Metastatic Breast Cancer: Cytology Diagnosis with Implications for Treatment
Abstract
:1. Introduction and Overview
2. How Does Metastatic Spread Occur?
3. Diagnosis of Breast Cancer Metastases
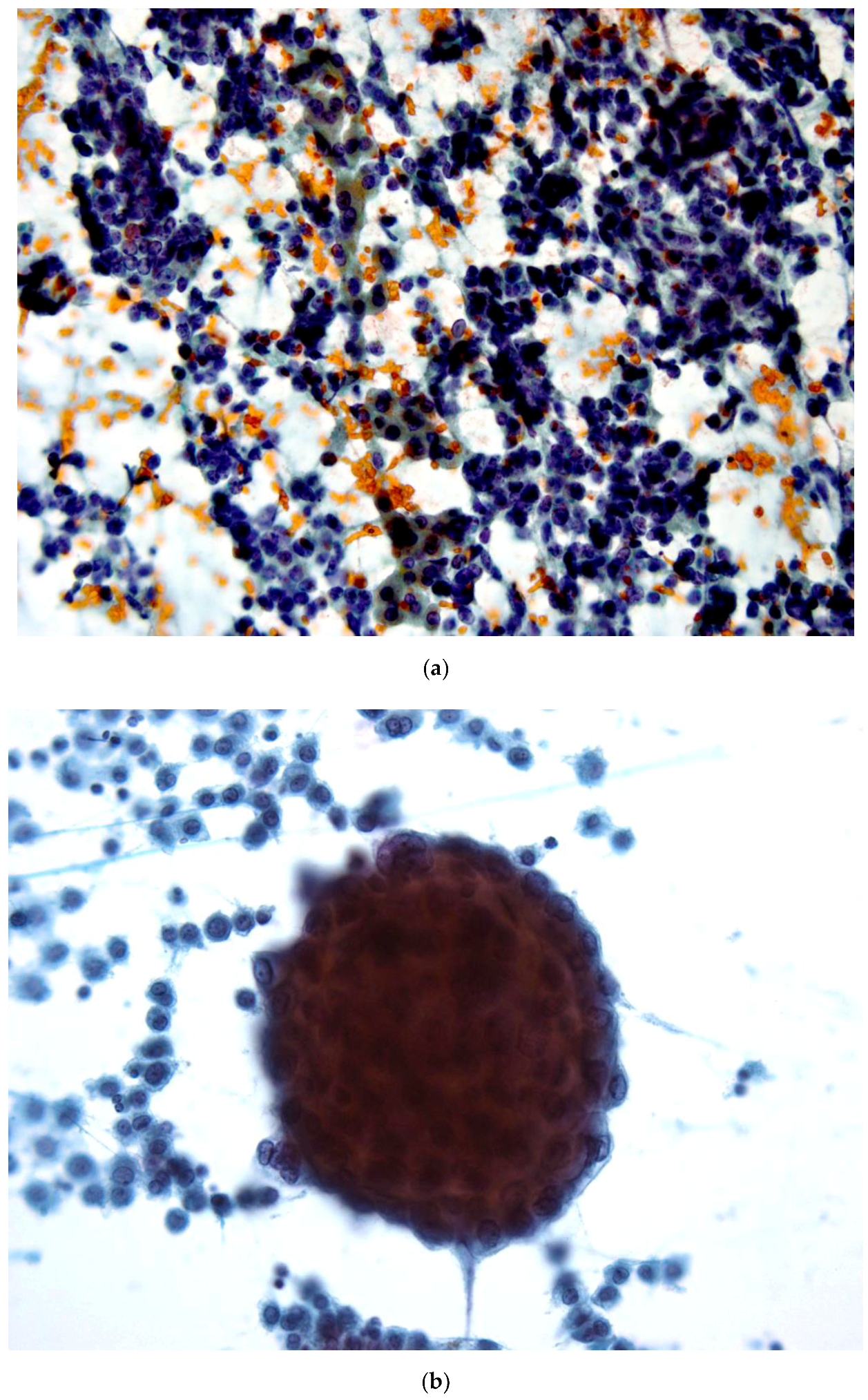
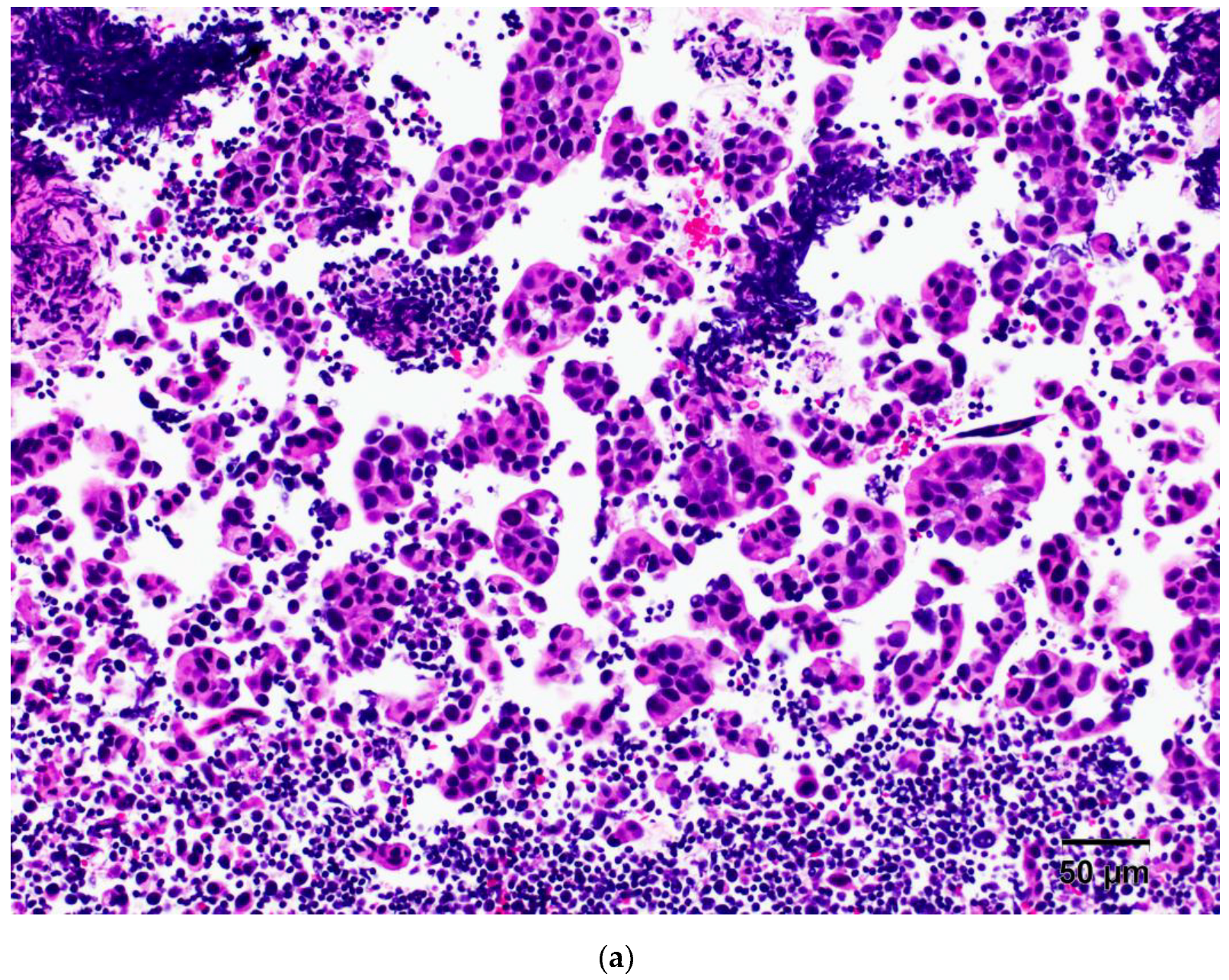
3.1. Metastatic Ductal Breast Cancer (No Special Type)
3.2. Metastatic Lobular Carcinoma
3.3. Metastatic Breast Cancer, Other Special Types
3.4. ER-Positive Metastatic Breast Cancer
3.5. HER2-Positive Metastatic Breast Cancer
3.6. Triple-Negative Metastatic Breast Cancer
3.7. Molecular Profiles in Cytology Samples and Liquid Biopsies
4. Novel Therapeutic Targets in Metastatic Breast Cancer
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Casasent, A.K.; Almekinders, M.M.; Mulder, C.; Bhattacharjee, P.; Collyar, D.; Thompson, A.M.; Jonkers, J.; Lips, E.H.; van Rheenen, J.; Hwang, E.S.; et al. Learning to distinguish progressive and non-progressive ductal carcinoma in situ. Nat. Rev. Cancer 2022, 22, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Giannakeas, V.; Sopik, V.; Narod, S.A. A comparison of two models for breast cancer mortality for women with ductal carcinoma in situ: An SEER-based analysis. Breast Cancer Res. Treat. 2018, 169, 587–594. [Google Scholar] [CrossRef]
- Hortogagyi, G.N.; Connoly, J.L.; D’Orsi, C.J.; Edge, S.B.; Mittendorf, S.A.; Rugo, H.S.; Solin, L.J.; Weaver, D.L.; Winchester, D.J.; Giuliano, A. Breast-AJCC Cancer Staging Manual, 8th ed.; American College of Surgeons: Chicago, IL, USA, 2018. [Google Scholar]
- Cardoso, F.; Spence, D.; Mertz, S.; Corneliussen-James, D.; Sabelko, K.; Gralow, J.; Cardoso, M.J.; Peccatori, F.; Paonessa, D.; Benares, A.; et al. Global analysis of advanced/metastatic breast cancer: Decade report (2005–2015). Breast 2018, 39, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Gennari, A.; Andre, F.; Barrios, C.H.; Cortes, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef]
- Ward, H.W. Anti-oestrogen therapy for breast cancer: A trial of tamoxifen at two dose levels. Br. Med. J. 1973, 1, 13–14. [Google Scholar] [CrossRef] [Green Version]
- Burstein, H.J.; Somerfield, M.R.; Barton, D.L.; Dorris, A.; Fallowfield, L.J.; Jain, D.; Johnston, S.R.D.; Korde, L.A.; Litton, J.K.; Macrae, E.R.; et al. Endocrine Treatment and Targeted Therapy for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 3959–3977. [Google Scholar] [CrossRef]
