Fluorescence In Situ Hybridization (FISH) for the Characterization and Monitoring of Primary Cultures from Human Tumors
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient and Cell Line Samples
2.2. Primary Cultures
2.3. Cell Smear Preparation and FISH
2.4. NGS and nCounter
3. Results
3.1. Samples and Protocol
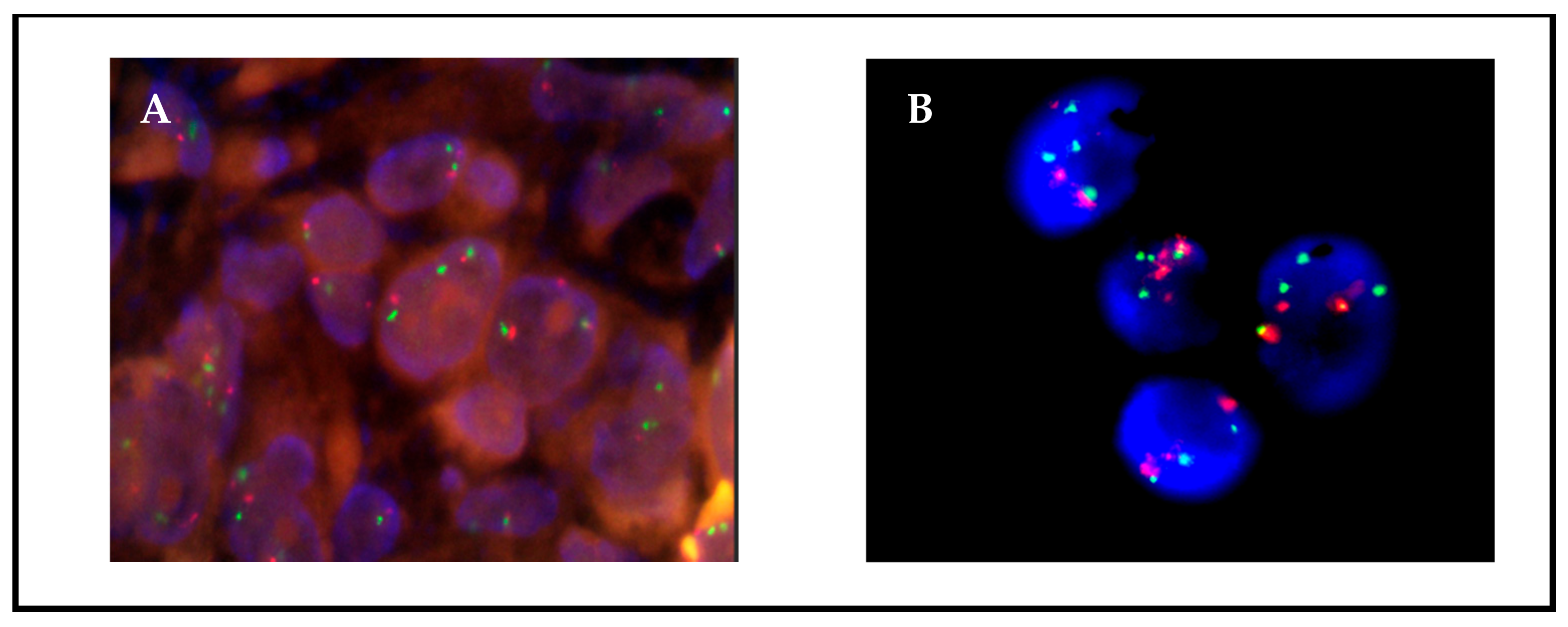
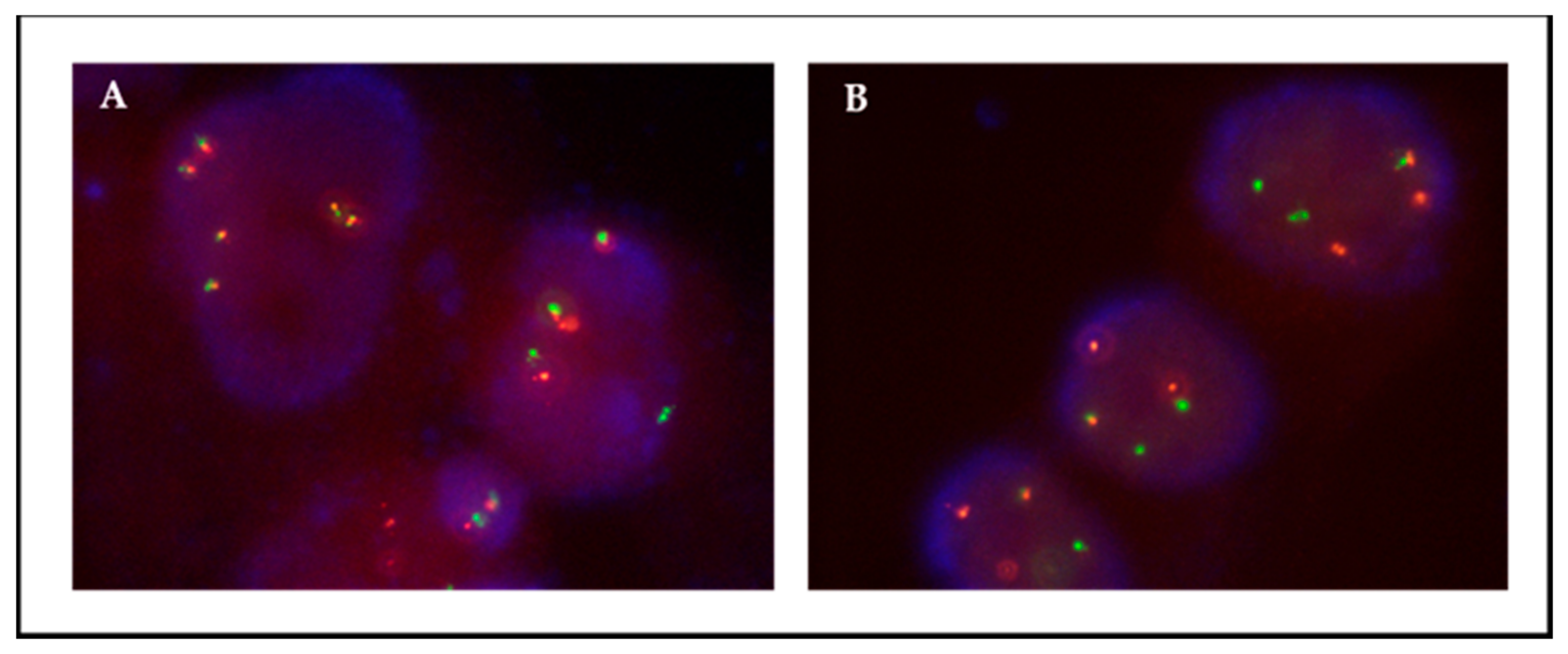
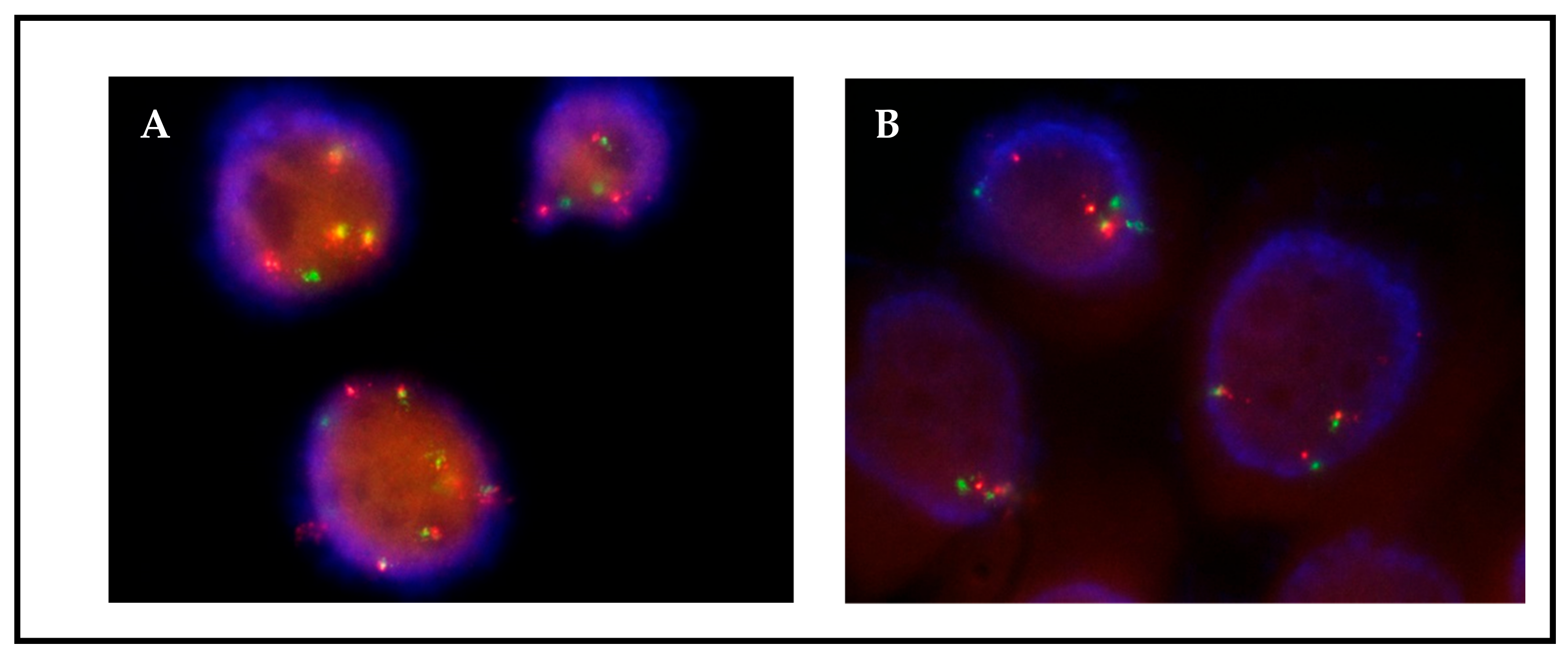
3.2. Detection of CNGs and Fusions in Low-Passage Primary Cultures
3.3. Monitoring of Tumor Cells in Primary Cultures by FISH
3.4. Comparison with NGS and nCounter
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zitter, R.; Chugh, R.M.; Saha, S. Patient Derived Ex-Vivo Cancer Models in Drug Development, Personalized Medicine, and Radiotherapy. Cancers 2022, 14, 3006. [Google Scholar] [CrossRef] [PubMed]
- Zanella, E.R.; Grassi, E.; Trusolino, L. Towards precision oncology with patient-derived xenografts. Nat. Rev. Clin. Oncol. 2022, 19, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Horan, J.C.; Tangpeerachaikul, A.; Besse, B.; Ou, S.-H.I.; Gadgeel, S.M.; Camidge, D.R.; van der Wekken, A.J.; Nguyen-Phuong, L.; Acker, A.; et al. NVL-520 Is a Selective, TRK-Sparing, and Brain-Penetrant Inhibitor of ROS1 Fusions and Secondary Resistance Mutations. Cancer Discov. 2023, 13, 598–615. [Google Scholar] [CrossRef] [PubMed]
- Domenici, G.; Trindade, G.; Estrada, M.F.; Cartaxo, A.L.; Alves, P.M.; André, S.; Brito, C. Patient-Derived Breast Cancer Tissue Cultures for Anti-Endocrine Drug Assays. Methods Mol. Biol. 2022, 2535, 11–31. [Google Scholar] [CrossRef]
- Mondielli, G.; Mougel, G.; Darriet, F.; Roche, C.; Querdray, A.; Lisbonis, C.; Appay, R.; Dufour, H.; Chinot, O.; Graillon, T.; et al. Co-Targeting MAP Kinase and Pi3K-Akt-mTOR Pathways in Meningioma: Preclinical Study of Alpelisib and Trametinib. Cancers 2022, 14, 4448. [Google Scholar] [CrossRef]
