What Is New in Biomarker Testing at Diagnosis of Advanced Non-Squamous Non-Small Cell Lung Carcinoma? Implications for Cytology and Liquid Biopsy
Abstract
:1. Introduction
2. Biomarkers Assessed at Diagnosis with Cytological Samples and/or Liquid Biopsies Obtained from Advanced Non-Squamous Non-Small-Cell Lung Cancer Patients
2.1. EGFR, ALK, ROS1, BRAF, and NTRK: What Is New for the “Big Five” When Using Cytological and Blood Samples?
2.1.1. EGFR
2.1.2. ALK
2.1.3. ROS1
2.1.4. BRAF
2.1.5. NTRK, a Very Recently Recommended Biomarker, Is One of the “Big Five”
2.2. Biomarkers Just Beyond the “Big Five” in 2021
2.2.1. RET Fusions
2.2.2. MET Mutations
2.3. Biomarkers in the Starting Block That Should Come Soon
2.3.1. KRAS G12C Mutations
2.3.2. HER2 Mutations
2.4. What about Other Biomarkers of Interest?
2.4.1. NRG1 Fusions
2.4.2. SMARCA4 Mutations
2.4.3. NUT (Nuclear Protein in Testis) Rearrangements
2.4.4. Others Potential Biomarkers of Interest
3. What Are the Consequences for Cytology and Liquid Biopsy Practices?
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Malapelle, U.; Muscarella, L.A.; Pisapia, P.; Rossi, A. Targeting emerging molecular alterations in the treatment of non-small cell lung cancer: Current challenges and the way forward. Expert Opin. Investig. Drugs 2020, 29, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Pisapia, P.; Pepe, F.; Troncone, G.; Malapelle, U. Predictive biomarkers for molecular pathology in lung cancer. Biomark. Med. 2020, 14, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Lopes, A.R.; McCusker, M.G.; Garrigues, S.G.; Ricciardi, G.R.; Arensmeyer, K.E.; Scilla, K.A.; Mehra, R.; Rolfo, C. New Targets in Lung Cancer (Excluding EGFR, ALK, ROS1). Curr. Oncol. Rep. 2020, 22, 48. [Google Scholar] [CrossRef]
- Tsakonas, G.; Ekman, S. Oncogene-addicted non-small cell lung cancer and immunotherapy. J. Thorac. Dis. 2018, 10, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Y.; Yang, J.C.-H.; Yang, P.-C. Precision Management of Advanced Non–Small Cell Lung Cancer. Annu. Rev. Med. 2020, 71, 117–136. [Google Scholar] [CrossRef] [Green Version]
- Clinical Practice Guidelines on Lung Cancer. Available online: https://www.esmo.org/guidelines/lung-and-chest-tumours (accessed on 9 November 2020).
- NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/guidelines/category_1 (accessed on 10 December 2020).
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch. Pathol. Lab. Med. 2018, 142, 321–346. [Google Scholar] [CrossRef] [Green Version]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.; Barlesi, F.; Lolkema, M.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef]
- De Toma, A.; Russo, G.L.; Signorelli, D.; Pagani, F.; Randon, G.; Galli, G.; Prelaj, A.; Ferrara, R.; Proto, C.; Ganzinelli, M.; et al. Uncommon targets in non-small cell lung cancer: Everyone wants a slice of cake. Crit. Rev. Oncol. 2021, 160, 103299. [Google Scholar] [CrossRef]
- Faber, E.; Grosu, H.; Sabir, S.; Lucas, F.A.S.; Barkoh, B.A.; Bassett, R.L.; Luthra, R.; Stewart, J.; Roy-Chowdhuri, S. Adequacy of small biopsy and cytology specimens for comprehensive genomic profiling of patients with non-small-cell lung cancer to determine eligibility for immune checkpoint inhibitor and targeted therapy. J. Clin. Pathol. 2021. [Google Scholar] [CrossRef]
- Aisner, D.L.; Rumery, M.D.; Merrick, D.T.; Kondo, K.L.; Nijmeh, H.; Linderman, D.J.; Doebele, R.C.; Thomas, N.; Chesnut, P.C.; Varella-Garcia, M.; et al. Do More With Less: Tips and Techniques for Maximizing Small Biopsy and Cytology Specimens for Molecular and Ancillary Testing: The University of Colorado Experience. Arch. Pathol. Lab. Med. 2016, 140, 1206–1220. [Google Scholar] [CrossRef] [Green Version]
- Angerilli, V.; Galuppini, F.; Pagni, F.; Fusco, N.; Malapelle, U.; Fassan, M. The Role of the Pathologist in the Next-Generation Era of Tumor Molecular Characterization. Diagnostics 2021, 11, 339. [Google Scholar] [CrossRef]
- Bubendorf, L.; Lantuejoul, S.; De Langen, A.J.; Thunnissen, E. Nonsmall cell lung carcinoma: Diagnostic difficulties in small biopsies and cytological specimens. Eur. Respir. Rev. 2017, 26, 170007. [Google Scholar] [CrossRef]
- Canberk, S.; Tischler, V.; Engels, M. Current Topics and Practical Considerations of Cytology Practice in Lung Cancer: Reflexions from the Lung Symposium at the 42nd European Congress of Cytology, Malmö. Acta Cytol. 2020, 64, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Canberk, S.; Engels, M. Cytology samples and molecular biomarker testing in lung cancer—Advantages and challenges. Virchows Arch. 2021, 478, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Chaddha, U.; Hogarth, D.K.; Murgu, S. The role of endobronchial ultrasound transbronchial needle aspiration for programmed death ligand 1 testing and next generation sequencing in advanced non-small cell lung cancer. Ann. Transl. Med. 2019, 7, 351. [Google Scholar] [CrossRef]
- Coley, S.M.; Crapanzano, J.P.; Saqi, A. FNA, core biopsy, or both for the diagnosis of lung carcinoma: Obtaining sufficient tissue for a specific diagnosis and molecular testing. Cancer Cytopathol. 2015, 123, 318–326. [Google Scholar] [CrossRef]
- Danakas, A.M.; Jones, C.E.; Magguilli, M.; Lada, M.J.; Plavnicky, J.; Parajuli, S.; Wizorek, J.J.; Peyre, C.G.; Ettel, M.; Sweeney, M.; et al. Optimising rapid on-site evaluation-assisted endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal lymph nodes: The real-time cytopathology intervention process. Cytopathology 2021, 32, 318–325. [Google Scholar] [CrossRef] [PubMed]
- El Messaoudi, S.; Rolet, F.; Mouliere, F.; Thierry, A.R. Circulating cell free DNA: Preanalytical considerations. Clin. Chim. Acta 2013, 424, 222–230. [Google Scholar] [CrossRef]
- Furuya, N.; Matsumoto, S.; Kakinuma, K.; Morikawa, K.; Inoue, T.; Saji, H.; Goto, K.; Mineshita, M. Suitability of transbronchial brushing cytology specimens for next-generation sequencing in peripheral lung cancer. Cancer Sci. 2021, 112, 380–387. [Google Scholar] [CrossRef]
- Hofman, P. The challenges of evaluating predictive biomarkers using small biopsy tissue samples and liquid biopsies from non-small cell lung cancer patients. J. Thorac. Dis. 2019, 11, S57–S64. [Google Scholar] [CrossRef]
- Ilie, M.; Hofman, P. Pros: Can tissue biopsy be replaced by liquid biopsy? Transl. Lung Cancer Res. 2016, 5, 420–423. [Google Scholar] [CrossRef] [Green Version]
- Jain, D.; Allen, T.C.; Aisner, D.L.; Beasley, M.B.; Cagle, P.T.; Capelozzi, V.L.; Hariri, L.P.; Lantuejoul, S.; Miller, R.; Mino-Kenudson, M.; et al. Rapid On-Site Evaluation of Endobronchial Ultrasound–Guided Transbronchial Needle Aspirations for the Diagnosis of Lung Cancer: A Perspective From Members of the Pulmonary Pathology Society. Arch. Pathol. Lab. Med. 2017, 142, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Keppens, C.; Van Royen, M.Y.; Brysse, A.; Cotteret, S.; Høgdall, E.; Kuhlmann, T.P.; O’Sullivan, B.; Pauwels, P.; Pauwels, S.; Rot, M.; et al. Incidents in Molecular Pathology: Frequency and Causes During Routine Testing. Arch. Pathol. Lab. Med. 2021. [Google Scholar] [CrossRef]
- Leong, T.L.; Christie, M.; Kranz, S.; Pham, K.; Hsu, A.; Irving, L.B.; Asselin-Labat, M.-L.; Steinfort, D.P. Evaluating the Genomic Yield of a Single Endobronchial Ultrasound-guided Transbronchial Needle Aspiration in Lung Cancer: Meeting the Challenge of Doing More With Less. Clin. Lung Cancer 2017, 18, 467–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano, M.D.; Echeveste, J.I.; Abengozar, M.; Mejías, L.D.; Idoate-Gastearena, M.-Á.; Calvo, A.; De Andrea, C.E. Cytology Smears in the Era of Molecular Biomarkers in Non–Small Cell Lung Cancer: Doing More With Less. Arch. Pathol. Lab. Med. 2018, 142, 291–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisapia, P.; Malapelle, U.; Troncone, G. Liquid Biopsy and Lung Cancer. Acta Cytol. 2019, 63, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Pisapia, P.; Malapelle, U.; Roma, G.; Saddar, S.; Zheng, Q.; Pepe, F.; Bruzzese, D.; Vigliar, E.; Bellevicine, C.; Luthra, R.; et al. Consistency and reproducibility of next-generation sequencing in cytopathology: A second worldwide ring trial study on improved cytological molecular reference specimens. Cancer Cytopathol. 2019, 127, 285–296. [Google Scholar] [CrossRef]
- Sacher, G.A.; Komatsubara, K.M.; Oxnard, G.R. Application of Plasma Genotyping Technologies in Non–Small Cell Lung Cancer: A Practical Review. J. Thorac. Oncol. 2017, 12, 1344–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvianti, F.; Gelmini, S.; Costanza, F.; Mancini, I.; Sonnati, G.; Simi, L.; Pazzagli, M.; Pinzani, P. The pre-analytical phase of the liquid biopsy. N. Biotechnol. 2020, 55, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Roy-Chowdhuri, S.; Pisapia, P.; Salto-Tellez, M.; Savic, S.; Nacchio, M.; De Biase, D.; Tallini, G.; Troncone, G.; Schmitt, F. Invited review—next-generation sequencing: A modern tool in cytopathology. Virchows Arch. 2019, 475, 3–11. [Google Scholar] [CrossRef]
- Seto, K.; Masago, K.; Fujita, S.; Haneda, M.; Horio, Y.; Hida, T.; Kuroda, H.; Hosoda, W.; Okubo, K. Targeted RNA sequencing with touch imprint cytology samples for non-small cell lung cancer patients. Thorac. Cancer 2020, 11, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- Stoy, S.P.; Segal, J.P.; Mueller, J.; Furtado, L.V.; Vokes, E.E.; Patel, J.D.; Murgu, S. Feasibility of Endobronchial Ultrasound-guided Transbronchial Needle Aspiration Cytology Specimens for Next Generation Sequencing in Non–small-cell Lung Cancer. Clin. Lung Cancer 2018, 19, 230–238. [Google Scholar] [CrossRef]
- Troncone, G.; Roy-Chowdhuri, S. Key Issues in Molecular Cytopathology. Arch. Pathol. Lab. Med. 2018, 142, 289–290. [Google Scholar] [CrossRef]
- Xie, F.; Zheng, X.; Mao, X.; Zhao, R.; Ye, J.; Zhang, Y.; Sun, J. Next-Generation Sequencing for Genotyping of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration Samples in Lung Cancer. Ann. Thorac. Surg. 2019, 108, 219–226. [Google Scholar] [CrossRef]
