biomarkers 2011, 16, 193–205. [Google Scholar] [CrossRef]
- Holstein, E.; Dittmann, A.; Kääriäinen, A.; Pesola, V.; Koivunen, J.; Pihlajaniemi, T.; Naba, A.; Izzi, V. The Burden of Post-Translational Modification (PTM)-Disrupting Mutations in the Tumor Matrisome. Cancers 2021, 13, 1081. [Google Scholar] [CrossRef]
- Kurbatov, I.; Dolgalev, G.; Arzumanian, V.; Kiseleva, O.; Poverennaya, E. The knowns and unknowns in protein–metabolite interactions. Int. J. Mol. Sci. 2023, 24, 4155. [Google Scholar] [CrossRef]
- Elagamey, E.; Narula, K.; Sinha, A.; Aggarwal, P.R.; Ghosh, S.; Chakraborty, N.; Chakraborty, S. Extracellular Matrix Proteome and Phosphoproteome of Potato Reveals Functionally Distinct and Diverse Canonical and Non-Canonical Proteoforms. Proteomes 2016, 4, 20. [Google Scholar] [CrossRef]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef]
- Izzi, V.; Davis, M.N.; Naba, A. Pan-cancer analysis of the genomic alterations and mutations of the matrisome. Cancers 2020, 12, 2046. [Google Scholar] [CrossRef]
- Peng, C.; Xu, Y.; Wu, J.; Wu, D.; Zhou, L.; Xia, X. TME-related biomimetic strategies against cancer. Int. J. Nanomed. 2024, 19, 109–135. [Google Scholar] [CrossRef] [PubMed]
- Kutova, O.M.; Pospelov, A.D.; Balalaeva, I.V. The multifaceted role of connexins in tumor microenvironment initiation and maintenance. Biology 2023, 12, 204. [Google Scholar] [CrossRef]
- Teuscher, A.C.; Statzer, C.; Pantasis, S.; Bordoli, M.R.; Ewald, C.Y. Assessing Collagen Deposition During Aging in Mammalian Tissue and in Caenorhabditis elegans. Methods Mol. Biol. 2019, 1944, 169–188. [Google Scholar] [CrossRef]
- Prakash, J.; Shaked, Y. The Interplay between Extracellular Matrix Remodeling and Cancer Therapeutics. Cancer Discov. 2024, 14, 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- Pompili, S.; Latella, G.; Gaudio, E.; Sferra, R.; Vetuschi, A. The Charming World of the Extracellular Matrix: A Dynamic and Protective Network of the Intestinal Wall. Front. Med. 2021, 8, 610189. [Google Scholar] [CrossRef] [PubMed]
- Abyaneh, H.S.; Regenold, M.; McKee, T.D.; Allen, C.; Gauthier, M.A. Towards extracellular matrix normalization for improved treatment of solid tumors. Theranostics 2020, 10, 1960–1980. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Hiroshima, Y.; Matsuyama, R.; Homma, Y.; Hoffman, R.M.; Endo, I. Role of the tumor microenvironment in pancreatic cancer. Ann. Gastroenterol. Surg. 2019, 3, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Lepucki, A.; Orlińska, K.; Mielczarek-Palacz, A.; Kabut, J.; Olczyk, P.; Komosińska-Vassev, K. The Role of Extracellular Matrix Proteins in Breast Cancer. J. Clin. Med. 2022, 11, 1250. [Google Scholar] [CrossRef]
- Valkenburg, K.C.; de Groot, A.E.; Pienta, K.J. Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 2018, 15, 366–381. [Google Scholar] [CrossRef]
- Mohan, V.; Das, A.; Sagi, I. Emerging roles of ECM remodeling processes in cancer. Semin. Cancer Biol. 2020, 62, 192–200. [Google Scholar] [CrossRef]
- Girigoswami, K.; Saini, D.; Girigoswami, A. Extracellular Matrix Remodeling and Development of Cancer. Stem Cell Rev. Rep. 2020, 17, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Boland, E.; Quondamatteo, F.; Van Agtmael, T. The role of basement membranes in cardiac biology and disease. Biosci. Rep. 2021, 41, BSR20204185. [Google Scholar] [CrossRef] [PubMed]