- Goss, P.E.; Ingle, J.N.; Martino, S.; Robert, N.J.; Muss, H.B.; Piccart, M.J.; Castiglione, M.; Tu, D.; Shepherd, L.E.; Pritchard, K.I.; et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N. Engl. J. Med. 2003, 349, 1793–1802. [Google Scholar] [CrossRef]
- Sgroi, D.C.; Sestak, I.; Cuzick, J.; Zhang, Y.; Schnabel, C.A.; Schroeder, B.; Erlander, M.G.; Dunbier, A.; Sidhu, K.; Lopez-Knowles, E.; et al. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: A prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol. 2013, 14, 1067–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardia, A.; Hovnanian, M.D.; Brachtel, E.F.; Nardi, V. Case 35-2018: A 68-Year-Old Woman with Back Pain and a Remote History of Breast Cancer. N. Engl. J. Med. 2018, 379, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, M.R.; Wander, S.A.; Hamilton, E.; Razavi, P.; Bardia, A. Next-generation selective estrogen receptor degraders and other novel endocrine therapies for management of metastatic hormone receptor-positive breast cancer: Current and emerging role. Ther. Adv. Med. Oncol. 2022, 14, 17588359221113694. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, Y.; Yates, M.E.; Tasdemir, N.; Bahreini, A.; Chen, J.; Levine, K.M.; Priedigkeit, N.M.; Nasrazadani, A.; Ali, S.; et al. Hotspot ESR1 Mutations Are Multimodal and Contextual Modulators of Breast Cancer Metastasis. Cancer Res. 2022, 82, 1321–1339. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spring, L.M.; Clark, S.L.; Li, T.; Goel, S.; Tayob, N.; Viscosi, E.; Abraham, E.; Juric, D.; Isakoff, S.J.; Mayer, E.; et al. Phase 1b clinical trial of ado-trastuzumab emtansine and ribociclib for HER2-positive metastatic breast cancer. NPJ Breast Cancer 2021, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Verret, B.; Bottosso, M.; Hervais, S.; Pistilli, B. The Molecular Predictive and Prognostic Biomarkers in Metastatic Breast Cancer: The Contribution of Molecular Profiling. Cancers 2022, 14, 4203. [Google Scholar] [CrossRef] [PubMed]
- Hammerl, D.; Martens, J.W.M.; Timmermans, M.; Smid, M.; Trapman-Jansen, A.M.; Foekens, R.; Isaeva, O.I.; Voorwerk, L.; Balcioglu, H.E.; Wijers, R.; et al. Spatial immunophenotypes predict response to anti-PD1 treatment and capture distinct paths of T cell evasion in triple negative breast cancer. Nat. Commun. 2021, 12, 5668. [Google Scholar] [CrossRef]