- Wang, K.; Schütze, I.; Gulde, S.; Bechmann, N.; Richter, S.; Helm, J.; Lauseker, M.; Maurer, J.; Reul, A.; Spoettl, G.; et al. Personalized drug testing in human pheochromocytoma/paraganglioma primary cultures. Endocr.-Relat. Cancer 2022, 29, 285–306. [Google Scholar] [CrossRef]
- Kodack, D.P.; Farago, A.F.; Dastur, A.; Held, M.A.; Dardaei, L.; Friboulet, L.; von Flotow, F.; Damon, L.J.; Lee, D.; Parks, M.; et al. Primary Patient-Derived Cancer Cells and Their Potential for Personalized Cancer Patient Care. Cell Rep. 2017, 21, 3298–3309. [Google Scholar] [CrossRef]
- Ouden, J.E.D.; Zaman, G.J.; Dylus, J.; van Doornmalen, A.M.; Mulder, W.R.; Grobben, Y.; van Riel, W.E.; de Hullu, J.A.; Buijsman, R.C.; van Altena, A.M. Chemotherapy sensitivity testing on ovarian cancer cells isolated from malignant ascites. Oncotarget 2020, 11, 4570–4581. [Google Scholar] [CrossRef]
- Ruiz, C.; Kustermann, S.; Pietilae, E.; Vlajnic, T.; Baschiera, B.; Arabi, L.; Lorber, T.; Oeggerli, M.; Savic, S.; Obermann, E.; et al. Culture and Drug Profiling of Patient Derived Malignant Pleural Effusions for Personalized Cancer Medicine. PLoS ONE 2016, 11, e0160807. [Google Scholar] [CrossRef]
- Wu, M.; Hong, G.; Chen, Y.; Ye, L.; Zhang, K.; Cai, K.; Yang, H.; Long, X.; Gao, W.; Li, H. Personalized drug testing in a patient with non-small-cell lung cancer using cultured cancer cells from pleural effusion. J. Int. Med. Res. 2020, 48, 300060520955058. [Google Scholar] [CrossRef]
- Roscilli, G.; De Vitis, C.; Ferrara, F.F.; Noto, A.; Cherubini, E.; Ricci, A.; Mariotta, S.; Giarnieri, E.; Giovagnoli, M.R.; Torrisi, M.R.; et al. Human lung adenocarcinoma cell cultures derived from malignant pleural effusions as model system to predict patients chemosensitivity. J. Transl. Med. 2016, 14, 61. [Google Scholar] [CrossRef]
- Kar, R.; Chawla, D.; Gupta, B.; Mehndiratta, M.; Wadhwa, N.; Agarwal, R. Establishment of Primary Cell Culture From Ascitic Fluid and Solid Tumor Obtained From Epithelial Ovarian Carcinoma Patients. Int. J. Gynecol. Cancer 2017, 27, 2000–2005. [Google Scholar] [CrossRef]