- Young, K.; Santos, G.D.C.; Card, P.; Leighl, N. The role of cytology in molecular testing and personalized medicine in lung cancer: A clinical perspective. Cancer Cytopathol. 2019, 127, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Bellevicine, C.; Malapelle, U.; Vigliar, E.; Pisapia, P.; Vita, G.; Troncone, G. How to prepare cytological samples for molecular testing. J. Clin. Pathol. 2017, 70, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Gosney, J.R.; Boothman, A.-M.; Ratcliffe, M.; Kerr, K.M. Cytology for PD-L1 testing: A systematic review. Lung Cancer 2020, 141, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Hofman, P.; Heeke, S.; Alix-Panabières, C.; Pantel, K. Liquid biopsy in the era of immuno-oncology: Is it ready for prime-time use for cancer patients? Ann. Oncol. 2019, 30, 1448–1459. [Google Scholar] [CrossRef] [Green Version]
- Rolfo, C.; Cardona, A.F.; Cristofanilli, M.; Paz-Ares, L.; Diaz Mochon, J.J.; Duran, I.; Raez, L.E.; Russo, A.; Lorente, J.A.; Malapelle, U.; et al. Challenges and opportunities of cfDNA analysis implementation in clinical practice: Perspective of the International Society of Liquid Biopsy (ISLB). Crit. Rev. Oncol. Hematol. 2020, 151, 102978. [Google Scholar] [CrossRef]
- Imyanitov, E.N.; Iyevleva, A.G.; Levchenko, E.V. Molecular testing and targeted therapy for non-small cell lung cancer: Current status and perspectives. Crit. Rev. Oncol. 2021, 157, 103194. [Google Scholar] [CrossRef]
- Ilié, M.; Mazières, J.; Chamorey, E.; Heeke, S.; Benzaquen, J.; Thamphya, B.; Boutros, J.; Tiotiu, A.; Fayada, J.; Cadranel, J.; et al. Prospective Multicenter Validation of the Detection of ALK Rearrangements of Circulating Tumor Cells for Noninvasive Longitudinal Management of Patients With Advanced NSCLC. J. Thorac. Oncol. 2021, 16, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Leighl, N.B.; Page, R.D.; Raymond, V.M.; Daniel, D.B.; Divers, S.G.; Reckamp, K.L.; Villalona-Calero, M.A.; Dix, D.; Odegaard, J.I.; Lanman, R.B.; et al. Clinical Utility of Comprehensive Cell-free DNA Analysis to Identify Genomic Biomarkers in Patients with Newly Diagnosed Metastatic Non–small Cell Lung Cancer. Clin. Cancer Res. 2019, 25, 4691–4700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heeke, S.; Ilié, M.; Allegra, M.; Vallée, A.; Salacroup, C.; Tanga, V.; Hofman, V.; Rajamani, J.; Lee, M.; Ordinario, E.; et al. Abstract 5299: Detection of ALK fusion transcripts in plasma of non-small cell lung cancer patients using a novel RT-PCR based assay. Exp. Mol. Ther. 2020, 80, 5299. [Google Scholar] [CrossRef]
- Heeke, S.; Benzaquen, J.; Hofman, V.; Ilié, M.; Allegra, M.; Long-Mira, E.; Lassalle, S.; Tanga, V.; Salacroup, C.; Bonnetaud, C.; et al. Critical Assessment in Routine Clinical Practice of Liquid Biopsy for EGFR Status Testing in Non–Small-Cell Lung Cancer: A Single-Laboratory Experience (LPCE, Nice, France). Clin. Lung Cancer 2020, 21, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Ilie, M.; Juco, J.; Huang, L.; Hofman, V.; Khambata-Ford, S.; Hofman, P. Use of the 22C3 anti-programmed death-ligand 1 antibody to determine programmed death-ligand 1 expression in cytology samples obtained from non-small cell lung cancer patients. Cancer Cytopathol. 2018, 126, 264–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, D.; Nambirajan, A.; Borczuk, A.; Chen, G.; Minami, Y.; Moreira, A.L.; Motoi, N.; Papotti, M.; Rekhtman, N.; Russell, P.A.; et al. Immunocytochemistry for predictive biomarker testing in lung cancer cytology. Cancer Cytopathol. 2019, 127, 325–339. [Google Scholar] [CrossRef] [Green Version]
- Ilie, M.; Szafer-Glusman, E.; Hofman, V.; Chamorey, E.; Lalvée, S.; Selva, E.; Leroy, S.; Marquette, C.H.; Kowanetz, M.; Hedge, P.; et al. Detection of PD-L1 in circulating tumor cells and white blood cells from patients with advanced non-small-cell lung cancer. Ann. Oncol. 2018, 29, 193–199. [Google Scholar] [CrossRef]
- Guibert, N.; Jones, G.; Beeler, J.F.; Plagnol, V.; Morris, C.; Mourlanette, J.; Delaunay, M.; Keller, L.; Rouquette, I.; Favre, G.; et al. Targeted sequencing of plasma cell-free DNA to predict response to PD1 inhibitors in advanced non-small cell lung cancer. Lung Cancer 2019, 137, 1–6. [Google Scholar] [CrossRef]
- Dearden, S.; Stevens, J.; Wu, Y.-L.; Blowers, D. Mutation incidence and coincidence in non small-cell lung cancer: Meta-analyses by ethnicity and histology (mutMap). Ann. Oncol. 2013, 24, 2371–2376. [Google Scholar] [CrossRef]
- Graham, R.P.; Treece, A.L.; Lindeman, N.I.; Vasalos, P.; Shan, M.; Jennings, L.J.; Rimm, D.L. Worldwide Frequency of Commonly Detected EGFR Mutations. Arch. Pathol. Lab. Med. 2017, 142, 163–167. [Google Scholar] [CrossRef] [Green Version]
- Arrieta, O.; Ramírez-Tirado, L.-A.; Báez-Saldaña, R.; Peña-Curiel, O.; Soca-Chafre, G.; Macedo-Perez, E.-O. Different mutation profiles and clinical characteristics among Hispanic patients with non-small cell lung cancer could explain the “Hispanic paradox”. Lung Cancer 2015, 90, 161–166. [Google Scholar] [CrossRef]
- Gimbrone, N.; Sarcar, B.; Gordian, E.; Rivera, J.; Lopez, C.; Yoder, S.; Teer, J.; Welsh, E.; Chiaporri, A.; Schabath, M.; et al. Somatic Mutations and Ancestry Markers in Hispanic Lung Cancer Patients. J. Thorac. Oncol. 2017, 12, 1541. [Google Scholar] [CrossRef]
- Steuer, C.E.; Behera, M.; Berry, L.; Kim, S.; Rossi, M.; Sica, G.; Owonikoko, T.K.; Johnson, B.E.; Kris, M.G.; Bunn, P.A.; et al. Role of race in oncogenic driver prevalence and outcomes in lung adenocarcinoma: Results from the Lung Cancer Mutation Consortium. Cancer 2015, 122, 766–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araujo, L.H.; Lammers, P.E.; Matthews-Smith, V.; Eisenberg, R.; Gonzalez, A.; Schwartz, A.G.; Timmers, C.; Shilo, K.; Zhao, W.; Natarajan, T.G.; et al. Somatic Mutation Spectrum of Non–Small-Cell Lung Cancer in African Americans: A Pooled Analysis. J. Thorac. Oncol. 2015, 10, 1430–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, J.D.; Lathan, C.; Sholl, L.; Ducar, M.; Vega, M.; Sunkavalli, A.; Lin, L.; Hanna, M.; Schubert, L.; Thorner, A.; et al. Comparison of Prevalence and Types of Mutations in Lung Cancers Among Black and White Populations. JAMA Oncol. 2017, 3, 801–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lusk, C.M.; Watza, D.; Dyson, G.; Craig, D.B.; Ratliff, V.; Wenzlaff, A.S.; Lonardo, F.; Bollig-Fischer, A.; Bepler, G.; Purrington, K.S.; et al. Profiling the Mutational Landscape in Known Driver Genes and Novel Genes in African American Non–Small Cell Lung Cancer Patients. Clin. Cancer Res. 2019, 25, 4300–4308. [Google Scholar] [CrossRef] [Green Version]
- Chougule, A.; Prabhash, K.; Noronha, V.; Joshi, A.; Thavamani, A.; Chandrani, P.; Upadhyay, P.; Utture, S.; Desai, S.; Jambhekar, N.; et al. Frequency of EGFR Mutations in 907 Lung Adenocarcioma Patients of Indian Ethnicity. PLoS ONE 2013, 8, e76164. [Google Scholar] [CrossRef] [Green Version]
- Nakra, T.; Mehta, A.; Bal, A.; Nambirajan, A.; Mishra, D.; Midha, D.; Gupta, N.; Arora, N.; Gupta, P.; Gupta, P.; et al. Epidermal growth factor receptor mutation status in pulmonary adenocarcinoma: Multi-institutional data discussion at national conference of “Lung Cancer Management in Indian context”. Curr. Probl. Cancer 2020, 44, 100561. [Google Scholar] [CrossRef]
- Hofman, V.; Hofman, P. Resistances to EGFR tyrosine kinase inhibitors in lung cancer—How to routinely track them in a molecular pathology laboratory? J. Thorac. Dis. 2019, 11, S65–S70. [Google Scholar] [CrossRef]
- Heeke, S.; Hofman, V.; Benzaquen, J.; Otto, J.; Tanga, V.; Zahaf, K.; Allegra, M.; Long-Mira, E.; Lassalle, S.; Marquette, C.-H.; et al. Detection of EGFR Mutations From Plasma of NSCLC Patients Using an Automatic Cartridge-Based PCR System. Front. Pharmacol. 2021, 12, 657743. [Google Scholar] [CrossRef]
- Lassalle, S.; Hofman, V.; Heeke, S.; Benzaquen, J.; Long, E.; Poudenx, M.; Lantéri, E.; Boutros, J.; Tanga, V.; Zahaf, K.; et al. Targeted Assessment of the EGFR Status as Reflex Testing in Treatment-Naive Non-Squamous Cell Lung Carcinoma Patients: A Single Laboratory Experience (LPCE, Nice, France). Cancers 2020, 12, 955. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, R.; Ohtsuka, K.; Ogura, W.; Arai, N.; Yoshida, T.; Nakazato, Y.; Tachibana, K.; Takata, S.; Fujiwara, M.; Kamma, H.; et al. Subtyping and EGFR mutation testing from blocks of cytological materials, based on liquid-based cytology for lung cancer at bronchoscopic examinations. Diagn. Cytopathol. 2020, 48, 516–523. [Google Scholar] [CrossRef]
- Hofman, P. Next-Generation Sequencing with Liquid Biopsies from Treatment-Naïve Non-Small Cell Lung Carcinoma Patients. Cancers 2021, 13, 2049. [Google Scholar] [CrossRef]
- Foggetti, G.; Li, C.; Cai, H.; Hellyer, J.A.; Lin, W.-Y.; Ayeni, D.; Hastings, K.; Choi, J.; Wurtz, A.; Andrejka, L.; et al. Genetic determinants of EGFR-Driven Lung Cancer Growth and Therapeutic Response In Vivo. Cancer Discov. 2021. [Google Scholar] [CrossRef]
- Qiao, M.; Jiang, T.; Liu, X.; Mao, S.; Zhou, F.; Li, X.; Zhao, C.; Chen, X.; Su, C.; Ren, S.; et al. Immune checkpoint inhibitors in EGFR-mutated non-small cell lung cancer: Dusk or Dawn? J. Thorac. Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.-W.; Kato, T.; et al. Osimertinib in Resected EGFR-Mutated Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Hofman, P. ALK in Non-Small Cell Lung Cancer (NSCLC) Pathobiology, Epidemiology, Detection from Tumor Tissue and Algorithm Diagnosis in a Daily Practice. Cancers 2017, 9, 107. [Google Scholar] [CrossRef] [Green Version]
- Hofman, P. Detecting Resistance to Therapeutic ALK Inhibitors in Tumor Tissue and Liquid Biopsy Markers: An Update to a Clinical Routine Practice. Cells 2021, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Jahanzeb, M.; Lin, H.M.; Pan, X.; Yin, Y.; Baumann, P.; Langer, C.J. Immunotherapy Treatment Patterns and Outcomes Among ALK-Positive Patients With Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2021, 22, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Hofman, P. ALK Status Assessment with Liquid Biopsies of Lung Cancer Patients. Cancers 2017, 9, 106. [Google Scholar] [CrossRef] [Green Version]
- Conde, E.; Rojo, F.; Gómez, J.; Enguita, A.B.; Abdulkader, I.; González, A.; Lozano, D.; Mancheño, N.; Salas, C.; Salido, M.; et al. Molecular diagnosis in non-small-cell lung cancer: Expert opinion on ALK and ROS1 testing. J. Clin. Pathol. 2021. [Google Scholar] [CrossRef]