- Schmid, P.; Salgado, R.; Park, Y.H.; Munoz-Couselo, E.; Kim, S.B.; Sohn, J.; Im, S.A.; Foukakis, T.; Kuemmel, S.; Dent, R.; et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: Results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann. Oncol. 2020, 31, 569–581. [Google Scholar] [CrossRef]
- Barchiesi, G.; Roberto, M.; Verrico, M.; Vici, P.; Tomao, S.; Tomao, F. Emerging Role of PARP Inhibitors in Metastatic Triple Negative Breast Cancer. Current Scenario and Future Perspectives. Front. Oncol. 2021, 11, 769280. [Google Scholar] [CrossRef]
- Aftimos, P.; Oliveira, M.; Irrthum, A.; Fumagalli, D.; Sotiriou, C.; Gal-Yam, E.N.; Robson, M.E.; Ndozeng, J.; Di Leo, A.; Ciruelos, E.M.; et al. Genomic and Transcriptomic Analyses of Breast Cancer Primaries and Matched Metastases in AURORA, the Breast International Group (BIG) Molecular Screening Initiative. Cancer Discov. 2021, 11, 2796–2811. [Google Scholar] [CrossRef] [PubMed]
- Najjar, S.; Allison, K.H. Updates on breast biomarkers. Virchows Arch. 2022, 480, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Brachtel, E.F.; Operana, T.N.; Sullivan, P.S.; Kerr, S.E.; Cherkis, K.A.; Schroeder, B.E.; Dry, S.M.; Schnabel, C.A. Molecular classification of cancer with the 92-gene assay in cytology and limited tissue samples. Oncotarget 2016, 7, 27220–27231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beca, F.; Schmitt, F.C. Ancillary Tests in Breast Cytology: A Practical Guide. Acta Cytol. 2019, 63, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Souza da Silva, R.; Schmitt, F. Optimal assessment of metastatic breast carcinoma: The value of cytopathology combined with molecular analysis. J. Mol. Pathol. 2022, 3, 329–338. [Google Scholar] [CrossRef]
- Medeiros, B.; Allan, A.L. Molecular Mechanisms of Breast Cancer Metastasis to the Lung: Clinical and Experimental Perspectives. Int. J. Mol. Sci. 2019, 20, 2272. [Google Scholar] [CrossRef] [Green Version]
- Harper, K.L.; Sosa, M.S.; Entenberg, D.; Hosseini, H.; Cheung, J.F.; Nobre, R.; Avivar-Valderas, A.; Nagi, C.; Girnius, N.; Davis, R.J.; et al. Mechanism of early dissemination and metastasis in Her2(+) mammary cancer. Nature 2016, 540, 588–592. [Google Scholar] [CrossRef]
- Laakmann, E.; Witzel, I.; Fasching, P.A.; Rezai, M.; Schem, C.; Solbach, C.; Tesch, H.; Klare, P.; Schneeweiss, A.; Salat, C.; et al. Development of central nervous system metastases as a first site of metastatic disease in breast cancer patients treated in the neoadjuvant trials GeparQuinto and GeparSixto. Breast Cancer Res. 2019, 21, 60. [Google Scholar] [CrossRef] [Green Version]