- Lira, M.E.; Kim, T.M.; Huang, D.; Deng, S.; Koh, Y.; Jang, B.; Go, H.; Lee, S.-H.; Chung, D.H.; Kim, W.H.; et al. Multiplexed Gene Expression and Fusion Transcript Analysis to Detect ALK Fusions in Lung Cancer. J. Mol. Diagn. 2012, 15, 51–61. [Google Scholar] [CrossRef]
- Lehmann, L.; Greulich, K.M.; Zitzelsberger, H.; Negele, T.; Spelsberg, F.; Bauchinger, M.; Weier, H.-U.G. Cytogenetic and molecular genetic characterization of a chromosome 2 rearrangement in a case of human papillary thyroid carcinoma with radiation history. Cancer Genet. Cytogenet. 1997, 96, 30–36. [Google Scholar] [CrossRef]
- Lerner, C.; Ketter, R.; Linsler, S.; Henn, W.; Oertel, J.; Urbschat, S. Establishment of a molecular cytogenetic analysis for native tumor tissue of meningiomas-suitable for clinical application. Mol. Cytogenet. 2014, 7, 12. [Google Scholar] [CrossRef]
- Villatoro, S.; Mayo-De-Las-Casas, C.; Jordana-Ariza, N.; Viteri-Ramírez, S.; Garzón-Ibañez, M.; Moya-Horno, I.; García-Peláez, B.; González-Cao, M.; Malapelle, U.; Balada-Bel, A.; et al. Prospective detection of mutations in cerebrospinal fluid, pleural effusion, and ascites of advanced cancer patients to guide treatment decisions. Mol. Oncol. 2019, 13, 2633–2645. [Google Scholar] [CrossRef]
- Vives-Usano, M.; García Pelaez, B.; Román Lladó, R.; Garzón Ibañez, M.; Aldeguer, E.; Rodriguez, S.; Aguilar, A.; Pons, F.; Viteri, S.; Cabrera, C.; et al. Analysis of Copy Number Variations in Solid Tumors Using a Next Generation Sequencing Custom Panel. J. Mol. Pathol. 2021, 2, 123–134. [Google Scholar] [CrossRef]
- Aguado, C.; Teixido, C.; Román, R.; Reyes, R.; Giménez-Capitán, A.; Marin, E.; Cabrera, C.; Viñolas, N.; Castillo, S.; Muñoz, S.; et al. Multiplex RNA-based detection of clinically relevant MET alterations in advanced non-small cell lung cancer. Mol. Oncol. 2020, 15, 350–363. [Google Scholar] [CrossRef]