- Ancsin, J.B.; Kisilevsky, R. Laminin interactions important for basement membrane assembly are promoted by zinc and implicate laminin zinc finger-like sequences. J. Biol. Chem. 1996, 271, 6845–6851. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, L.M.; Macara, I.G. Epithelial organization, cell polarity and tumorigenesis. Trends Cell Biol. 2011, 21, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Chaudhuri, O. Beyond proteases: Basement membrane mechanics and cancer invasion. J. Cell Biol. 2019, 218, 2456–2469. [Google Scholar] [CrossRef]
- Catalano, V.; Turdo, A.; Di Franco, S.; Dieli, F.; Todaro, M.; Stassi, G. Tumor and its microenvironment: A synergistic interplay. Semin. Cancer Biol. 2013, 23, 522–532. [Google Scholar] [CrossRef]
- Shen, Q.; Reedijk, M. Notch Signaling and the Breast Cancer Microenvironment. Adv. Exp. Med. Biol. 2021, 1287, 183–200. [Google Scholar] [CrossRef]
- Bateman, A. Division of labour in a matrix, rather than phagocytosis or endosymbiosis, as a route for the origin of eukaryotic cells. Biol Direct. 2020, 15, 8. [Google Scholar] [CrossRef]
- Leight, J.L.; Drain, A.P.; Weaver, V.M. Extracellular matrix remodeling and stiffening modulate tumor phenotype and treatment response. Annu. Rev. Cancer Biol. 2017, 1, 313–334. [Google Scholar] [CrossRef]
- Manou, D.; Caon, I.; Bouris, P.; Triantaphyllidou, I.E.; Giaroni, C.; Passi, A.; Karamanos, N.K.; Vigetti, D.; Theocharis, A.D. The complex interplay between extracellular matrix and cells in tissues. Extracell. Matrix: Methods Protoc. 2019, 1952, 1–20. [Google Scholar]
- Man, Y.-G.; Stojadinovic, A.; Mason, J.; Avital, I.; Bilchik, A.; Bruecher, B.; Protic, M.; Nissan, A.; Izadjoo, M.; Zhang, X.; et al. Tumor-infiltrating immune cells promoting tumor invasion and metastasis: Existing theories. J. Cancer 2013, 4, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, T.E.; Dyer, D.P.; Allen, J.E. The extracellular matrix and the immune system: A mutually dependent relationship. Science 2023, 379, eabp8964. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef] [PubMed]
- Feldinghabermann, B.; Cheresh, D.A. Vitronectin and its receptors. Curr. Opin. Cell Biol. 1993, 5, 864–868. [Google Scholar] [CrossRef]
- Takada, Y.; Ye, X.; Simon, S. The integrins. Genome Biol. 2007, 8, 215. [Google Scholar] [CrossRef]
- Nieberler, M.; Reuning, U.; Reichart, F.; Notni, J.; Wester, H.-J.; Schwaiger, M.; Weinmüller, M.; Räder, A.; Steiger, K.; Kessler, H. Exploring the Role of RGD-Recognizing Integrins in Cancer. Cancers 2017, 9, 116. [Google Scholar] [CrossRef]
- Lee, B.M.; Park, S.J.; Noh, I.; Kim, C.-H. The effects of the molecular weights of hyaluronic acid on the immune responses. Biomater. Res. 2021, 25, 27. [Google Scholar] [CrossRef]
- Burgos-Panadero, R.; Noguera, I.; Cañete, A.; Navarro, S.; Noguera, R. Vitronectin as a molecular player of the tumor microenvironment in neuroblastoma. BMC Cancer 2019, 19, 479. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, H.; Wang, J.; Liu, Y.; Luo, T.; Hua, H. Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J. Hematol. Oncol. 2022, 15, 34. [Google Scholar] [CrossRef]
- McGranahan, N.; Swanton, C. Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer Cell 2015, 27, 15–26. [Google Scholar] [CrossRef]
- Vendramin, R.; Litchfield, K.; Swanton, C. Cancer evolution: Darwin and beyond. EMBO J. 2021, 40, e108389. [Google Scholar] [CrossRef] [PubMed]
- Kabakov, A.; Yakimova, A.; Matchuk, O. Molecular chaperones in cancer stem cells: Determinants of stemness and potential targets for antitumor therapy. Cells 2020, 9, 892. [Google Scholar] [CrossRef] [PubMed]