- Hofman, V.; Lassalle, S.; Bence, C.; Long-Mira, E.; Nahon-Estève, S.; Heeke, S.; Lespinet-Fabre, V.; Butori, C.; Ilie, M.; Hofman, P. Any Place for Immunohistochemistry within the Predictive Biomarkers of Treatment in Lung Cancer Patients? Cancers 2018, 10, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisapia, P.; Lozano, M.D.; Vigliar, E.; Bellevicine, C.; Pepe, F.; Malapelle, U.; Troncone, G. ALK and ROS1 testing on lung cancer cytologic samples: Perspectives. Cancer Cytopathol. 2017, 125, 817–830. [Google Scholar] [CrossRef] [Green Version]
- Roy-Chowdhuri, S. Immunocytochemistry of cytology specimens for predictive biomarkers in lung cancer. Transl. Lung Cancer Res. 2020, 9, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Marino, F.Z.; Rossi, G.; Cozzolino, I.; Montella, M.; Micheli, M.; Bogina, G.; Munari, E.; Brunelli, M.; Franco, R. Multiplex fluorescence in situ hybridisation to detect anaplastic lymphoma kinase and ROS proto-oncogene 1 receptor tyrosine kinase rearrangements in lung cancer cytological samples. J. Clin. Pathol. 2019, 73, 96–101. [Google Scholar] [CrossRef]
- Amemiya, K.; Hirotsu, Y.; Nagakubo, Y.; Mochizuki, H.; Higuchi, R.; Tsutsui, T.; Kakizaki, Y.; Miyashita, Y.; Oyama, T.; Omata, M. Actionable driver DNA variants and fusion genes can be detected in archived cytological specimens with the Oncomine Dx Target Test Multi-CDx system in lung cancer. Cancer Cytopathol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Aguado, C.; Giménez-Capitán, A.; Román, R.; Rodríguez, S.; Jordana-Ariza, N.; Aguilar, A.; Cabrera-Gálvez, C.; Rivas-Corredor, C.; Lianes, P.; Viteri, S.; et al. RNA-Based Multiplexing Assay for Routine Testing of Fusion and Splicing Variants in Cytological Samples of NSCLC Patients. Diagnostics 2020, 11, 15. [Google Scholar] [CrossRef]
- Alidousty, C.; Duerbaum, N.; Wagener-Ryczek, S.; Baar, T.; Martelotto, L.G.; Heydt, C.; Siemanowski, J.; Holz, B.; Binot, E.; Fassunke, J.; et al. Prevalence and potential biological role of TERT amplifications in ALK translocated adenocarcinoma of the lung. Histopathology 2021, 78, 578–585. [Google Scholar] [CrossRef]
- Drilon, A.; Jenkins, C.; Iyer, S.; Schoenfeld, A.; Keddy, C.; Davare, M.A. ROS1-dependent cancers—biology, diagnostics and therapeutics. Nat. Rev. Clin. Oncol. 2021, 18, 35–55. [Google Scholar] [CrossRef] [PubMed]
- Landi, L.; Cappuzzo, F. How selecting best upfront therapy for metastatic disease?—Focus on ROS1-rearranged disease. Transl. Lung Cancer Res. 2020, 9, 2686–2695. [Google Scholar] [CrossRef]
- Hofman, V.; Rouquette, I.; Long-Mira, E.; Piton, N.; Chamorey, E.; Heeke, S.; Vignaud, J.M.; Yguel, C.; Mazières, J.; Lepage, A.-L.; et al. Multicenter Evaluation of a Novel ROS1 Immunohistochemistry Assay (SP384) for Detection of ROS1 Rearrangements in a Large Cohort of Lung Adenocarcinoma Patients. J. Thorac. Oncol. 2019, 14, 1204–1212. [Google Scholar] [CrossRef]
- Roviello, G.; D’Angelo, A.; Sirico, M.; Pittacolo, M.; Conter, F.U.; Sobhani, N. Advances in anti-BRAF therapies for lung cancer. Investig. N. Drugs 2021, 39, 879–890. [Google Scholar] [CrossRef]
- Carneiro, J.G.; Couto, P.G.; Bastos-Rodrigues, L.; Bicalho, M.A.C.; Vidigal, P.V.; Vilhena, A.; Amaral, N.F.; Bale, A.E.; Friedman, E.; De Marco, L. Spectrum of somatic EGFR, KRAS, BRAF, PTEN mutations and TTF-1 expression in Brazilian lung cancer patients. Genet. Res. 2014, 96, 2. [Google Scholar] [CrossRef]
- Hofman, V.; Benzaquen, J.; Heeke, S.; Lassalle, S.; Poudenx, M.; Long, E.; Lantéri, E.; Bordone, O.; Lespinet, V.; Tanga, V.; et al. Real-world assessment of the BRAF status in non-squamous cell lung carcinoma using VE1 immunohistochemistry: A single laboratory experience (LPCE, Nice, France). Lung Cancer 2020, 145, 58–62. [Google Scholar] [CrossRef]
- Jain, D.; Roy-Chowdhuri, S. Molecular Pathology of Lung Cancer Cytology Specimens: A Concise Review. Arch. Pathol. Lab. Med. 2018, 142, 1127–1133. [Google Scholar] [CrossRef] [Green Version]
- Iaccarino, A.; Pisapia, P.; Pepe, F.; Sgariglia, R.; Nacchio, M.; Russo, G.; Gragnano, G.; De Luca, C.; Troncone, G.; Malapelle, U. Liquid biopsy for BRAF mutations testing in non-small cell lung cancer: A retrospective study. J. Clin. Pathol. 2020. [Google Scholar] [CrossRef]
- Amatu, A.; Sartore-Bianchi, A.; Siena, S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. ESMO Open 2016, 1, 000023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amatu, A.; Sartore-Bianchi, A.; Bencardino, K.; Pizzutilo, E.; Tosi, F.; Siena, S. Tropomyosin receptor kinase (TRK) biology and the role of NTRK gene fusions in cancer. Ann. Oncol. 2019, 30, 5–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chetty, R. Neurotrophic tropomyosin or tyrosine receptor kinase (NTRK) genes. J. Clin. Pathol. 2019, 72, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Farago, A.F.; Taylor, M.S.; Doebele, R.C.; Zhu, V.W.; Kummar, S.; Spira, A.I.; Boyle, T.A.; Haura, E.B.; Arcila, M.E.; Benayed, R.; et al. Clinicopathologic Features of Non-Small-Cell Lung Cancer Harboring an NTRK Gene Fusion. JCO Precis. Oncol. 2018, 18, 37. [Google Scholar] [CrossRef]
- Li, H.; Yan, S.; Liu, Y.; Ma, L.; Liu, X.; Liu, Y.; Cheng, Y. Analysis of NTRK mutation and clinicopathologic factors in lung cancer patients in northeast China. Int. J. Biol. Markers 2020, 35, 36–40. [Google Scholar] [CrossRef]
- Si, X.; Pan, R.; Ma, S.; Li, L.; Liang, L.; Zhang, P.; Chu, Y.; Wang, H.; Wang, M.; Zhang, X.; et al. Genomic characteristics of driver genes in Chinese patients with non-small cell lung cancer. Thorac. Cancer 2021, 12, 357–363. [Google Scholar] [CrossRef]
- Volckmar, A.-L.; Christopoulos, P.; Kirchner, M.; Allgäuer, M.; Neumann, O.; Budczies, J.; Rempel, E.; Horak, P.; Glade, J.; Goldschmid, H.; et al. Targeting rare and non-canonical driver variants in NSCLC—An uncharted clinical field. Lung Cancer 2021, 154, 131–141. [Google Scholar] [CrossRef]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Drilon, A. TRK inhibitors in TRK fusion-positive cancers. Ann. Oncol. 2019, 30, 23–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haratake, N.; Seto, T. NTRK Fusion-positive Non–small-cell Lung Cancer: The Diagnosis and Targeted Therapy. Clin. Lung Cancer 2021, 22, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.W.; Feng, F.Y. A Tumor-Agnostic NTRK (TRK) Inhibitor. Cell 2019, 177, 8. [Google Scholar] [CrossRef] [PubMed]
- Ricciuti, B.; Brambilla, M.; Metro, G.; Baglivo, S.; Matocci, R.; Pirro, M.; Chiari, R. Targeting NTRK fusion in non-small cell lung cancer: Rationale and clinical evidence. Med. Oncol. 2017, 34, 105. [Google Scholar] [CrossRef]
- Rolfo, C. NTRK gene fusions: A rough diamond ready to sparkle. Lancet Oncol. 2020, 21, 472–474. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Pizzutilo, E.G.; Marrapese, G.; Tosi, F.; Cerea, G.; Siena, S. Entrectinib for the treatment of metastatic NSCLC: Safety and efficacy. Expert Rev. Anticancer. Ther. 2020, 20, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Pentheroudakis, G.; Mishima, S.; Overman, M.; Yeh, K.-H.; Baba, E.; Naito, Y.; Calvo, F.; Saxena, A.; Chen, L.-T.; et al. JSCO—ESMO—ASCO—JSMO—TOS: International expert consensus recommendations for tumour-agnostic treatments in patients with solid tumours with microsatellite instability or NTRK fusions. Ann. Oncol. 2020, 31, 861–872. [Google Scholar] [CrossRef]
- Rozlytrek. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/rozlytrek (accessed on 10 April 2021).
- Vitrakvi. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/vitrakvi (accessed on 10 April 2021).
- Matter, M.S.; Chijioke, O.; Savic, S.; Bubendorf, L. Narrative review of molecular pathways of kinase fusions and diagnostic approaches for their detection in non-small cell lung carcinomas. Transl. Lung Cancer Res. 2020, 9, 2645–2655. [Google Scholar] [CrossRef] [PubMed]
- Conde, E.; Hernandez, S.; Sanchez, E.; Regojo, R.M.; Camacho, C.; Alonso, M.; Martinez, R.; Lopez-Rios, F. Pan-TRK Immunohistochemistry. Arch. Pathol. Lab. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Pepe, F.; Iaccarino, A.; Pisapia, P.; Righi, L.; Listì, A.; Greco, L.; Gragnano, G.; Campione, S.; De Dominicis, G.; et al. RNA-Based Assay for Next-Generation Sequencing of Clinically Relevant Gene Fusions in Non-Small Cell Lung Cancer. Cancers 2021, 13, 139. [Google Scholar] [CrossRef]
- Abi-Raad, R.; Prasad, M.L.; Adeniran, A.J.; Cai, G. Fine-needle aspiration cytomorphology of papillary thyroid carcinoma withNTRKgene rearrangement from a case series with predominantly indeterminate cytology. Cancer Cytopathol. 2020, 128, 803–811. [Google Scholar] [CrossRef]
- Hrudka, J.; Drozenová, J.; Sýba, J.; Gregová, M.; Dundr, P. Secretory carcinoma of salivary type in a lymph node presenting as a neck cyst diagnosed by cytology: A case report. Diagn. Cytopathol. 2021, 49, 1–6. [Google Scholar] [CrossRef]
- Labourier, E.; Fahey, T.J. Preoperative molecular testing in thyroid nodules with Bethesda VI cytology: Clinical experience and review of the literature. Diagn. Cytopathol. 2021, 49, 175–180. [Google Scholar] [CrossRef]
- Ramani, N.S.; Chen, H.; Broaddus, R.R.; Lazar, A.J.; Luthra, R.; Medeiros, L.J.; Patel, K.P.; Rashid, A.; Routbort, M.J.; Stewart, J.; et al. Utilization of cytology smears improves success rates of RNA-based next-generation sequencing gene fusion assays for clinically relevant predictive biomarkers. Cancer Cytopathol. 2021, 129, 374–382. [Google Scholar] [CrossRef]
- De Winne, K.; Sorber, L.; Lambin, S.; Siozopoulou, V.; Beniuga, G.; Dedeurwaerdere, F.; D’Haene, N.; Habran, L.; Libbrecht, L.; Van Huysse, J.; et al. Immunohistochemistry as a screening tool for NTRK gene fusions: Results of a first Belgian ring trial. Virchows Arch. 2021, 478, 283–291. [Google Scholar] [CrossRef]
- Elfving, H.; Broström, E.; Moens, L.N.; Almlöf, J.; Cerjan, D.; Lauter, G.; Nord, H.; Mattsson, J.S.; Ullenhag, G.J.; Strell, C.; et al. Evaluation of NTRK immunohistochemistry as a screening method for NTRK gene fusion detection in non-small cell lung cancer. Lung Cancer 2021, 151, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, I.; Ačkar, L.; Mohammadi, P.M.; Pantel, K. Liquid biopsies: Potential and challenges. Int. J. Cancer 2021, 148, 528–545. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Aisner, D.L.; Sonett, J.R.; Turk, A.T.; Weintraub, J.L.; Lindeman, N.I. Practical Considerations Relating to Routine Clinical Biomarker Testing for Non–small Cell Lung Cancer: Focus on Testing for RET Fusions. Front. Med. 2021, 7, 562480. [Google Scholar] [CrossRef]