- Reguart, N.; Teixidó, C.; Giménez-Capitán, A.; Paré, L.; Galván, P.; Viteri, S.; Rodríguez, S.; Peg, V.; Aldeguer, E.; Viñolas, N.; et al. Identification of ALK, ROS1, and RET Fusions by a Multiplexed mRNA-Based Assay in Formalin-Fixed, Paraffin-Embedded Samples from Advanced Non–Small-Cell Lung Cancer Patients. Clin. Chem. 2017, 63, 751–760. [Google Scholar] [CrossRef]
- Wolff, D.J.; Bagg, A.; Cooley, L.D.; Dewald, G.W.; Hirsch, B.A.; Jacky, P.B.; Rao, K.W.; Rao, P.N.; Association for Molecular Pathology Clinical Practice Committee; American College of Medical Genetics Laboratory Quality Assurance Committee. Guidance for Fluorescence in Situ Hybridization Testing in Hematologic Disorders. J. Mol. Diagn. 2007, 9, 134–143. [Google Scholar] [CrossRef]
- Kijima, T.; Nakagawa, H.; Shimonosono, M.; Chandramouleeswaran, P.M.; Hara, T.; Sahu, V.; Kasagi, Y.; Kikuchi, O.; Tanaka, K.; Giroux, V.; et al. Three-Dimensional Organoids Reveal Therapy Resistance of Esophageal and Oropharyngeal Squamous Cell Carcinoma Cells. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, Y.; Chung, C. Scientific Validation and Clinical Application of Lung Cancer Organoids. Cells 2021, 10, 3012. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Noel, P.; Borazanci, E.H.; Lee, J.; Amini, A.; Han, I.W.; Heo, J.S.; Jameson, G.S.; Fraser, C.; Steinbach, M.; et al. Single-cell transcriptome analysis of tumor and stromal compartments of pancreatic ductal adenocarcinoma primary tumors and metastatic lesions. Genome Med. 2020, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.; Park, D. Challenges in genomic analysis of model systems and primary tumors of pancreatic ductal adenocarcinoma. Comput. Struct. Biotechnol. J. 2022, 20, 4806–4815. [Google Scholar] [CrossRef]