- Vitos, N.; Gerlee, P. Model-based inference of metastatic seeding rates in de novo metastatic breast cancer reveals the impact of secondary seeding and molecular subtype. Sci. Rep. 2022, 12, 9455. [Google Scholar] [CrossRef]
- Bombonati, A.; Lerwill, M.F. Metastases to and from the Breast. Surg. Pathol. Clin. 2012, 5, 719–747. [Google Scholar] [CrossRef]
- Hui, Y.; Wang, Y.; Nam, G.; Fanion, J.; Sturtevant, A.; Lombardo, K.A.; Resnick, M.B. Differentiating breast carcinoma with signet ring features from gastrointestinal signet ring carcinoma: Assessment of immunohistochemical markers. Hum. Pathol. 2018, 77, 11–19. [Google Scholar] [CrossRef] [PubMed]
- WHO. Breast Tumours, 5th ed.; Board of Editors, WHO Classification of Tumours; International Agency for Research on Cancer: Lyon, France, 2019. [Google Scholar]
- Ni, Y.B.; Tsang, J.Y.S.; Shao, M.M.; Chan, S.K.; Cheung, S.Y.; Tong, J.; To, K.F.; Tse, G.M. GATA-3 is superior to GCDFP-15 and mammaglobin to identify primary and metastatic breast cancer. Breast Cancer Res. Treat. 2018, 169, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Tozbikian, G.H.; Zynger, D.L. A combination of GATA3 and SOX10 is useful for the diagnosis of metastatic triple-negative breast cancer. Hum. Pathol. 2019, 85, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahed, M.; Yurtsever, N.; Savant, D.; Karam, P.; Gimenez, C.; Das, K.; Sheikh-Fayyaz, S.; Khutti, S. Utility of TRPS-1 immunohistochemistry in diagnosis of metastatic breast carcinoma in cytology specimens. J. Am. Soc. Cytopathol. 2022, 11, 345–351. [Google Scholar] [CrossRef]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Guideline Update. Arch. Pathol. Lab. Med. 2020, 144, 545–563. [Google Scholar] [CrossRef] [Green Version]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef] [Green Version]
- Verret, B.; Sourisseau, T.; Stefanovska, B.; Mosele, F.; Tran-Dien, A.; Andre, F. The Influence of Cancer Molecular Subtypes and Treatment on the Mutation Spectrum in Metastatic Breast Cancers. Cancer Res. 2020, 80, 3062–3069. [Google Scholar] [CrossRef] [Green Version]
- Borst, M.J.; Ingold, J.A. Metastatic patterns of invasive lobular versus invasive ductal carcinoma of the breast. Surgery 1993, 114, 637–641, discussion 641–632. [Google Scholar]