- Seclì, L.; Fusella, F.; Avalle, L.; Brancaccio, M. The dark-side of the outside: How extracellular heat shock proteins promote cancer. Cell. Mol. Life Sci. 2021, 78, 4069–4083. [Google Scholar] [CrossRef]
- Chen, J.-W.E.; Pedron, S.; Shyu, P.; Hu, Y.; Sarkaria, J.N.; Harley, B.A.C. Influence of Hyaluronic Acid Transitions in Tumor Microenvironment on Glioblastoma Malignancy and Invasive Behavior. Front. Mater. 2018, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Mesrati, M.H.; Syafruddin, S.E.; Mohtar, M.A.; Syahir, A. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules 2021, 11, 1850. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Azpurua, J.; Hine, C.; Vaidya, A.; Myakishev-Rempel, M.; Ablaeva, J.; Mao, Z.; Nevo, E.; Gorbunova, V.; Seluanov, A. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 2013, 499, 346–349. [Google Scholar] [CrossRef]
- Hassan, N.; Efing, J.; Kiesel, L.; Bendas, G.; Götte, M. The tissue factor pathway in cancer: Overview and role of heparan sulfate proteoglycans. Cancers 2023, 15, 1524. [Google Scholar] [CrossRef]
- Keely, P.J. Mechanisms by which the extracellular matrix and integrin signaling act to regulate the switch between tumor suppression and tumor promotion. J. Mammary Gland. Biol. Neoplasia 2011, 16, 205–219. [Google Scholar] [CrossRef]
- Acerbi, I.; Cassereau, L.; Dean, I.; Shi, Q.; Au, A.; Park, C.; Chen, Y.Y.; Liphardt, J.; Hwang, E.S.; Weaver, V.M. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. 2015, 7, 1120–1134. [Google Scholar] [CrossRef]
- Alfano, M.; Nebuloni, M.; Allevi, R.; Zerbi, P.; Longhi, E.; Lucianò, R.; Locatelli, I.; Pecoraro, A.; Indrieri, M.; Speziali, C.; et al. Linearized texture of three-dimensional extracellular matrix is mandatory for bladder cancer cell invasion. Sci. Rep. 2016, 6, 36128. [Google Scholar] [CrossRef]
- Hui, L.; Chen, Y. Tumor microenvironment: Sanctuary of the devil. Cancer Lett. 2015, 368, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, J.G.; Koch, D.L.; Paszek, M.J. Equilibrium modeling of the mechanics and structure of the cancer glycocalyx. Biophysical Journal 2019, 116(4), 694–708. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wu, M.; Xu, X.; Zhang, L.; Huang, Y.; Xu, Z.; He, K.; Wang, H.; Wang, H.; Teng, L. COL12A1, a novel potential prognostic factor and therapeutic target in gastric cancer. Mol. Med. Rep. 2019, 20, 3103–3112. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.R.; Erler, J.T. Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. Dis. Models Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Kruegel, J.; Miosge, N. Basement membrane components are key players in specialized extracellular matrices. Cell. Mol. Life Sci. 2010, 67, 2879–2895. [Google Scholar] [CrossRef]
- Tremblay, E.; Ménard, D. Differential expression of extracellular matrix components during the morphogenesis of human gastric mucosa. Anat. Rec. 1996, 245, 668–676. [Google Scholar] [CrossRef]
- Shieh, A.C. Biomechanical forces shape the tumor microenvironment. Ann. Biomed. Eng. 2011, 39, 1379–1389. [Google Scholar] [CrossRef]
- Park, J.; Kim, D.H.; Levchenko, A. Topotaxis: A New Mechanism of Directed Cell Migration in Topographic ECM Gradients. Biophys. J. 2018, 114, 1257–1263. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, Y.; Liu, J.; Liu, P.; Yang, J.; Guo, D.; Tang, A.; Tao, J. Matrix hardness regulates the cancer cell malignant progression through cytoskeletal network. Biochem. Biophys. Res. Commun. 2021, 541, 95–101. [Google Scholar] [CrossRef]