- Mazzocchi, A.; Devarasetty, M.; Herberg, S.; Petty, W.J.; Marini, F.; Miller, L.; Kucera, G.; Dukes, D.K.; Ruiz, J.; Skardal, A.; et al. Pleural Effusion Aspirate for Use in 3D Lung Cancer Modeling and Chemotherapy Screening. ACS Biomater. Sci. Eng. 2019, 5, 1937–1943. [Google Scholar] [CrossRef]
- Mou, Y.; Huang, J.; Yang, W.; Wan, Y.; Pu, Z.; Zhang, J.; Liu, J.; Li, Q.; Zhang, P.; Tian, Y.; et al. Patient-derived primary breast cancer cells and their potential for predicting sensitivity to chemotherapy. Front. Oncol. 2022, 12, 1023391. [Google Scholar] [CrossRef]
- Papp, E.; Steib, A.; Abdelwahab, E.M.; Meggyes-Rapp, J.; Jakab, L.; Smuk, G.; Schlegl, E.; Moldvay, J.; Sárosi, V.; Pongracz, J.E. Feasibility study of in vitro drug sensitivity assay of advanced non-small cell lung adenocarcinomas. BMJ Open Respir. Res. 2020, 7, e000505. [Google Scholar] [CrossRef]
- Coleman, N.; Hong, L.; Zhang, J.; Heymach, J.; Hong, D.; Le, X. Beyond epidermal growth factor receptor: MET amplification as a general resistance driver to targeted therapy in oncogene-driven non-small-cell lung cancer. ESMO Open 2021, 6, 100319. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Yoda, S.; Lennerz, J.K.; Langenbucher, A.; Lin, J.J.; Rooney, M.M.; Prutisto-Chang, K.; Oh, A.; Adams, N.A.; Yeap, B.Y.; et al. MET Alterations Are a Recurring and Actionable Resistance Mechanism in ALK-Positive Lung Cancer. Clin. Cancer Res. 2020, 26, 2535–2545. [Google Scholar] [CrossRef]


| Sample | Type of Tumor | Collection Time | Fluid | Known Fusion/CNGs in Previous Material |
|---|---|---|---|---|
| 1 | Lung Adenocarcinoma | Progression | Pleural | ALK fusion/MET CNG |
| 2 | Lung Adenocarcinoma | Progression | Pleural | ALK fusion/MET CNG |
| 3 | High-Grade Serous Ovarian Carcinoma | Progression | Ascitic | EGFR CNG |
| 4 | Lung Adenocarcinoma | Basal | Pleural | FGFR1 CNG |
| 5 | Lung Adenocarcinoma | Basal | Pleural | MET CNG |
| 6 | Lung Adenocarcinoma | Progression | Pleural | MET CNG |
| 7 | Melanoma | Progression | Ascitic | MET CNG |