- Bennett, J.A.; Young, R.H.; Chuang, A.Y.; Lerwill, M.F. Ovarian Metastases of Breast Cancers With Signet Ring Cells: A Report of 17 Cases Including 14 Krukenberg Tumors. Int. J. Gynecol. Pathol. 2018, 37, 507–515. [Google Scholar] [CrossRef]
- Pinto, D.; Schmitt, F.C. Immunohistochemistry Applied to Breast Cytological Material. Pathobiology 2022, 89, 343–358. [Google Scholar] [CrossRef]
- Cheng, J.; Cao, Y.; MacLeay, A.; Lennerz, J.K.; Baig, A.; Frazier, R.P.; Lee, J.; Hu, K.; Pacula, M.; Meneses, E.; et al. Clinical Validation of a Cell-Free DNA Gene Panel. J. Mol. Diagn. 2019, 21, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Fribbens, C.; Garcia Murillas, I.; Beaney, M.; Hrebien, S.; O’Leary, B.; Kilburn, L.; Howarth, K.; Epstein, M.; Green, E.; Rosenfeld, N.; et al. Tracking evolution of aromatase inhibitor resistance with circulating tumour DNA analysis in metastatic breast cancer. Ann. Oncol. 2018, 29, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Galvano, A.; Castellana, L.; Gristina, V.; La Mantia, M.; Insalaco, L.; Barraco, N.; Perez, A.; Cutaia, S.; Calo, V.; Bazan Russo, T.D.; et al. The diagnostic accuracy of PIK3CA mutations by circulating tumor DNA in breast cancer: An individual patient data meta-analysis. Ther. Adv. Med. Oncol. 2022, 14, 17588359221110162. [Google Scholar] [CrossRef] [PubMed]
- Boire, A.; Brandsma, D.; Brastianos, P.K.; Le Rhun, E.; Ahluwalia, M.; Junck, L.; Glantz, M.; Groves, M.D.; Lee, E.Q.; Lin, N.; et al. Liquid biopsy in central nervous system metastases: A RANO review and proposals for clinical applications. Neuro-Oncology 2019, 21, 571–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristofanilli, M.; Pierga, J.Y.; Reuben, J.; Rademaker, A.; Davis, A.A.; Peeters, D.J.; Fehm, T.; Nole, F.; Gisbert-Criado, R.; Mavroudis, D.; et al. The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): International expert consensus paper. Crit. Rev. Oncol. Hematol. 2019, 134, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Eigeliene, N.; Saarenheimo, J.; Jekunen, A. Potential of Liquid Biopsies for Breast Cancer Screening, Diagnosis, and Response to Treatment. Oncology 2019, 96, 115–124. [Google Scholar] [CrossRef]