- Vasudevan, J.; Jiang, K.; Fernandez, J.G.; Lim, C.T. Extracellular matrix mechanobiology in cancer cell migration. Acta Biomater. 2023, 163, 351–364. [Google Scholar] [CrossRef]
- White, K.A.; Grillo-Hill, B.K.; Barber, D.L. Cancer cell behaviors mediated by dysregulated pH dynamics at a glance. J. Cell Sci. 2017, 130, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.R.; Rathmell, W.K.; Rathmell, J.C. The tumor microenvironment as a metabolic barrier to effector T cells and immunotherapy. Elife 2020, 9, e55185. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.T.; Murray, G.I. Current mechanistic insights into the roles of matrix metalloproteinases in tumour invasion and metastasis. J. Pathol. 2015, 237, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Fricke, H.; Morse, S. The electric capacity of tumours of the breast. J. Cancer Res. 1926, 10, 340–376. [Google Scholar]
- Haemmerich, D.; Schutt, D.J.; Wright, A.S.; Webster, J.G.; Mahvi, D.M. Electrical conductivity measurement of excised human metastatic liver tumours before and after thermal ablation. Physiol. Meas. 2009, 30, 459–466. [Google Scholar] [CrossRef]
- Vahidi, M.; Rizkalla, A.S.; Mequanint, K. Extracellular Matrix-Surrogate Advanced Functional Composite Biomaterials for Tissue Repair and Regeneration. Adv. Heal. Mater. 2024; Early View. [Google Scholar]
- Sieni, E.; Dettin, M.; Zamuner, A.; Conconi, M.T.; Bazzolo, B.; Balducci, C.; Di Barba, P.; Forzan, M.; Lamberti, P.; Mognaschi, M.E. Finite element evaluation of the electric field distribution in a non-homogeneous environment. Bioengineering 2023, 10, 1062. [Google Scholar] [CrossRef]
- Lin, Q.Y.; Yang, H.Q.; Xie, S.S.; Wang, Y.H.; Ye, Z.; Chen, S.Q. Detecting early breast tumour by finite element thermal analysis. J. Med Eng. Technol. 2009, 33, 274–280. [Google Scholar] [CrossRef]
- Al Husaini, M.A.S.; Habaebi, M.H.; Hameed, S.A.; Islam, R.; Gunawan, T.S. A systematic review of breast cancer detection using thermography and neural networks. IEEE Access 2020, 8, 208922–208937. [Google Scholar] [CrossRef]
- Fujimura, T.; Okabe, T.; Tanita, K.; Sato, Y.; Lyu, C.; Kambayashi, Y.; Maruyama, S.; Aiba, S. A novel technique to diagnose non-melanoma skin cancer by thermal conductivity measurements: Correlations with cancer stromal factors. Exp. Dermatol. 2019, 28, 1029–1035. [Google Scholar] [CrossRef]
- Eble, J.A.; Niland, S. The extracellular matrix in tumor progression and metastasis. Clin. Exp. Metastasis 2019, 36, 171–198. [Google Scholar] [CrossRef]
- Chighizola, M.; Dini, T.; Marcotti, S.; D’Urso, M.; Piazzoni, C.; Borghi, F.; Previdi, A.; Ceriani, L.; Folliero, C.; Stramer, B.; et al. The glycocalyx affects the mechanotransductive perception of the topographical microenvironment. J. Nanobiotechnol. 2022, 20, 418. [Google Scholar] [CrossRef] [PubMed]
- Cross, V.L.; Zheng, Y.; Choi, N.W.; Verbridge, S.S.; Sutermaster, B.A.; Bonassar, L.J.; Fischbach, C.; Stroock, A.D. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials 2010, 31, 8596–8607. [Google Scholar] [CrossRef] [PubMed]
- Matte, B.F.; Kumar, A.; Placone, J.K.; Zanella, V.G.; Martins, M.D.; Engler, A.J.; Lamers, M.L. Matrix stiffness mechanically conditions EMT and migratory behavior of oral squamous cell carcinoma. J. Cell Sci. 2019, 132, jcs224360. [Google Scholar] [CrossRef] [PubMed]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.T.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Chakraborty, P.; Jolly, M.K.; Levine, H. A theoretical approach to coupling the epithelial-mesenchymal transition (EMT) to extracellular matrix (ECM) stiffness via LOXL2. Cancers 2021, 13, 1609. [Google Scholar] [CrossRef]