- Hsiao, S.J.; Zehir, A.; Sireci, A.N.; Aisner, D.L. Detection of Tumor NTRK Gene Fusions to Identify Patients Who May Benefit from Tyrosine Kinase (TRK) Inhibitor Therapy. J. Mol. Diagn. 2019, 21, 553–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirchner, M.; Glade, J.; Lehmann, U.; Merkelbach-Bruse, S.; Hummel, M.; Lehmann, A.; Trautmann, M.; Kumbrink, J.; Jung, A.; Dietmaier, W.; et al. NTRK testing: First results of the QuiP-EQA scheme and a comprehensive map of NTRK fusion variants and their diagnostic coverage by targeted RNA-based NGS assays. Genes Chromosom. Cancer 2020, 59, 445–453. [Google Scholar] [CrossRef]
- Marchio’, C.; Scaltriti, M.; Ladanyi, M.; Iafrate, A.; Bibeau, F.; Dietel, M.; Hechtman, J.; Troiani, T.; López-Rios, F.; Douillard, J.-Y.; et al. ESMO recommendations on the standard methods to detect NTRK fusions in daily practice and clinical research. Ann. Oncol. 2019, 30, 1417–1427. [Google Scholar] [CrossRef] [Green Version]
- Pfarr, N.; Kirchner, M.; Lehmann, U.; Leichsenring, J.; Merkelbach-Bruse, S.; Glade, J.; Hummel, M.; Stögbauer, F.; Lehmann, A.; Trautmann, M.; et al. Testing NTRK testing: Wet-lab and in silico comparison of RNA-based targeted sequencing assays. Genes Chromosomes Cancer 2020, 59, 178–188. [Google Scholar] [CrossRef]
- Solomon, J.P.; Linkov, I.; Rosado, A.; Mullaney, K.; Rosen, E.Y.; Frosina, D.; Jungbluth, A.A.; Zehir, A.; Benayed, R.; Drilon, A.; et al. NTRK fusion detection across multiple assays and 33,997 cases: Diagnostic implications and pitfalls. Mod. Pathol. 2020, 33, 38–46. [Google Scholar] [CrossRef]
- Gautschi, O.; Bubendorf, L.; Leyvraz, S.; Menon, R.; Diebold, J. Challenges in the Diagnosis of NTRK Fusion-Positive Cancers. J. Thorac. Oncol. 2020, 15, 108–110. [Google Scholar] [CrossRef]
- Gatalica, Z.; Xiu, J.; Swensen, J.; Vranic, S. Molecular characterization of cancers with NTRK gene fusions. Mod. Pathol. 2019, 32, 147–153. [Google Scholar] [CrossRef]
- Hechtman, J.F.; Benayed, R.; Hyman, D.M.; Drilon, A.; Zehir, A.; Frosina, D.; Arcila, M.E.; Dogan, S.; Klimstra, D.S.; Ladanyi, M.; et al. Pan-Trk Immunohistochemistry Is an Efficient and Reliable Screen for the Detection of NTRK Fusions. Am. J. Surg. Pathol. 2017, 41, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.P.; Sholl, L.M. Diagnostic and Predictive Immunohistochemistry for Non–Small Cell Lung Carcinomas. Adv. Anat. Pathol. 2018, 25, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Solomon, J.P.; Hechtman, J.F. Detection of NTRK Fusions: Merits and Limitations of Current Diagnostic Platforms. Cancer Res. 2019, 79, 3163–3168. [Google Scholar] [CrossRef]
- Bruno, R.; Fontanini, G. Next Generation Sequencing for Gene Fusion Analysis in Lung Cancer: A Literature Review. Diagnostics 2020, 10, 521. [Google Scholar] [CrossRef]
- Kato, S.; Subbiah, V.; Marchlik, E.; Elkin, S.K.; Carter, J.L.; Kurzrock, R. RET Aberrations in Diverse Cancers: Next-Generation Sequencing of 4871 Patients. Clin. Cancer Res. 2017, 23, 1988–1997. [Google Scholar] [CrossRef] [Green Version]
- Platt, A.; Morten, J.; Ji, Q.; Elvin, P.; Womack, C.; Su, X.; Donald, E.; Gray, N.; Read, J.; Bigley, G.; et al. A retrospective analysis of RET translocation, gene copy number gain and expression in NSCLC patients treated with vandetanib in four randomized Phase III studies. BMC Cancer 2015, 15, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Guo, L.; Liu, Y.; Dong, L.; Yang, L.; Chen, L.; Liu, K.; Shao, Y.; Ying, J. Potential Unreliability of Uncommon ALK, ROS1, and RET Genomic Breakpoints in Predicting the Efficacy of Targeted Therapy in NSCLC. J. Thorac. Oncol. 2021, 16, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, A.; Cirenajwis, H.; Ericson-Lindquist, K.; Brunnström, H.; Reuterswärd, C.; Jönsson, M.; Ortiz-Villalón, C.; Hussein, A.; Bergman, B.; Vikström, A.; et al. A combined gene expression tool for parallel histological prediction and gene fusion detection in non-small cell lung cancer. Sci. Rep. 2019, 9, 5207. [Google Scholar] [CrossRef]
- Wang, R.; Pan, Y.; Li, C.; Zhang, H.; Garfield, D.; Li, Y.; Ye, T.; Hu, H.; Luo, X.; Li, H.; et al. Analysis of Major Known Driver Mutations and Prognosis in Resected Adenosquamous Lung Carcinomas. J. Thorac. Oncol. 2014, 9, 760–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrara, R.; Auger, N.; Auclin, E.; Besse, B. Clinical and Translational Implications of RET Rearrangements in Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 27–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Offin, M.; Guo, R.; Wu, S.L.; Sabari, J.; Land, J.D.; Ni, A.; Montecalvo, J.; Halpenny, D.F.; Buie, L.W.; Pak, T.; et al. Immunophenotype and Response to Immunotherapy of RET-Rearranged Lung Cancers. JCO Precis. Oncol. 2019, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y. Molecular diagnostic characteristics based on the next generation sequencing in lung cancer and its relationship with the expression of PD-L. Pathol. Res. Pract. 2020, 216, 152797. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, T.; Shiraishi, K.; Shimada, Y.; Ogiwara, H.; Tsuta, K.; Ichikawa, H.; Sakamoto, H.; Kato, M.; Shibata, T.; Nakano, T.; et al. Molecular Mechanisms Underlying Oncogenic RET Fusion in Lung Adenocarcinoma. J. Thorac. Oncol. 2014, 9, 622–630. [Google Scholar] [CrossRef] [Green Version]
- Belli, C.; Anand, S.; Gainor, J.F.; Penault-Llorca, F.; Subbiah, V.; Drilon, A.; Andrè, F.; Curigliano, G. Progresses Toward Precision Medicine in RET-altered Solid Tumors. Clin. Cancer Res. 2020, 26, 6102–6111. [Google Scholar] [CrossRef]
- Choudhury, N.J.; Drilon, A. Decade in review: A new era for RET-rearranged lung cancers. Transl. Lung Cancer Res. 2020, 9, 2571–2580. [Google Scholar] [CrossRef]
- Drilon, A.; Lin, J.J.; Filleron, T.; Ni, A.; Milia, J.; Bergagnini, I.; Hatzoglou, V.; Velcheti, V.; Offin, M.; Li, B.; et al. Frequency of Brain Metastases and Multikinase Inhibitor Outcomes in Patients With RET–Rearranged Lung Cancers. J. Thorac. Oncol. 2018, 13, 1595–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drilon, A.; Hu, Z.I.; Lai, G.G.Y.; Tan, D.S.W. Targeting RET-driven cancers: Lessons from evolving preclinical and clinical landscapes. Nat. Rev. Clin. Oncol. 2018, 15, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Drusbosky, L.M.; Rodriguez, E.; Dawar, R.; Ikpeazu, C.V. Therapeutic strategies in RET gene rearranged non-small cell lung cancer. J. Hematol. Oncol. 2021, 14, 50. [Google Scholar] [CrossRef]
- Jia, C.-C.; Chen, W.; Feng, Z.-L.; Liu, Z.-P. Recent developments of RET protein kinase inhibitors with diverse scaffolds as hinge binders. Futur. Med. Chem. 2021, 13, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Yang, D.; Velcheti, V.; Drilon, A.; Meric-Bernstam, F. State-of-the-Art Strategies for Targeting RET-Dependent Cancers. J. Clin. Oncol. 2020, 38, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Cote, G.J. Advances in Targeting RET-Dependent Cancers. Cancer Discov. 2020, 10, 498–505. [Google Scholar] [CrossRef] [Green Version]
- Baglivo, S.; Ludovini, V.; Moretti, R.; Bellezza, G.; Sidoni, A.; Roila, F.; Metro, G. RET Rearrangement as a Predictor of Unresponsiveness to Immunotherapy in Non-Small Cell Lung Cancer: Report of Two Cases with Review of the Literature. Oncol. Ther. 2020, 8, 333–339. [Google Scholar] [CrossRef]
- Hegde, A.; Andreev-Drakhlin, A.Y.; Roszik, J.; Huang, L.; Liu, S.; Hess, K.; Cabanillas, M.; Hu, M.I.; Busaidy, N.L.; Sherman, S.I.; et al. Responsiveness to immune checkpoint inhibitors versus other systemic therapies in RET-aberrant malignancies. ESMO Open 2020, 5, 000799. [Google Scholar] [CrossRef] [PubMed]
- Belli, C.; Penault-Llorca, F.; Ladanyi, M.; Normanno, N.; Scoazec, J.-Y.; Lacroix, L.; Reis-Filho, J.; Subbiah, V.; Gainor, J.; Endris, V.; et al. ESMO recommendations on the standard methods to detect RET fusions and mutations in daily practice and clinical research. Ann. Oncol. 2021, 32, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Yatabe, Y.; Dacic, S.; Borczuk, A.C.; Warth, A.; Russell, P.A.; Lantuejoul, S.; Beasley, M.B.; Thunnissen, E.; Pelosi, G.; Rekhtman, N.; et al. Best Practices Recommendations for Diagnostic Immunohistochemistry in Lung Cancer. J. Thorac. Oncol. 2019, 14, 377–407. [Google Scholar] [CrossRef] [Green Version]
- Alì, G.; Bruno, R.; Savino, M.; Giannini, R.; Pelliccioni, S.; Menghi, M.; Boldrini, L.; Proietti, A.; Chella, A.; Ribechini, A.; et al. Analysis of Fusion Genes by NanoString System: A Role in Lung Cytology? Arch. Pathol. Lab. Med. 2018, 142, 480–489. [Google Scholar] [CrossRef] [Green Version]
- Tan, A.C.; Seet, A.O.; Lai, G.G.; Lim, T.H.; Lim, A.S.; Tan, G.S.; Takano, A.; Tai, D.W.-M.; Tan, T.J.; Lam, J.Y.; et al. Molecular Characterization and Clinical Outcomes in RET-Rearranged NSCLC. J. Thorac. Oncol. 2020, 15, 1928–1934. [Google Scholar] [CrossRef]
- Benayed, R.; Offin, M.; Mullaney, K.; Sukhadia, P.; Rios, K.; Desmeules, P.; Ptashkin, R.; Won, H.; Chang, J.; Halpenny, D.; et al. High Yield of RNA Sequencing for Targetable Kinase Fusions in Lung Adenocarcinomas with No Mitogenic Driver Alteration Detected by DNA Sequencing and Low Tumor Mutation Burden. Clin. Cancer Res. 2019, 25, 4712–4722. [Google Scholar] [CrossRef]
- Esagian, S.M.; Grigoriadou, G.Ι.; Nikas, I.P.; Boikou, V.; Sadow, P.M.; Won, J.-K.; Economopoulos, K.P. Comparison of liquid-based to tissue-based biopsy analysis by targeted next generation sequencing in advanced non-small cell lung cancer: A comprehensive systematic review. J. Cancer Res. Clin. Oncol. 2020, 146, 2051–2066. [Google Scholar] [CrossRef]