| 8 | Lung Adenocarcinoma | Progression | Pleural | MET CNG |
| 9 | Lung Adenocarcinoma | Progression | Pleural | MYC CNG |
| 10 | NSCLC | Basal | Pleural | MYC CNG |
| 11 | Lung Adenocarcinoma | Progression | Pleural | ROS1 fusion |
| 12 | Lung Adenocarcinoma | Progression | Pleural | ROS1 fusion |
| Sample | Passage | Alteration | % Translocated Cells |
|---|---|---|---|
| Sample 1 | 2 | ALK fusion | 50 |
| Sample 1 | 3 | ALK fusion | 17 |
| Sample 1 | 5 | ALK fusion | 50 |
| Sample 1 | 8 | ALK fusion | 90 |
| Sample 1 | 9 | ALK fusion | 75 |
| Sample 1 | 11 | ALK fusion | 65 |
| Sample 1 | 13 | ALK fusion | 50 |
| Sample 2 | 2 | ALK fusion | 50 |
| Sample 2 | 3 | ALK fusion | 72 |
| Sample 2 | 5 | ALK fusion | 37 |
| Sample 11 | 1 | ROS1 fusion | 9 |
| Sample 11 | 2 | ROS1 fusion | 87 |
| Sample 11 | 5 | ROS1 fusion | 78 |
| Sample 11 | 7 | ROS1 fusion | 99 |
| Sample 12 AC | 1 | ROS1 fusion | 76 |
| Sample 12 AC | 2 | ROS1 fusion | 99 |
| Sample 12 AC | 5 | ROS1 fusion | 100 |
| Sample 12 FC | 1 | ROS1 fusion | 100 |
| Sample 12 FC | 2 | ROS1 fusion | 100 |
| Sample 12 FC | 5 | ROS1 fusion | 100 |
| Sample | Passage | Alteration | Ratio (gen/cen) | Copies | % Cells with ≥5 Gene Copies |
|---|---|---|---|---|---|
| Sample 1 | 5 | MET CNG | 1.45 | 5.1 | 77 |
| Sample 1 | 8 | MET CNG | 1.3 | 4.4 | 50 |
| Sample 1 | 9 | MET CNG | 1.3 | 4.5 | 53 |
| Sample 1 | 11 | MET CNG | 1 | 3.2 | 27 |
| Sample 1 | 13 | MET CNG | 1 | 3.0 | 5 |
| Sample 2 | 2 | MET CNG | 2.4 | 7.3 | 27 |
| Sample 2 | 3 | MET CNG | 1.2 | 3.5 | 7 |
| Sample 2 | 5 | MET CNG | 1.3 | 4.1 | 10 |
| Sample 5 | 2 | MET CNG | 0.9 | 10.2 | 100 |
| Sample 5 | 3 | MET CNG | 0.9 | 8.4 | 100 |
| Sample 6 AC | 1 | MET CNG | 8.5 | 17.1 | 36 |
| Sample 6 AC | 2 | MET CNG | 2.8 | 5.5 | 20 |
| Sample 6 FC | 1 | MET CNG | >10 | >20 | 81 |
| Sample 6 FC | 2 | MET CNG | >10 | >20 | 96 |
| Sample 6 FC | 8 | MET CNG | >10 | >20 | 100 |