- Fitzpatrick, A.; Iravani, M.; Mills, A.; Childs, L.; Alaguthurai, T.; Clifford, A.; Garcia-Murillas, I.; Van Laere, S.; Dirix, L.; Harries, M.; et al. Assessing CSF ctDNA to Improve Diagnostic Accuracy and Therapeutic Monitoring in Breast Cancer Leptomeningeal Metastasis. Clin. Cancer Res. 2022, 28, 1180–1191. [Google Scholar] [CrossRef]
- Main, S.C.; Cescon, D.W.; Bratman, S.V. Liquid biopsies to predict CDK4/6 inhibitor efficacy and resistance in breast cancer. Cancer Drug Resist. 2022, 5, 727–748. [Google Scholar] [CrossRef]
- Engelman, J.A.; Luo, J.; Cantley, L.C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006, 7, 606–619. [Google Scholar] [CrossRef]
- Andre, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Tognon, C.; Knezevich, S.R.; Huntsman, D.; Roskelley, C.D.; Melnyk, N.; Mathers, J.A.; Becker, L.; Carneiro, F.; MacPherson, N.; Horsman, D.; et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell 2002, 2, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Angus, L.; Smid, M.; Wilting, S.M.; van Riet, J.; Van Hoeck, A.; Nguyen, L.; Nik-Zainal, S.; Steenbruggen, T.G.; Tjan-Heijnen, V.C.G.; Labots, M.; et al. The genomic landscape of metastatic breast cancer highlights changes in mutation and signature frequencies. Nat. Genet. 2019, 51, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Bertucci, F.; Ng, C.K.Y.; Patsouris, A.; Droin, N.; Piscuoglio, S.; Carbuccia, N.; Soria, J.C.; Dien, A.T.; Adnani, Y.; Kamal, M.; et al. Genomic characterization of metastatic breast cancers. Nature 2019, 569, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Ribnikar, D.; Volovat, S.R.; Cardoso, F. Targeting CDK4/6 pathways and beyond in breast cancer. Breast 2019, 43, 8–17. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hrizat, A.; Brachtel, E. Metastatic Breast Cancer: Cytology Diagnosis with Implications for Treatment. J. Mol. Pathol. 2023, 4, 1-14. https://doi.org/10.3390/jmp4010001
Hrizat A, Brachtel E. Metastatic Breast Cancer: Cytology Diagnosis with Implications for Treatment. Journal of Molecular Pathology. 2023; 4(1):1-14. https://doi.org/10.3390/jmp4010001
Chicago/Turabian StyleHrizat, Alaa, and Elena Brachtel. 2023. "Metastatic Breast Cancer: Cytology Diagnosis with Implications for Treatment" Journal of Molecular Pathology 4, no. 1: 1-14. https://doi.org/10.3390/jmp4010001
APA StyleHrizat, A., & Brachtel, E. (2023). Metastatic Breast Cancer: Cytology Diagnosis with Implications for Treatment. Journal of Molecular Pathology, 4(1), 1-14. https://doi.org/10.3390/jmp4010001