- Supplee, J.G.; Milan, M.S.; Lim, L.P.; Potts, K.T.; Sholl, L.M.; Oxnard, G.R.; Paweletz, C.P. Sensitivity of next-generation sequencing assays detecting oncogenic fusions in plasma cell-free DNA. Lung Cancer 2019, 134, 96–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuong, H.G.; Ho, A.T.N.; Altibi, A.M.; Nakazawa, T.; Katoh, R.; Kondo, T. Clinicopathological implications of MET exon 14 mutations in non-small cell lung cancer—A systematic review and meta-analysis. Lung Cancer 2018, 123, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Paik, P.K.; Felip, E.; Veillon, R.; Sakai, H.; Cortot, A.B.; Garassino, M.C.; Mazieres, J.; Viteri, S.; Senellart, H.; Van Meerbeeck, J.; et al. Tepotinib in Non–Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N. Engl. J. Med. 2020, 383, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Seto, T.; Han, J.-Y.; Reguart, N.; Garon, E.B.; Groen, H.J.; Tan, D.S.; Hida, T.; de Jonge, M.; Orlov, S.V.; et al. Capmatinib inMETExon 14–Mutated orMET-Amplified Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 944–957. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Smit, E.F.; Bauer, T.M. Capmatinib for patients with non-small cell lung cancer with MET exon 14 skipping mutations: A review of preclinical and clinical studies. Cancer Treat. Rev. 2021, 95, 102173. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Zhang, J.; Heymach, J.V.; Le, X. Current and future treatment options for MET exon 14 skipping alterations in non-small cell lung cancer. Ther. Adv. Med. Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Guibert, N.; Ilie, M.; Long, E.; Hofman, V.; Bouhlel, L.; Brest, P.; Mograbi, B.; Marquette, C.H.; Didier, A.; Mazieres, J.; et al. KRAS Mutations in Lung Adenocarcinoma: Molecular and Epidemiological Characteristics, Methods for Detection, and Therapeutic Strategy Perspectives. Curr. Mol. Med. 2015, 15, 418–432. [Google Scholar] [CrossRef]
- McQuitty, E.; Zhang, W.; Hendrickson, H.; Tio, F.O.; Jagirdar, J.; Olsen, R.; Cagle, P.T. Lung Adenocarcinoma Biomarker Incidence in Hispanic Versus Non-Hispanic White Patients. Arch. Pathol. Lab. Med. 2013, 138, 390–394. [Google Scholar] [CrossRef] [PubMed]
- El Osta, B.; Behera, M.; Kim, S.; Berry, L.D.; Sica, G.; Pillai, R.N.; Owonikoko, T.K.; Kris, M.G.; Johnson, B.E.; Kwiatkowski, D.J.; et al. Characteristics and Outcomes of Patients With Metastatic KRAS-Mutant Lung Adenocarcinomas: The Lung Cancer Mutation Consortium Experience. J. Thorac. Oncol. 2019, 14, 876–889. [Google Scholar] [CrossRef]
- Griesinger, F.; Eberhardt, W.; Nusch, A.; Reiser, M.; Zahn, M.O.; Maintz, C.; Bernhardt, C.; Losem, C.; Stenzinger, A.; Heukamp, L.C.; et al. Biomarker testing in non-small cell lung cancer in routine care: Analysis of the first 3,717 patients in the German prospective, observational, nation-wide CRISP Registry (AIO-TRK-0315). Lung Cancer 2021, 152, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-Y.; Sun, H.; Zhou, J.-Y.; Jie, G.-L.; Xie, Z.; Shao, Y.; Zhang, X.; Ye, J.-Y.; Chen, C.-X.; Zhang, X.-C.; et al. Clinical characteristics and prognostic value of the KRAS G12C mutation in Chinese non-small cell lung cancer patients. Biomark. Res. 2020, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Barlesi, F.; Mazieres, J.; Merlio, J.-P.; Debieuvre, D.; Mosser, J.; Lena, H.; Ouafik, L.H.; Besse, B.; Rouquette, I.; Westeel, V.; et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: Results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016, 387, 1415–1426. [Google Scholar] [CrossRef]
- Nadal, E.; Chen, G.; Prensner, J.R.; Shiratsuchi, H.; Sam, C.; Zhao, L.; Kalemkerian, G.P.; Brenner, D.; Lin, J.; Reddy, R.M.; et al. KRAS-G12C Mutation Is Associated with Poor Outcome in Surgically Resected Lung Adenocarcinoma. J. Thorac. Oncol. 2014, 9, 1513–1522. [Google Scholar] [CrossRef] [Green Version]
- Gao, G.; Liao, W.; Ma, Q.; Zhang, B.; Chen, Y.; Wang, Y. KRAS G12D mutation predicts lower TMB and drives immune suppression in lung adenocarcinoma. Lung Cancer 2020, 149, 41–45. [Google Scholar] [CrossRef]
- Lindsay, C.; Jamal-Hanjani, M.; Forster, M.; Blackhall, F. KRAS: Reasons for optimism in lung cancer. Eur. J. Cancer 2018, 99, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Arbour, K.C.; Rizvi, H.; Plodkowski, A.J.; Hellmann, M.D.; Knezevic, A.; Heller, G.; Yu, H.A.; Ladanyi, M.; Kris, M.G.; Arcila, M.E.; et al. Treatment Outcomes and Clinical Characteristics of Patients with KRAS-G12C–Mutant Non–Small Cell Lung Cancer. Clin. Cancer Res. 2021, 27, 2209–2215. [Google Scholar] [CrossRef]
- Aredo, J.V.; Padda, S.K. Management of KRAS-Mutant Non-Small Cell Lung Cancer in the Era of Precision Medicine. Curr. Treat. Options Oncol. 2018, 19, 43. [Google Scholar] [CrossRef]
- Burns, T.F.; Borghaei, H.; Ramalingam, S.S.; Mok, T.S.; Peters, S. Targeting KRAS-Mutant Non–Small-Cell Lung Cancer: One Mutation at a Time, With a Focus on KRAS G12C Mutations. J. Clin. Oncol. 2020, 38, 4208–4218. [Google Scholar] [CrossRef] [PubMed]
- Cagir, A.; Azmi, A.S. KRASG12C inhibitors on the horizon. Futur. Med. Chem. 2019, 11, 923–925. [Google Scholar] [CrossRef]
- Canon, J.; Rex, K.; Saiki, A.Y.; Mohr, C.; Cooke, K.; Bagal, D.; Gaida, K.; Holt, T.; Knutson, C.G.; Koppada, N.; et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 2019, 575, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.G.; Olson, P.; Briere, T.; Wiel, C.; Bergo, M.O. Targeting Kras g12c -mutant cancer with a mutation-specific inhibitor. J. Intern. Med. 2020, 288, 183–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elez, F.E.; Tabernero, J. The Effective Targeting of KRASG12C Elusiveness. Cancer Cell 2020, 38, 785–787. [Google Scholar] [CrossRef] [PubMed]
- Fedele, C.; Li, S.; Teng, K.W.; Foster, C.J.; Peng, D.; Ran, H.; Mita, P.; Geer, M.J.; Hattori, T.; Koide, A.; et al. SHP2 inhibition diminishes KRASG12C cycling and promotes tumor microenvironment remodeling. J. Exp. Med. 2021, 218, 20201414. [Google Scholar] [CrossRef]
- Ferrer, I.; Zugazagoitia, J.; Herbertz, S.; John, W.; Paz-Ares, L.; Schmid-Bindert, G. KRAS-Mutant non-small cell lung cancer: From biology to therapy. Lung Cancer 2018, 124, 53–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghimessy, A.; Radeczky, P.; Laszlo, V.; Hegedus, B.; Renyi-Vamos, F.; Fillinger, J.; Klepetko, W.; Lang, C.; Dome, B.; Megyesfalvi, Z. Current therapy of KRAS-mutant lung cancer. Cancer Metastasis Rev. 2020, 39, 1159–1177. [Google Scholar] [CrossRef]
- Hallin, J.; Engstrom, L.D.; Hargis, L.; Calinisan, A.; Aranda, R.; Briere, D.M.; Sudhakar, N.; Bowcut, V.; Baer, B.R.; Ballard, J.A.; et al. The KRAS(G12C) Inhibitor MRTX849 Provides Insight toward Therapeutic Susceptibility of KRAS-Mutant Cancers in Mouse Models and Patients. Cancer Discov. 2020, 10, 54–71. [Google Scholar] [CrossRef] [Green Version]
- Hong, D.S.; Fakih, M.G.; Strickler, J.H.; Desai, J.; Durm, G.A.; Shapiro, G.I.; Falchook, G.S.; Price, T.J.; Sacher, A.; Denlinger, C.S.; et al. KRASG12C Inhibition with Sotorasib in Advanced Solid Tumors. N. Engl. J. Med. 2020, 383, 1207–1217. [Google Scholar] [CrossRef]
- Kettle, J.G.; Cassar, D.J. Covalent inhibitors of the GTPase KRASG12C: A review of the patent literature. Expert Opin. Ther. Pat. 2020, 30, 103–120. [Google Scholar] [CrossRef]
- Kim, D.; Xue, J.Y.; Lito, P. Targeting KRAS(G12C): From Inhibitory Mechanism to Modulation of Antitumor Effects in Patients. Cell 2020, 183, 850–859. [Google Scholar] [CrossRef]
- Klempner, S.J.; Hata, A.N. Can the Help Match the Hype? KRASG12C-Specific Inhibitors and Beyond. Cancer Discov. 2020, 10, 20–22. [Google Scholar] [CrossRef]
- Janes, M.R.; Zhang, J.; Li, L.-S.; Hansen, R.; Peters, U.; Guo, X.; Chen, Y.; Babbar, A.; Firdaus, S.J.; Darjania, L.; et al. Targeting KRAS Mutant Cancers with a Covalent G12C-Specific Inhibitor. Cell 2018, 172, 578–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanman, B.A.; Allen, J.R.; Allen, J.G.; Amegadzie, A.K.; Ashton, K.S.; Booker, S.K.; Chen, J.J.; Chen, N.; Frohn, M.J.; Goodman, G.; et al. Discovery of a Covalent Inhibitor of KRASG12C (AMG 510) for the Treatment of Solid Tumors. J. Med. Chem. 2019, 63, 52–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, A.R.; Rosenberg, S.C.; McCormick, F.; Malek, S. RAS-targeted therapies: Is the undruggable drugged? Nat. Rev. Drug Discov. 2020, 19, 533–552. [Google Scholar] [CrossRef]
- Nagasaka, M.; Li, Y.; Sukari, A.; Ou, S.-H.I.; Al-Hallak, M.N.; Azmi, A.S. KRAS G12C Game of Thrones, which direct KRAS inhibitor will claim the iron throne? Cancer Treat. Rev. 2020, 84, 101974. [Google Scholar] [CrossRef] [PubMed]
- Passiglia, F.; Malapelle, U.; Del Re, M.; Righi, L.; Pagni, F.; Furlan, D.; Danesi, R.; Troncone, G.; Novello, S. KRAS inhibition in non–small cell lung cancer: Past failures, new findings and upcoming challenges. Eur. J. Cancer 2020, 137, 57–68. [Google Scholar] [CrossRef]
- Seton-Rogers, S. KRAS-G12C in the crosshairs. Nat. Rev. Cancer 2019, 20, 3. [Google Scholar] [CrossRef]
- Salgia, R.; Pharaon, R.; Mambetsariev, I.; Nam, A.; Sattler, M. The improbable targeted therapy: KRAS as an emerging target in non-small cell lung cancer (NSCLC). Cell Rep. Med. 2021, 2, 100186. [Google Scholar] [CrossRef]
- Thein, K.Z.; Biter, A.B.; Hong, D.S. Therapeutics Targeting Mutant KRAS. Annu. Rev. Med. 2021, 72, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Uprety, D.; Adjei, A.A. KRAS: From undruggable to a druggable Cancer Target. Cancer Treat. Rev. 2020, 89, 102070. [Google Scholar] [CrossRef]
- Xu, K.; Park, D.; Magis, A.T.; Zhang, J.; Zhou, W.; Sica, G.L.; Ramalingam, S.S.; Curran, W.J.; Deng, X. Small Molecule KRAS Agonist for Mutant KRAS Cancer Therapy. Mol. Cancer 2019, 18, 85. [Google Scholar] [CrossRef] [Green Version]
- Jones, G.D.; Caso, R.; Tan, K.S.; Mastrogiacomo, B.; Sanchez-Vega, F.; Liu, Y.; Connolly, J.G.; Murciano-Goroff, Y.R.; Bott, M.J.; Adusumilli, P.S.; et al. KRASG12C Mutation Is Associated with Increased Risk of Recurrence in Surgically Resected Lung Adenocarcinoma. Clin. Cancer Res. 2021, 27, 2604–2612. [Google Scholar] [CrossRef]
- Nacchio, M.; Sgariglia, R.; Gristina, V.; Pisapia, P.; Pepe, F.; De Luca, C.; Migliatico, I.; Clery, E.; Greco, L.; Vigliar, E.; et al. KRAS mutations testing in non-small cell lung cancer: The role of Liquid biopsy in the basal setting. J. Thorac. Dis. 2020, 12, 3836–3843. [Google Scholar] [CrossRef]