| Sample 7 | 1 | MET CNG | 1 | 2 | 0 |
| Sample 8 | 1 | MET CNG | 1 | 3.5 | 20 |
| Sample 8 | 3 | MET CNG | 1 | 2 | 0 |
| Sample 3 | 1 | EGFR CNG | 1 | 6.7 | 100 |
| Sample 3 | 3 | EGFR CNG | 1 | 6.2 | 90 |
| Sample 4 | 1 | FGFR1 CNG | 3.6 | 15.1 | 67 |
| Sample 4 | 3 | FGFR1 CNG | 3 | 11.2 | 70 |
| Sample 9 | 1 | MYC CNG | - | >6 | 72 |
| Sample 9 | 2 | MYC CNG | - | >6 | 63 |
| Sample 9 | 6 | MYC CNG | - | 7.8 | 90 |
| Sample 10 | 2 | MYC CNG | - | >6 | 100 |
| Sample 10 | 6 | MYC CNG | - | >6 | 100 |
| FISH | nCounter | ||||||
|---|---|---|---|---|---|---|---|
| Sample | Passage | Alteration | Ratio | Copies | % Positive Cells | Fusion | Exons |
| Sample 1 | 3 | ALK | - | - | 17 | EML4-ALK | v1 (E13:A20) |
| Sample 1 | 8 | ALK | - | - | 90 | EML4-ALK | v1 (E13:A20) |
| Sample 1 | 9 | ALK | - | - | 75 | EML4-ALK | v1 (E13:A20) |
| Sample 1 | 13 | ALK | - | - | 50 | EML4-ALK | v1 (E13:A20) |
| Sample 2 | 2 | ALK | - | - | 50 | EML4-ALK | v1 (E13:A20) |
| Sample 2 | 3 | ALK | - | - | 72 | EML4-ALK | v1 (E13:A20) |
| Sample 11 | 2 | ROS1 | - | - | 87 | ROS1 | not identified * |
| Sample 11 | 5 | ROS1 | - | - | 78 | ROS1 | not identified * |
| Sample 11 | 7 | ROS1 | - | - | 99 | ROS1 | not identified * |
| Sample 12 AC | 1 | ROS1 | - | - | 76 | CD74-ROS1 | C6-E34 |
| Sample 12 FC | 1 | ROS1 | - | - | 100 | CD74-ROS1 | C6-E34 |
| Sample 12 FC | 24 | ROS1 | - | - | 100 | CD74-ROS1 | C6-E34 |
| Sample | Passage | Alteration | Ratio | Copies | % Cells with ≥5 | NGS | |
| Sample 1 | 5 | MET CNG | 1.45 | 5.1 | 77 | MET | 10 copies |
| Sample 1 | 11 | MET CNG | 1 | 3.2 | 27 | ND | ND |
| Sample 2 | 2 | MET CNG | 2.4 | 7.3 | 25 | MET | 15 copies |
| Sample 3 | 1 | EGFR CNG | 1 | 6.7 | 100 | EGFR | 5 copies |
| Sample 4 | 1 | FGFR1 CNG | 3.6 | 15.1 | 90 | FGFR1 | 16 copies |
| Sample 5 | 2 | MET CNG | 0.9 | 10.2 | 100 | ND | ND |
| Sample 6 FC | 1 | MET CNG | >10 | >20 | 81 | MET | >50 copies |
| Sample 7 | 1 | MET CNG | 1 | 2 | 100 | ND | ND |