- Nicolas-Boluda, A.; Vaquero, J.; Vimeux, L.; Guilbert, T.; Barrin, S.; Kantari-Mimoun, C.; Ponzo, M.; Renault, G.; Deptula, P.; Pogoda, K.; et al. Tumor stiffening reversion through collagen crosslinking inhibition improves T cell migration and anti-PD-1 treatment. Elife 2021, 10, e58688. [Google Scholar] [CrossRef]
- Mai, Z.; Lin, Y.; Lin, P.; Zhao, X.; Cui, L. Modulating extracellular matrix stiffness: A strategic approach to boost cancer immunotherapy. Cell Death Dis. 2024, 15, 307. [Google Scholar] [CrossRef]
- Deng, B.; Zhao, Z.; Kong, W.; Han, C.; Shen, X.; Zhou, C. Biological role of matrix stiffness in tumor growth and treatment. J. Transl. Med. 2022, 20, 540. [Google Scholar] [CrossRef]
- Kai, F.; Drain, A.P.; Weaver, V.M. The extracellular matrix modulates the metastatic journey. Dev. Cell 2019, 49, 332–346. [Google Scholar] [CrossRef]
- Betteridge, K.B.; Arkill, K.P.; Neal, C.R.; Harper, S.J.; Foster, R.R.; Satchell, S.C.; Bates, D.O.; Salmon, A.H. Sialic acids regulate microvessel permeability, revealed by novel in vivo studies of endothelial glycocalyx structure and function. J. Physiol. 2017, 595(15), 5015–5035. [Google Scholar] [CrossRef]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix metalloproteinases shape the tumor microenvironment in cancer progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular matrix remodeling in tumor progression and immune escape: From mechanisms to treatments. Mol. Cancer 2023, 22, 48. [Google Scholar] [CrossRef] [PubMed]
- Popova, N.V.; Jücker, M. The functional role of extracellular matrix proteins in cancer. Cancers 2022, 14, 238. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.L.; Ling, L.; Fischbach, C. Engineering strategies to capture the biological and biophysical tumor microenvironment in vitro. Adv. Drug Deliv. Rev. 2021, 176, 113852. [Google Scholar] [CrossRef] [PubMed]
- Hosonuma, M.; Yoshimura, K. Association between pH regulation of the tumor microenvironment and immunological state. Front. Oncol. 2023, 13, 1175563. [Google Scholar] [CrossRef]
- Ward, C.; Meehan, J.; Gray, M.E.; Murray, A.F.; Argyle, D.J.; Kunkler, I.H.; Langdon, S.P. The impact of tumour pH on cancer progression: Strategies for clinical intervention. Explor. Target. Anti-Tumor Ther. 2020, 1, 71. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; la Rosa, C.C.-D.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, K.; Tan, X.; Li, Z.; Wang, H. Immunomodulatory role of metalloproteases in cancers: Current progress and future trends. Front. Immunol. 2022, 13, 1064033. [Google Scholar] [CrossRef]
- Siddhartha, R.; Garg, M. Interplay Between Extracellular Matrix Remodeling and Angiogenesis in Tumor Ecosystem. Mol. Cancer Ther. 2023, 22, 291–305. [Google Scholar] [CrossRef]
- Andreuzzi, E.; Capuano, A.; Poletto, E.; Pivetta, E.; Fejza, A.; Favero, A.; Doliana, R.; Cannizzaro, R.; Spessotto, P.; Mongiat, M. Role of extracellular matrix in gastrointestinal cancer-associated angiogenesis. Int. J. Mol. Sci. 2020, 21, 3686. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Ge, H.; Ghadban, T.; Reeh, M.; Guengoer, C. The extracellular matrix: A key accomplice of cancer stem cell migration, metastasis formation, and drug resistance in PDAC. Cancers 2022, 14, 3998. [Google Scholar] [CrossRef] [PubMed]