- Bauml, J.; Levy, B. Clonal Hematopoiesis: A New Layer in the Liquid Biopsy Story in Lung Cancer. Clin. Cancer Res. 2018, 24, 4352–4354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, H.T.; Nagayama, S.; Chin, Y.M.; Otaki, M.; Hayashi, R.; Kiyotani, K.; Fukunaga, Y.; Ueno, M.; Nakamura, Y.; Low, S. Clinical significance of clonal hematopoiesis in the interpretation of blood liquid biopsy. Mol. Oncol. 2020, 14, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ulrich, B.C.; Supplee, J.; Kuang, Y.; Lizotte, P.H.; Feeney, N.B.; Guibert, N.M.; Awad, M.M.; Wong, K.K.; Jänne, P.A.; et al. False-Positive Plasma Genotyping Due to Clonal Hematopoiesis. Clin. Cancer Res. 2018, 24, 4437–4443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shlush, L.I. Age-related clonal hematopoiesis. Blood 2018, 131, 496–504. [Google Scholar] [CrossRef] [Green Version]
- Holmes, M.; Mahar, A.; Lum, T.; Kao, S.; Cooper, W.A. Real-world programmed death-ligand 1 prevalence rates in non-small cell lung cancer: Correlation with clinicopathological features and tumour mutation status. J. Clin. Pathol. 2021, 74, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Jeanson, A.; Tomasini, P.; Souquet-Bressand, M.; Brandone, N.; Boucekine, M.; Grangeon, M.; Chaleat, S.; Khobta, N.; Milia, J.; Mhanna, L.; et al. Efficacy of Immune Checkpoint Inhibitors in KRAS-Mutant Non-Small Cell Lung Cancer (NSCLC). J. Thorac. Oncol. 2019, 14, 1095–1101. [Google Scholar] [CrossRef]
- Lamberti, G.; Spurr, L.F.; Li, Y.; Ricciuti, B.; Recondo, G.; Umeton, R.; Nishino, M.; Sholl, L.M.; Meyerson, M.L.; Cherniack, A.D.; et al. Clinicopathological and genomic correlates of programmed cell death ligand 1 (PD-L1) expression in nonsquamous non-small-cell lung cancer. Ann. Oncol. 2020, 31, 807–814. [Google Scholar] [CrossRef]
- Adderley, H.; Blackhall, F.H.; Lindsay, C.R. KRAS-mutant non-small cell lung cancer: Converging small molecules and immune checkpoint inhibition. EBioMedicine 2019, 41, 711–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Gao, G.F.; Minna, J.D.; Williams, N.S.; Westover, K.D. Loss of wild type KRAS in KRAS lung adenocarcinoma is associated with cancer mortality and confers sensitivity to FASN inhibitors. Lung Cancer 2021, 153, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Molina-Arcas, M.; Moore, C.; Rana, S.; van Maldegem, F.; Mugarza, E.; Romero-Clavijo, P.; Herbert, E.; Horswell, S.; Li, L.-S.; Janes, M.R.; et al. Development of combination therapies to maximize the impact of KRAS-G12C inhibitors in lung cancer. Sci. Transl. Med. 2019, 11, 7999. [Google Scholar] [CrossRef]
- Xia, M.; Li, X.; Diao, Y.; Du, B.; Li, Y. Targeted inhibition of glutamine metabolism enhances the antitumor effect of selumetinib in KRAS-mutant NSCLC. Transl. Oncol. 2021, 14, 100920. [Google Scholar] [CrossRef] [PubMed]
- Amanam, I.; Mambetsariev, I.; Gupta, R.; Achuthan, S.; Wang, Y.; Pharaon, R.; Massarelli, E.; Koczywas, M.; Reckamp, K.; Salgia, R. Role of immunotherapy and co-mutations on KRAS-mutant non- small cell lung cancer survival. J. Thorac. Dis. 2020, 12, 5086–5095. [Google Scholar] [CrossRef]
- Arbour, K.C.; Jordan, E.; Kim, H.R.; Dienstag, J.; Yu, H.A.; Sanchez-Vega, F.; Lito, P.; Berger, M.; Solit, D.B.; Hellmann, M.; et al. Effects of Co-occurring Genomic Alterations on Outcomes in Patients with KRAS-Mutant Non–Small Cell Lung Cancer. Clin. Cancer Res. 2018, 24, 334–340. [Google Scholar] [CrossRef] [Green Version]
- Aredo, J.V.; Padda, S.K.; Kunder, C.A.; Han, S.S.; Neal, J.W.; Shrager, J.B.; Wakelee, H.A. Impact of KRAS mutation subtype and concurrent pathogenic mutations on non-small cell lung cancer outcomes. Lung Cancer 2019, 133, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Bange, E.; Marmarelis, M.E.; Hwang, W.-T.; Yang, Y.-X.; Thompson, J.C.; Rosenbaum, J.; Bauml, J.M.; Ciunci, C.; Alley, E.W.; Cohen, R.B.; et al. Impact of KRAS and TP53 Co-Mutations on Outcomes After First-Line Systemic Therapy Among Patients With STK11-Mutated Advanced Non–Small-Cell Lung Cancer. JCO Precis. Oncol. 2019, 3, 1–11. [Google Scholar] [CrossRef]
- Biton, J.; Mansuet-Lupo, A.; Pécuchet, N.; Alifano, M.; Ouakrim, H.; Arrondeau, J.; Boudou-Rouquette, P.; Goldwasser, F.; Leroy, K.; Goc, J.; et al. TP53, STK11, and EGFR Mutations Predict Tumor Immune Profile and the Response to Anti–PD-1 in Lung Adenocarcinoma. Clin. Cancer Res. 2018, 24, 5710–5723. [Google Scholar] [CrossRef] [Green Version]
- Cai, D.; Hu, C.; Li, L.; Deng, S.; Yang, J.; Han-Zhang, H.; Li, M. The prevalence and prognostic value of KRAS co-mutation subtypes in Chinese advanced non-small cell lung cancer patients. Cancer Med. 2019, 9, 84–93. [Google Scholar] [CrossRef]
- La Fleur, L.; Falk-Sörqvist, E.; Smeds, P.; Berglund, A.; Sundström, M.; Mattsson, J.S.; Brandén, E.; Koyi, H.; Isaksson, J.; Brunnström, H.; et al. Mutation patterns in a population-based non-small cell lung cancer cohort and prognostic impact of concomitant mutations in KRAS and TP53 or STK. Lung Cancer 2019, 130, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Skoulidis, F.; Byers, L.A.; Diao, L.; Papadimitrakopoulou, V.A.; Tong, P.; Izzo, J.G.; Behrens, C.; Kadara, H.; Parra, E.R.; Canales, J.R.; et al. Co-occurring Genomic Alterations Define Major Subsets of KRAS-Mutant Lung Adenocarcinoma with Distinct Biology, Immune Profiles, and Therapeutic Vulnerabilities. Cancer Discov. 2015, 5, 860–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheffler, M.; Ihle, M.A.; Hein, R.; Merkelbach-Bruse, S.; Scheel, A.H.; Siemanowski, J.; Brägelmann, J.; Kron, A.; Abedpour, N.; Ueckeroth, F.; et al. K-ras Mutation Subtypes in NSCLC and Associated Co-occuring Mutations in Other Oncogenic Pathways. J. Thorac. Oncol. 2019, 14, 606–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skoulidis, F.; Heymach, J.V. Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nat. Rev. Cancer 2019, 19, 495–509. [Google Scholar] [CrossRef]
- Skoulidis, F.; Goldberg, M.E.; Greenawalt, D.M.; Hellmann, M.D.; Awad, M.M.; Gainor, J.F.; Schrock, A.B.; Hartmaier, R.J.; Trabucco, S.E.; Gay, L.; et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer Discov. 2018, 8, 822–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibert, J.; Clavé, S.; Hardy-Werbin, M.; Taus, Á.; Rocha, P.; Longarón, R.; Piquer, G.; Chaib, I.; Carcereny, E.; Morán, T.; et al. Concomitant genomic alterations in KRAS mutant advanced lung adenocarcinoma. Lung Cancer 2020, 140, 42–45. [Google Scholar] [CrossRef]
- Domingues, I.; Cedres, S.; Callejo, A.; Vivancos, A.; Martinez-Marti, A.; Felip, E. Long duration of immunotherapy in a STK11 mutated/KRAS wild-type non-small cell lung cancer patient. Pulmonology 2020, 26, 49–50. [Google Scholar] [CrossRef] [PubMed]
- Nadal, E.; Heeke, S.; Benzaquen, J.; Vilariño, N.; Navarro, A.; Azuara, D.; Varela, M.; Otto, J.; Baixeras, N.; Shahbazian, D.; et al. Two Patients With Advanced-Stage Lung Adenocarcinoma With Radiologic Complete Response to Nivolumab Treatment Harboring an STK11/LKB1 Mutation. JCO Precis. Oncol. 2020, 4, 1239–1245. [Google Scholar] [CrossRef]
- Adachi, Y.; Ito, K.; Hayashi, Y.; Kimura, R.; Tan, T.Z.; Yamaguchi, R.; Ebi, H. Epithelial-to-Mesenchymal Transition is a Cause of Both Intrinsic and Acquired Resistance to KRAS G12C Inhibitor in KRAS G12C–Mutant Non–Small Cell Lung Cancer. Clin. Cancer Res. 2020, 26, 5962–5973. [Google Scholar] [CrossRef]
- Cannataro, V.L.; Gaffney, S.G.; Stender, C.; Zhao, Z.-M.; Philips, M.; Greenstein, A.E.; Townsend, J.P. Heterogeneity and mutation in KRAS and associated oncogenes: Evaluating the potential for the evolution of resistance to targeting of KRAS G12C. Oncogene 2018, 37, 2444–2455. [Google Scholar] [CrossRef] [PubMed]
- Dunnett-Kane, V.; Nicola, P.; Blackhall, F.; Lindsay, C. Mechanisms of Resistance to KRASG12C Inhibitors. Cancers 2021, 13, 151. [Google Scholar] [CrossRef]
- Hata, A.N.; Shaw, A.T. Resistance looms for KRASG12C inhibitors. Nat. Med. 2020, 26, 169–170. [Google Scholar] [CrossRef] [PubMed]
- Yaeger, R.; Solit, D.B. Overcoming Adaptive Resistance to KRAS Inhibitors Through Vertical Pathway Targeting. Clin. Cancer Res. 2020, 26, 1538–1540. [Google Scholar] [CrossRef] [Green Version]
- Mehta, A.; Nathany, S.; Tripathi, R.; Sharma, S.K.; Saifi, M.; Batra, U. Non-amplification genetic alterations ofHER2gene in non-small cell lung carcinoma. J. Clin. Pathol. 2021, 74, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Landi, L.; Cappuzzo, F. HER2 and lung cancer. Expert Rev. Anticancer. Ther. 2013, 13, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Mazières, J.; Peters, S.; Lepage, B.; Cortot, A.; Barlesi, F.; Beau-Faller, M.; Besse, B.; Blons, H.; Mansuet-Lupo, A.; Urban, T.; et al. Lung Cancer That Harbors an HER2 Mutation: Epidemiologic Characteristics and Therapeutic Perspectives. J. Clin. Oncol. 2013, 31, 1997–2003. [Google Scholar] [CrossRef] [Green Version]
- Fois, S.S.; Paliogiannis, P.; Zinellu, A.; Fois, A.G.; Cossu, A.; Palmieri, G. Molecular Epidemiology of the Main Druggable Genetic Alterations in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 612. [Google Scholar] [CrossRef]
- Xu, F.; Yang, G.; Xu, H.; Yang, L.; Qiu, W.; Wang, Y. Treatment outcome and clinical characteristics of HER2 mutated advanced non-small cell lung cancer patients in China. Thorac. Cancer 2020, 11, 679–685. [Google Scholar] [CrossRef]
- Offin, M.; Feldman, D.; Ni, A.; Myers, M.L.; Lai, W.V.; Pentsova, E.; Boire, A.; Daras, M.; Jordan, E.J.; Solit, D.B.; et al. Frequency and outcomes of brain metastases in patients with HER2 -mutant lung cancers. Cancer 2019, 125, 4380–4387. [Google Scholar] [CrossRef]
- Baraibar, I.; Mezquita, L.; Gil-Bazo, I.; Planchard, D. Novel drugs targeting EGFR and HER2 exon 20 mutations in metastatic NSCLC. Crit. Rev. Oncol. 2020, 148, 102906. [Google Scholar] [CrossRef]
- Del Re, M.; Cucchiara, F.; Petrini, I.; Fogli, S.; Passaro, A.; Crucitta, S.; Attili, I.; De Marinis, F.; Chella, A.; Danesi, R. erbB in NSCLC as a molecular target: Current evidences and future directions. ESMO Open 2020, 5, 724. [Google Scholar] [CrossRef]
- Ekman, S. HER2: Defining a Neu target in non-small-cell lung cancer. Ann. Oncol. 2019, 30, 353–355. [Google Scholar] [CrossRef]
- Horvath, L.; Pircher, A. ASCO 2020 non-small lung cancer (NSCLC) personal highlights. Memo. Mag. Eur. Med. Oncol. 2021, 14, 66–69. [Google Scholar] [CrossRef]