| Sample 8 | 1 | MET CNG | 1 | 3,5 | 0 | ND | ND |
| Sample 9 | 2 | MYC CNG | - | >6 | 63 | MYC | 7 copies |
| Sample 10 | 2 | MYC CNG | - | >6 | 100 | MYC | >50 copies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Román-Lladó, R.; Aguado, C.; Jordana-Ariza, N.; Roca-Arias, J.; Rodríguez, S.; Aldeguer, E.; Garzón-Ibañez, M.; García-Peláez, B.; Vives-Usano, M.; Giménez-Capitán, A.; et al. Fluorescence In Situ Hybridization (FISH) for the Characterization and Monitoring of Primary Cultures from Human Tumors. J. Mol. Pathol. 2023, 4, 57-68. https://doi.org/10.3390/jmp4010007
Román-Lladó R, Aguado C, Jordana-Ariza N, Roca-Arias J, Rodríguez S, Aldeguer E, Garzón-Ibañez M, García-Peláez B, Vives-Usano M, Giménez-Capitán A, et al. Fluorescence In Situ Hybridization (FISH) for the Characterization and Monitoring of Primary Cultures from Human Tumors. Journal of Molecular Pathology. 2023; 4(1):57-68. https://doi.org/10.3390/jmp4010007
Chicago/Turabian StyleRomán-Lladó, Ruth, Cristina Aguado, Núria Jordana-Ariza, Jaume Roca-Arias, Sonia Rodríguez, Erika Aldeguer, Mónica Garzón-Ibañez, Beatriz García-Peláez, Marta Vives-Usano, Ana Giménez-Capitán, and et al. 2023. "Fluorescence In Situ Hybridization (FISH) for the Characterization and Monitoring of Primary Cultures from Human Tumors" Journal of Molecular Pathology 4, no. 1: 57-68. https://doi.org/10.3390/jmp4010007
APA StyleRomán-Lladó, R., Aguado, C., Jordana-Ariza, N., Roca-Arias, J., Rodríguez, S., Aldeguer, E., Garzón-Ibañez, M., García-Peláez, B., Vives-Usano, M., Giménez-Capitán, A., Aguilar, A., Martinez-Bueno, A., Cao, M. G., García-Casabal, F., Viteri, S., Mayo de las Casas, C., Rosell, R., & Molina-Vila, M. A. (2023). Fluorescence In Situ Hybridization (FISH) for the Characterization and Monitoring of Primary Cultures from Human Tumors. Journal of Molecular Pathology, 4(1), 57-68. https://doi.org/10.3390/jmp4010007