- Pally, D.; Naba, A. Extracellular matrix dynamics: A key regulator of cell migration across length-scales and systems. Curr. Opin. Cell Biol. 2024, 86, 102309. [Google Scholar] [CrossRef] [PubMed]
- Bandzerewicz, A.; Gadomska-Gajadhur, A. Into the tissues: Extracellular matrix and its artificial substitutes: Cell signalling mechanisms. Cells 2022, 11, 914. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Kabakov, A.E.; Yakimova, A.O. Hypoxia-Induced Cancer Cell Responses Driving Radioresistance of Hypoxic Tumors: Approaches to Targeting and Radiosensitizing. Cancers 2021, 13, 1102. [Google Scholar] [CrossRef]
- Emblem, K.E.; Farrar, C.T.; Gerstner, E.R.; Batchelor, T.T.; Borra, R.J.H.; Rosen, B.R.; Sorensen, A.G.; Jain, R.K. Vessel caliber—A potential MRI biomarker of tumour response in clinical trials. Nat. Rev. Clin. Oncol. 2014, 11, 566–584. [Google Scholar] [CrossRef]
- Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Epithelial-mesenchymal transition (EMT): The type-2 EMT in wound healing, tissue regeneration and organ fibrosis. Cells 2021, 10, 1587. [Google Scholar] [CrossRef]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef]
- Huang, T.; Song, X.; Xu, D.; Tiek, D.; Goenka, A.; Wu, B.; Sastry, N.; Hu, B.; Cheng, S.-Y. Stem cell programs in cancer initiation, progression, and therapy resistance. Theranostics 2020, 10, 8721. [Google Scholar] [CrossRef]
- Ju, F.; Atyah, M.M.; Horstmann, N.; Gul, S.; Vago, R.; Bruns, C.J.; Zhao, Y.; Dong, Q.-Z.; Ren, N. Characteristics of the cancer stem cell niche and therapeutic strategies. Stem Cell Res. Ther. 2022, 13, 233. [Google Scholar] [CrossRef]
- Tzanakakis, G.; Kavasi, R.; Voudouri, K.; Berdiaki, A.; Spyridaki, I.; Tsatsakis, A.; Nikitovic, D. Role of the extracellular matrix in cancer-associated epithelial to mesenchymal transition phenomenon. Dev. Dyn. 2018, 247, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Toole, B.P.; Zoltan-Jones, A.; Misra, S.; Ghatak, S. Hyaluronan: A critical component of epithelial-mesenchymal and epithelial-carcinoma transitions. Cells Tissues Organs 2005, 179, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Jariyal, H.; Gupta, C.; Srivastava, A. Hyaluronic acid induction on breast cancer stem cells unfolds subtype specific variations in stemness and epithelial-to-mesenchymal transition. Int. J. Biol. Macromol. 2020, 160, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, A.; Anaka, M.; Prithviraj, P.; Hudson, C.; McKeown, S.J.; Lo, P.-H.; Vella, L.J.; Goding, C.R.; Cebon, J.; Behren, A. Thrombospondin 1 promotes an aggressive phenotype through epithelial-to-mesenchymal transition in human melanoma. Oncotarget 2014, 5, 5782–5797. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, C.; Qi, W.; Cui, C.; Cui, Y.; Xuan, Y. Tenascin-C as a prognostic determinant of colorectal cancer through induction of epithelial-to-mesenchymal transition and proliferation. Exp. Mol. Pathol. 2018, 105, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jiang, K.; Kang, X.; Gao, D.; Sun, C.; Li, Y.; Sun, L.; Zhang, S.; Liu, X.; Wu, W.; et al. Tumor-derived secretory clusterin induces epithelial-mesenchymal transition and facilitates hepatocellular carcinoma metastasis. Int. J. Biochem. Cell Biol. 2012, 44, 2308–2320. [Google Scholar] [CrossRef]
- Wei, S.C.; Fattet, L.; Tsai, J.H.; Guo, Y.; Pai, V.H.; Majeski, H.E.; Chen, A.C.; Sah, R.L.; Taylor, S.S.; Engler, A.J.; et al. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat. Cell Biol. 2015, 17, 678–688. [Google Scholar] [CrossRef]