- Koga, T.; Kobayashi, Y.; Tomizawa, K.; Suda, K.; Kosaka, T.; Sesumi, Y.; Fujino, T.; Nishino, M.; Ohara, S.; Chiba, M.; et al. Activity of a novel HER2 inhibitor, poziotinib, for HER2 exon 20 mutations in lung cancer and mechanism of acquired resistance: An in vitro study. Lung Cancer 2018, 126, 72–79. [Google Scholar] [CrossRef]
- Liu, S.; Li, S.; Hai, J.; Wang, X.; Chen, T.; Quinn, M.M.; Gao, P.; Zhang, Y.; Ji, H.; Cross, D.A.; et al. Targeting HER2 Aberrations in Non–Small Cell Lung Cancer with Osimertinib. Clin. Cancer Res. 2018, 24, 2594–2604. [Google Scholar] [CrossRef] [Green Version]
- Oh, D.-Y.; Bang, Y.-J. HER2-targeted therapies—a role beyond breast cancer. Nat. Rev. Clin. Oncol. 2020, 17, 33–48. [Google Scholar] [CrossRef]
- Patil, T.; Mushtaq, R.; Marsh, S.; Azelby, C.; Pujara, M.; Davies, K.D.; Aisner, D.L.; Purcell, W.T.; Schenk, E.L.; Pacheco, J.M.; et al. Clinicopathologic Characteristics, Treatment Outcomes, and Acquired Resistance Patterns of Atypical EGFR Mutations and HER2 Alterations in Stage IV Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2020, 21, 191–204. [Google Scholar] [CrossRef]
- Pillai, R.N.; Behera, M.; Berry, L.D.; Rossi, M.R.; Kris, M.G.; Johnson, B.E.; Bunn, P.A.; Ramalingam, S.S.; Khuri, F.R. HER2 mutations in lung adenocarcinomas: A report from the Lung Cancer Mutation Consortium. Cancer 2017, 123, 4099–4105. [Google Scholar] [CrossRef] [Green Version]
- Hotta, K.; Yanai, H.; Ohashi, K.; Ninomiya, K.; Nakashima, H.; Kayatani, H.; Takata, M.; Kiura, K. Pilot evaluation of a HER2 testing in non-small-cell lung cancer. J. Clin. Pathol. 2019, 73, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Li, B.T.; Ross, D.S.; Aisner, D.L.; Chaft, J.E.; Hsu, M.; Kako, S.L.; Kris, M.G.; Varella-Garcia, M.; Arcila, M.E. HER2 Amplification and HER2 Mutation Are Distinct Molecular Targets in Lung Cancers. J. Thorac. Oncol. 2016, 11, 414–419. [Google Scholar] [CrossRef] [Green Version]
- Cadranel, J.; Liu, S.V.; Duruisseaux, M.; Branden, E.; Goto, Y.; Weinberg, B.A.; Heining, C.; Schlenk, R.F.; Cheema, P.; Jones, M.R.; et al. Therapeutic Potential of Afatinib in NRG1 Fusion-Driven Solid Tumors: A Case Series. Oncologist 2021, 26, 7–16. [Google Scholar] [CrossRef]
- Fernandez-Cuesta, L.; Thomas, R.K. Molecular Pathways: Targeting NRG1 Fusions in Lung Cancer. Clin. Cancer Res. 2015, 21, 1989–1994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laskin, J.; Liu, S.; Tolba, K.; Heining, C.; Schlenk, R.; Cheema, P.; Cadranel, J.; Jones, M.; Drilon, A.; Cseh, A.; et al. NRG1 fusion-driven tumors: Biology, detection, and the therapeutic role of afatinib and other ErbB-targeting agents. Ann. Oncol. 2020, 31, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Somwar, R.; Mangatt, B.P.; Edgren, H.; Desmeules, P.; Ruusulehto, A.; Smith, R.S.; Delasos, L.; Vojnic, M.; Plodkowski, A.J.; et al. Response to ERBB3-Directed Targeted Therapy in NRG1-Rearranged Cancers. Cancer Discov. 2018, 8, 686–695. [Google Scholar] [CrossRef] [Green Version]
- Duruisseaux, M.; McLeer-Florin, A.; Antoine, M.; Alavizadeh, S.; Poulot, V.; Lacave, R.; Rabbe, N.; Cadranel, J.; Wislez, M. NRG1 fusion in a French cohort of invasive mucinous lung adenocarcinoma. Cancer Med. 2016, 5, 3579–3585. [Google Scholar] [CrossRef]
- Agaimy, A.; Fuchs, F.; Moskalev, E.A.; Sirbu, H.; Hartmann, A.; Haller, F. SMARCA4-deficient pulmonary adenocarcinoma: Clinicopathological, immunohistochemical, and molecular characteristics of a novel aggressive neoplasm with a consistent TTF1neg/CK7pos/HepPar-1pos immunophenotype. Virchows Arch. 2017, 471, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Agaimy, A.; Daum, O.; Michal, M.; Schmidt, M.W.; Stoehr, R.; Hartmann, A.; Lauwers, G.Y. Undifferentiated large cell/rhabdoid carcinoma presenting in the intestines of patients with concurrent or recent non-small cell lung cancer (NSCLC): Clinicopathologic and molecular analysis of 14 cases indicates an unusual pattern of dedifferentiated metastases. Virchows Arch. 2021. [Google Scholar] [CrossRef]
- Bell, E.H.; Chakraborty, A.R.; Mo, X.; Liu, Z.; Shilo, K.; Kirste, S.; Stegmaier, P.; McNulty, M.; Karachaliou, N.; Rosell, R.; et al. SMARCA4/BRG1 Is a Novel Prognostic Biomarker Predictive of Cisplatin-Based Chemotherapy Outcomes in Resected Non–Small Cell Lung Cancer. Clin. Cancer Res. 2016, 22, 2396–2404. [Google Scholar] [CrossRef] [Green Version]
- Chatzopoulos, K.; Boland, J.M. Update on genetically defined lung neoplasms: NUT carcinoma and thoracic SMARCA4-deficient undifferentiated tumors. Virchows Arch. 2021, 478, 21–30. [Google Scholar] [CrossRef]
- Chetty, R.; Serra, S. SMARCA family of genes. J. Clin. Pathol. 2020, 73, 257–260. [Google Scholar] [CrossRef]
- Geng, H.; Li, S.; Guo, Y.; Yan, F.; Han, Y.; Xu, M.; Cui, Y. Survival prediction for patients with lung adenocarcinoma: A prognostic risk model based on gene mutations. Cancer Biomark. 2020, 27, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ahmed, T.; Petty, W.J.; Grant, S.; Ruiz, J.; Lycan, T.W.; Topaloglu, U.; Chou, P.; Miller, L.D.; Hawkins, G.A.; et al. SMARCA4 mutations in KRAS-mutant lung adenocarcinoma: A multi-cohort analysis. Mol. Oncol. 2021, 15, 462–472. [Google Scholar] [CrossRef]
- Rekhtman, N.; Montecalvo, J.; Chang, J.C.; Alex, D.; Ptashkin, R.N.; Ai, N.; Sauter, J.L.; Kezlarian, B.; Jungbluth, A.; Desmeules, P.; et al. SMARCA4-Deficient Thoracic Sarcomatoid Tumors Represent Primarily Smoking-Related Undifferentiated Carcinomas Rather Than Primary Thoracic Sarcomas. J. Thorac. Oncol. 2020, 15, 231–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sesboue, C.; Le Loarer, F. SWI/SNF-deficient thoraco-pulmonary neoplasms. Semin. Diagn. Pathol. 2021, 38, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Schrock, A.B.; Kem, M.; Jessop, N.; Lee, J.; Ali, S.M.; Ross, J.S.; Lennerz, J.K.; Shaw, A.T.; Mino-Kenudson, M. Clinicopathologic Characteristics of BRG1-Deficient NSCLC. J. Thorac. Oncol. 2020, 15, 766–776. [Google Scholar] [CrossRef] [Green Version]
- Fernando, T.M.; Piskol, R.; Bainer, R.; Sokol, E.S.; Trabucco, S.E.; Zhang, Q.; Trinh, H.; Maund, S.; Kschonsak, M.; Chaudhuri, S.; et al. Functional characterization of SMARCA4 variants identified by targeted exome-sequencing of 131,668 cancer patients. Nat. Commun. 2020, 11, 5551. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Bandlamudi, C.; Lavery, J.A.; Montecalvo, J.; Namakydoust, A.; Rizvi, H.; Egger, J.V.; Concepcion, C.P.; Paul, S.; Arcila, M.E.; et al. The Genomic Landscape of SMARCA4 Alterations and Associations with Outcomes in Patients with Lung Cancer. Clin. Cancer Res. 2020, 26, 5701–5708. [Google Scholar] [CrossRef]
- Tagal, V.; Wei, S.; Zhang, W.; Brekken, R.A.; Posner, B.A.; Peyton, M.; Girard, L.; Hwang, T.; Wheeler, D.A.; Minna, J.D.; et al. SMARCA4-inactivating mutations increase sensitivity to Aurora kinase A inhibitor VX-680 in non-small cell lung cancers. Nat. Commun. 2017, 8, 14098. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Meehan, B.; Fu, Z.; Wang, X.Q.D.; Fiset, P.O.; Rieker, R.; Levins, C.; Kong, T.; Zhu, X.; Morin, G.; et al. SMARCA4 loss is synthetic lethal with CDK4/6 inhibition in non-small cell lung cancer. Nat. Commun. 2019, 10, 557. [Google Scholar] [CrossRef]
- Deribe, Y.L.; Sun, Y.; Terranova, C.; Khan, F.; Martinez-Ledesma, J.; Gay, J.; Gao, G.; Mullinax, R.A.; Khor, T.; Feng, N.; et al. Mutations in the SWI/SNF complex induce a targetable dependence on oxidative phosphorylation in lung cancer. Nat. Med. 2018, 24, 1047–1057. [Google Scholar] [CrossRef]
- Papillon, J.P.N.; Nakajima, K.; Adair, C.D.; Hempel, J.; Jouk, A.O.; Karki, R.G.; Mathieu, S.; Moebitz, H.; Ntaganda, R.; Smith, T.; et al. Discovery of Orally Active Inhibitors of Brahma Homolog (BRM)/SMARCA2 ATPase Activity for the Treatment of Brahma Related Gene 1 (BRG1)/SMARCA4-Mutant Cancers. J. Med. Chem. 2018, 61, 10155–10172. [Google Scholar] [CrossRef]
- Yamagishi, M.; Uchimaru, K. Targeting EZH2 in cancer therapy. Curr. Opin. Oncol. 2017, 29, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Henon, C.; Blay, J.-Y.; Massard, C.; Mir, O.; Bahleda, R.; Dumont, S.; Postel-Vinay, S.; Adam, J.; Soria, J.-C.; Le Cesne, A. Long lasting major response to pembrolizumab in a thoracic malignant rhabdoid-like SMARCA4-deficient tumor. Ann. Oncol. 2019, 30, 1401–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naito, T.; Umemura, S.; Nakamura, H.; Zenke, Y.; Udagawa, H.; Kirita, K.; Matsumoto, S.; Yoh, K.; Niho, S.; Motoi, N.; et al. Successful treatment with nivolumab for SMARCA4-deficient non-small cell lung carcinoma with a high tumor mutation burden: A case report. Thorac. Cancer 2019, 10, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Shen, J.; Liu, J.; Fang, W.; Zhang, L. Efficacy of Immune Checkpoint Inhibitors in SMARCA4-Mutant NSCLC. J. Thorac. Oncol. 2020, 15, 133–136. [Google Scholar] [CrossRef]
- Chiba, Y.; Kawanami, T.; Yamasaki, K.; Uchimura, K.; Matsuyama, A.; Yatera, K. Hyper-progressive disease after immune checkpoint inhibitor in SMARCA4 -deficient small-cell lung carcinoma. Respirol. Case Rep. 2020, 8, 00667. [Google Scholar] [CrossRef] [PubMed]
- Orlando, K.A.; Nguyen, V.; Raab, J.R.; Walhart, T.; Weissman, B.E. Remodeling the cancer epigenome: Mutations in the SWI/SNF complex offer new therapeutic opportunities. Expert Rev. Anticancer. Ther. 2019, 19, 375–391. [Google Scholar] [CrossRef]
- Early, C.A.; Wangsiricharoen, S.; Jones, R.M.; VandenBussche, C.J. Review of SMARCA4 (BRG1)-deficient carcinomas following a malignant pleural effusion specimen confounded by reduced claudin-4 expression. J. Am. Soc. Cytopathol. 2021, 10, 197–207. [Google Scholar] [CrossRef]
- Herpel, E.; Rieker, R.J.; Dienemann, H.; Muley, T.; Meister, M.; Hartmann, A.; Warth, A.; Agaimy, A. SMARCA4 and SMARCA2 deficiency in non–small cell lung cancer: Immunohistochemical survey of 316 consecutive specimens. Ann. Diagn. Pathol. 2017, 26, 47–51. [Google Scholar] [CrossRef]
- Nambirajan, A.; Singh, V.; Bhardwaj, N.; Mittal, S.; Kumar, S.; Jain, D. SMARCA4/BRG1–Deficient Non–Small Cell Lung Carcinomas: A Case Series and Review of the Literature. Arch. Pathol. Lab. Med. 2021, 145, 90–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nambirajan, A.; Dutta, R.; Malik, P.S.; Bubendorf, L.; Jain, D. Cytology of SMARCA4-Deficient Thoracic Neoplasms: Comparative Analysis of SMARCA4-Deficient Non-Small Cell Lung Carcinomas and SMARCA4-Deficient Thoracic Sarcomas. Acta Cytol. 2021, 65, 67–74. [Google Scholar] [CrossRef]