- Dong, Y.; Zheng, Q.; Wang, Z.; Lin, X.; You, Y.; Wu, S.; Wang, Y.; Hu, C.; Xie, X.; Chen, J.; et al. Higher matrix stiffness as an independent initiator triggers epithelial-mesenchymal transition and facilitates HCC metastasis. J. Hematol. Oncol. 2019, 12, 112. [Google Scholar] [CrossRef]
- To, K.K.; Cho, W.C. Exosome secretion from hypoxic cancer cells reshapes the tumor microenvironment and mediates drug resistance. Cancer Drug Resist. 2022, 5, 577. [Google Scholar] [CrossRef]
- Chen, S.; Sun, J.; Zhou, H.; Lei, H.; Zang, D.; Chen, J. New roles of tumor-derived exosomes in tumor microenvironment. Chin. J. Cancer Res. 2024, 36, 151. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Koshy, S.T.; da Cunha, C.B.; Shin, J.-W.; Verbeke, C.S.; Allison, K.H.; Mooney, D.J. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat. Mater. 2014, 13, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Jiao, Y.; Fan, Q.; Hai, M.; Yang, J.; Hu, Z.; Yang, Y.; Shuai, J.; Chen, G.; Liu, R.; et al. Modeling three-dimensional invasive solid tumor growth in heterogeneous microenvironment under chemotherapy. PLoS ONE. 2018, 13, e0206292. [Google Scholar] [CrossRef] [PubMed]
- Eke, I.; Cordes, N. Radiobiology goes 3D: How ECM and cell morphology impact on cell survival after irradiation. Radiother. Oncol. 2011, 99, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Suwa, T.; Kobayashi, M.; Nam, J.M.; Harada, H. Tumor microenvironment and radioresistance. Exp. Mol. Med. 2021, 53, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Brett, E.; Rosemann, M.; Azimzadeh, O.; Pagani, A.; Prahm, C.; Daigeler, A.; Duscher, D.; Kolbenschlag, J. Irradiated Triple-Negative Breast Cancer Co-Culture Produces a Less Oncogenic Extracellular Matrix. Int. J. Mol. Sci. 2022, 23, 8265. [Google Scholar] [CrossRef] [PubMed]
- Mottareale, R.; Frascogna, C.; La Verde, G.; Arrichiello, C.; Muto, P.; Netti, P.A.; Fusco, S.; Panzetta, V.; Pugliese, M. Impact of ionizing radiation on cell-ECM mechanical crosstalk in breast cancer. Front. Bioeng. Biotechnol. 2024, 12, 1408789. [Google Scholar] [CrossRef]
- Buffone Jr, A.; Weaver, V.M. Don’t sugarcoat it: How glycocalyx composition influences cancer progression. J. Cell Biol. 2019, 219(1), e201910070. [Google Scholar] [CrossRef]
- Mortezaee, K.; Majidpoor, J.; Kharazinejad, E. The impact of hypoxia on tumor-mediated bypassing anti-PD-(L) 1 therapy. Biomed. Pharmacother. 2023, 162, 114646. [Google Scholar] [CrossRef]
- Jiang, H.; Zuo, J.; Li, B.; Chen, R.; Luo, K.; Xiang, X.; Lu, S.; Huang, C.; Liu, L.; Tang, J.; et al. Drug-induced oxidative stress in cancer treatments: Angel or devil? Redox Biol. 2023, 63, 102754. [Google Scholar] [CrossRef]
- Simpson, R.C.; Shanahan, E.R.; Scolyer, R.A.; Long, G.V. Towards modulating the gut microbiota to enhance the efficacy of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2023, 20, 697–715. [Google Scholar] [CrossRef]
- Pham, F.; Moinard-Butot, F.; Coutzac, C.; Chaput, N. Cancer and immunotherapy: A role for microbiota composition. Eur. J. Cancer 2021, 155, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Kolesov, D.; Astakhova, A.; Galdobina, M.; Moskovtsev, A.; Kubatiev, A.; Sokolovskaya, A.; Ukrainskiy, L.; Morozov, S. Scanning Probe Microscopy Techniques for Studying the Cell Glycocalyx. Cells 2023, 12, 2778. [Google Scholar] [CrossRef] [PubMed]
- Archer, M.; Dasari, P.; Evdokiou, A.; Ingman, W.V. Biological mechanisms and therapeutic opportunities in mammographic density and breast cancer risk. Cancers 2021, 13, 5391. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, Y. Fourier Transform Infrared Spectroscopy in Oral Cancer Diagnosis. Int. J. Mol. Sci. 2021, 22, 1206. [Google Scholar] [CrossRef]
- de Boer, L.L.; Bydlon, T.M.; van Duijnhoven, F.; Peeters, M.-J.T.F.D.V.; Loo, C.E.; Winter-Warnars, G.A.O.; Sanders, J.; Sterenborg, H.J.C.M.; Hendriks, B.H.W.; Ruers, T.J.M. Towards the use of diffuse reflectance spectroscopy for real-time in vivo detection of breast cancer during surgery. J. Transl. Med. 2018, 16, 367. [Google Scholar] [CrossRef]
- Ding, H.; Nyman, J.S.; Sterling, J.A.; Perrien, D.S.; Mahadevan-Jansen, A.; Bi, X. Development of Raman spectral markers to assess metastatic bone in breast cancer. J. Biomed. Opt. 2014, 19, 111606. [Google Scholar] [CrossRef]
- Ukkonen, H.; Vuokila, S.; Mikkonen, J.J.W.; Dekker, H.; Schulten, E.A.J.M.; Bloemena, E.; Koistinen, A.; Valdez, T.A.; Kullaa, A.M.; Singh, S.P. Biochemical Changes in Irradiated Oral Mucosa: A FTIR Spectroscopic Study. Biosensors 2019, 9, 12. [Google Scholar] [CrossRef]
- Belkov, S.A.; Kochemasov, G.G.; Lyubynskaya, T.E.; Maslov, N.V.; Nuzhny, A.S.; Da Silva, L.B.; Rubenchik, A. Optical spectra analysis for breast cancer diagnostics. Appl. Phys. B Laser Opt. 2011, 105, 641–648. [Google Scholar] [CrossRef]
- Baranovskii, D.; Demner, J.; Nürnberger, S.; Lyundup, A.; Redl, H.; Hilpert, M.; Pigeot, S.; Krasheninnikov, M.; Krasilnikova, O.; Klabukov, I.; et al. Engineering of Tracheal Grafts Based on Recellularization of Laser-Engraved Human Airway Cartilage Substrates. Cartilage 2022, 13, 19476035221075951. [Google Scholar] [CrossRef]
- Klabukov, I.D.; Lyundup, A.V.; Dyuzheva, T.G.; Tyakht, A.V. Biliary Microbiota and Bile Duct Diseases. Ann. Russ. Acad. Med. Sci. 2017, 72, 172–179. [Google Scholar] [CrossRef]
- Bultman, S.J. Emerging roles of the microbiome in cancer. Carcinogenesis 2014, 35, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Gentilin, E.; D’Angelo, E.; Agostini, M.; Astolfi, L. Decellularized normal and cancer tissues as tools for cancer research. Cancer Gene Ther. 2022, 29, 879–888. [Google Scholar] [CrossRef]
- Klabukov, I.; Tenchurin, T.; Shepelev, A.; Baranovskii, D.; Mamagulashvili, V.; Dyuzheva, T.; Krasilnikova, O.; Balyasin, M.; Lyundup, A.; Krasheninnikov, M.; et al. Biomechanical Behaviors and Degradation Properties of Multilayered Polymer Scaffolds: The Phase Space Method for Bile Duct Design and Bioengineering. Biomedicines 2023, 11, 745. [Google Scholar] [CrossRef] [PubMed]
- Albelda, S.M. CAR T cell therapy for patients with solid tumours: Key lessons to learn and unlearn. Nat. Rev. Clin. Oncol. 2024, 21, 47–66. [Google Scholar] [CrossRef]
- Marofi, F.; Motavalli, R.; Safonov, V.A.; Thangavelu, L.; Yumashev, A.V.; Alexander, M.; Khiavi, F.M. CAR T cells in solid tumors: Challenges and opportunities. Stem Cell Res. Ther. 2021, 12, 81. [Google Scholar] [CrossRef]
- Gordon-Weeks, A.; Yuzhalin, A.E. Cancer Extracellular Matrix Proteins Regulate Tumour Immunity. Cancers 2020, 12, 3331. [Google Scholar] [CrossRef]
- Wu, X.; Wang, J.; Liang, Q.; Tong, R.; Huang, J.; Yang, X.; Xu, Y.; Wang, W.; Sun, M.; Shi, J. Recent progress on FAK inhibitors with dual targeting capabilities for cancer treatment. Biomed. Pharmacother. 2022, 151, 113116. [Google Scholar] [CrossRef]
- Chuang, H.H.; Zhen, Y.Y.; Tsai, Y.C.; Chuang, C.H.; Hsiao, M.; Huang, M.S.; Yang, C.J. FAK in cancer: From mechanisms to therapeutic strategies. Int. J. Mol. Sci. 2022, 23, 1726. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).