- Cao, J.; Chen, D.; Yang, F.; Yao, J.; Zhu, W.; Zhao, C. NUT midline carcinoma as a primary lung tumor: A case report. J. Thorac. Dis. 2017, 9, 1045–1049. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.A.; Choi, Y.; Hwang, I.; Lee, K.; Cho, J.H.; Han, J. Clinicopathological characteristics of primary lung nuclear protein in testis carcinoma: A single-institute experience of 10 cases. Thorac. Cancer 2020, 11, 3205–3212. [Google Scholar] [CrossRef]
- Cho, H.J.; Lee, H. Lung nuclear protein in testis carcinoma in an elderly Korean woman: A case report with cytohistological analysis. Thorac. Cancer 2020, 11, 1724–1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harms, A.; Herpel, E.; Pfarr, N.; Penzel, R.; Heussel, C.-P.; Herth, F.J.; Dienemann, H.; Weichert, W.; Warth, A. NUT carcinoma of the thorax: Case report and review of the literature. Lung Cancer 2015, 90, 484–491. [Google Scholar] [CrossRef]
- Karakuş, E.; Poyraz, A.; Erdogan, A.S.O.; Emir, S.; Özyörük, D. NUT midline carcinoma of the lung in a six-year-old child. Fetal Pediatr. Pathol. 2017, 36, 472–474. [Google Scholar] [CrossRef]
- Lund-Iversen, M.; Grøholt, K.K.; Helland, Å.; Borgen, E.; Brustugun, O.T. NUT expression in primary lung tumours. Diagn. Pathol. 2015, 10, 156. [Google Scholar] [CrossRef] [Green Version]
- Sholl, L.M.; Nishino, M.; Pokharel, S.; Mino-Kenudson, M.; French, C.A.; Janne, P.A.; Lathan, C. Primary Pulmonary NUT Midline Carcinoma: Clinical, Radiographic, and Pathologic Characterizations. J. Thorac. Oncol. 2015, 10, 951–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- French, C.A. NUT Carcinoma: Clinicopathologic features, pathogenesis, and treatment. Pathol. Int. 2018, 68, 583–595. [Google Scholar] [CrossRef]
- Hakun, M.; Gu, B. Challenges and Opportunities in NUT Carcinoma Research. Genes 2021, 12, 235. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, D.; Kishaba, Y.; Ishikawa, S.; Sakatani, T.; Oguni, S.; Tamura, T.; Hoshino, H.; Sugiyama, Y.; Endo, S.; Murakami, Y.; et al. Lung cancer with loss of BRG1/BRM, shows epithelial mesenchymal transition phenotype and distinct histologic and genetic features. Cancer Sci. 2013, 104, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, N.; French, C.A.; Cameron, M.J.; Kratzke, R.A. Long-Term Survival of a Patient with Squamous Cell Carcinoma Harboring NUT Gene Rearrangement. J. Thorac. Oncol. 2010, 5, 1704–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baras, A.S.; Naidoo, J.; Hann, C.L.; Illei, P.B.; Iii, C.W.R.; Lauring, J. Rediagnosis of Lung Cancer as NUT Midline Carcinoma Based on Clues From Tumor Genomic Profiling. J. Natl. Compr. Cancer Netw. 2018, 16, 467–472. [Google Scholar] [CrossRef]
- Chau, N.G.; Ma, C.; Danga, K.; Al-Sayegh, H.; Nardi, V.; Barrette, R.; Lathan, C.S.; Dubois, S.G.; Haddad, R.I.; Shapiro, G.I.; et al. An Anatomical Site and Genetic-Based Prognostic Model for Patients With Nuclear Protein in Testis (NUT) Midline Carcinoma: Analysis of 124 Patients. JNCI Cancer Spectr. 2019, 4, 94. [Google Scholar] [CrossRef]
- Parikh, S.A.; French, C.A.; Costello, B.A.; Marks, R.S.; Dronca, R.S.; Nerby, C.L.; Roden, A.C.; Peddareddigari, V.G.; Hilton, J.; Shapiro, G.I.; et al. NUT Midline Carcinoma: An Aggressive Intrathoracic Neoplasm. J. Thorac. Oncol. 2013, 8, 1335–1338. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, S.; Kurabe, N.; Ohnishi, I.; Yasuda, K.; Aoshima, Y.; Naito, M.; Tanioka, F.; Sugimura, H. NSD3-NUT-expressing midline carcinoma of the lung: First characterization of primary cancer tissue. Pathol. Res. Pract. 2015, 211, 404–408. [Google Scholar] [CrossRef]
- Tanaka, M.; Kato, K.; Gomi, K.; Yoshida, M.; Niwa, T.; Aida, N.; Kigasawa, H.; Ohama, Y.; Tanaka, Y. NUT Midline Carcinoma. Am. J. Surg. Pathol. 2012, 36, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Haack, H.; Johnson, L.A.; Fry, C.J.; Crosby, K.; Polakiewicz, R.D.; Stelow, E.B.; Hong, S.-M.; Schwartz, B.E.; Cameron, M.J.; Rubin, M.A.; et al. Diagnosis of NUT Midline Carcinoma Using a NUT-specific Monoclonal Antibody. Am. J. Surg. Pathol. 2009, 33, 984–991. [Google Scholar] [CrossRef] [Green Version]
- Inamura, K. Update on Immunohistochemistry for the Diagnosis of Lung Cancer. Cancers 2018, 10, 72. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, S.; Suzuki, S.; Kurita, A.; Muraki, M.; Aoshima, Y.; Tanioka, F.; Sugimura, H. Cytological Features of a Variant NUT Midline Carcinoma of the Lung Harboring theNSD3-NUTFusion Gene: A Case Report and Literature Review. Case Rep. Pathol. 2015, 2015, 572951. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-K.; Louzada, S.; An, Y.; Kim, S.Y.; Youk, J.; Park, S.; Koo, S.H.; Keam, B.; Jeon, Y.K.; Ku, J.-L.; et al. Complex chromosomal rearrangements by single catastrophic pathogenesis in NUT midline carcinoma. Ann. Oncol. 2017, 28, 890–897. [Google Scholar] [CrossRef]
- Mack, P.C.; Ms, K.C.B.; Ms, C.R.E.; Ms, R.A.B.; Zill, O.A.; Ms, C.E.L.; Riess, J.W.; Mortimer, S.A.; Talasaz, A.; Lanman, R.B.; et al. Spectrum of driver mutations and clinical impact of circulating tumor DNA analysis in non–small cell lung cancer: Analysis of over 8000 cases. Cancer 2020, 126, 3219–3228. [Google Scholar] [CrossRef]
- Policarpio-Nicolas, M.L.C.; de Leon, E.M.B.; Jagirdar, J. Cytologic findings of NUT midline carcinoma in the hilum of the lung. Diagn. Cytopathol. 2015, 43, 739–742. [Google Scholar] [CrossRef]
- Bishop, J.A.; French, C.A.; Ali, S.Z. Cytopathologic features of NUT midline carcinoma: A series of 26 specimens from 13 patients. Cancer Cytopathol. 2016, 124, 901–908. [Google Scholar] [CrossRef]
- Dutta, R.; Nambirajan, A.; Mittal, S.; Roy-Chowdhuri, S.; Jain, D. Cytomorphology of primary pulmonary NUT carcinoma in different cytology preparations. Cancer Cytopathol. 2021, 129, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Kashima, J.; Yatabe, Y. The Isoform Matters in NUT Carcinoma: A Diagnostic Pitfall of p40 Immunohistochemistry. J. Thorac. Oncol. 2020, 15, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.P.; Chen, A.L.; Taylor, M.S.; Huynh, T.G.; Kem, M.; Selig, M.K.; Nielsen, G.P.; Lennerz, J.K.; Azzoli, C.G.; Dagogo-Jack, I.; et al. Thoracic nuclear protein in testis (NUT) carcinoma: Expanded pathological spectrum with expression of thyroid transcription factor-1 and neuroendocrine markers. Histopathology 2021, 78, 896–904. [Google Scholar] [CrossRef]
- Numakura, S.; Saito, K.; Motoi, N.; Mori, T.; Saito, Y.; Yokote, F.; Kanamoto, Y.; Asami, M.; Sakai, T.; Yamauchi, Y.; et al. P63-negative pulmonary NUT carcinoma arising in the elderly: A case report. Diagn. Pathol. 2020, 15, 134. [Google Scholar] [CrossRef]
- Pezzuto, F.; Fortarezza, F.; Mammana, M.; Pasello, G.; Pelosi, G.; Rea, F.; Calabrese, F. Immunohistochemical neuroendocrine marker expression in primary pulmonary NUT carcinoma: A diagnostic pitfall. Histopathology 2020, 77, 508–510. [Google Scholar] [CrossRef] [PubMed]
- Mbbs, K.D.S.; Stanzione, N.; Caron, J.E.; Fishbein, G.A.; Abtin, F.; Lluri, G.; Hirschowitz, S.L. Midline carcinoma expressing NUT in malignant effusion cytology. Diagn. Cytopathol. 2019, 47, 594–598. [Google Scholar] [CrossRef]
- Eagen, K.P.; French, C.A. Supercharging BRD4 with NUT in carcinoma. Oncogene 2021, 40, 1396–1408. [Google Scholar] [CrossRef]
- Sahai, V.; Redig, A.J.; Collier, K.A.; Eckerdt, F.D.; Munshi, H.G. Targeting BET bromodomain proteins in solid tumors. Oncotarget 2016, 7, 53997–54009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stathis, A.; Bertoni, F. BET Proteins as Targets for Anticancer Treatment. Cancer Discov. 2017, 8, 24–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, A.; Mahar, A.; Wong, K.; Barnett, M.; Kao, S. Prolonged Disease Control on Nivolumab for Primary Pulmonary NUT Carcinoma. Clin. Lung Cancer 2020. [Google Scholar] [CrossRef] [PubMed]
- Morrison-Smith, C.D.; Knox, T.M.; Filic, I.; Soroko, K.M.; Eschle, B.K.; Wilkens, M.K.; Gokhale, P.C.; Giles, F.; Griffin, A.; Brown, B.; et al. Combined Targeting of the BRD4-NUT-p300 Axis in NUT Midline Carcinoma by Dual Selective Bromodomain Inhibitor, NEO2734. Mol. Cancer Ther. 2020, 19, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Lewin, J.; Soria, J.-C.; Stathis, A.; Delord, J.-P.; Peters, S.; Awada, A.; Aftimos, P.G.; Bekradda, M.; Rezai, K.; Zeng, Z.; et al. Phase Ib Trial With Birabresib, a Small-Molecule Inhibitor of Bromodomain and Extraterminal Proteins, in Patients With Selected Advanced Solid Tumors. J. Clin. Oncol. 2018, 36, 3007–3014. [Google Scholar] [CrossRef]
- Zhang, X.; Zegar, T.; Lucas, A.; Morrison-Smith, C.; Knox, T.; French, C.A.; Knapp, S.; Müller, S.; Siveke, J.T. Therapeutic targeting of p300/CBP HAT domain for the treatment of NUT midline carcinoma. Oncogene 2020, 39, 4770–4779. [Google Scholar] [CrossRef]
- Mograbi, B.; Heeke, S.; Hofman, P. The Importance of STK11/LKB1 Assessment in Non-Small Cell Lung Carcinomas. Diagnostics 2021, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Yanagitani, N.; Ninomiya, H.; Sakamoto, H.; Tozuka, T.; Yoshida, H.; Amino, Y.; Uematsu, S.; Yoshizawa, T.; Ari-yasu, R.; et al. Association Between the Efficacy of Pembrolizumab and Low STK11/LKB1 Expression in High-PD-L1-expressing Non-small-cell Lung Cancer. In Vivo 2020, 34, 2997–3003. [Google Scholar] [CrossRef]
- Kwack, W.G.; Shin, S.Y.; Lee, S.H. Primary Resistance to Immune Checkpoint Blockade in an STK11/TP53/KRAS-Mutant Lung Adenocarcinoma with High PD-L1 Expression. Onco. Targets Ther. 2020, 13, 8901–8905. [Google Scholar] [CrossRef]
- Laderian, B.; Mundi, P.; Fojo, T.; Bates, S.E. Emerging Therapeutic Implications of STK11 Mutation: Case Series. Oncologist 2020, 25, 733–737. [Google Scholar] [CrossRef]
- Arauz, R.F.; Byun, J.S.; Tandon, M.; Sinha, S.; Kuhn, S.; Taylor, S.; Zingone, A.; Mitchell, K.A.; Pine, S.R.; Gardner, K.; et al. Whole-Exome Profiling of NSCLC Among African Americans. J. Thorac. Oncol. 2020, 15, 1880–1892. [Google Scholar] [CrossRef]
- Shen, H.; Zhu, M.; Wang, C. Precision oncology of lung cancer: Genetic and genomic differences in Chinese population. NPJ Precis. Oncol. 2019, 3, 14. [Google Scholar] [CrossRef]
- Adachi, Y.; Watanabe, K.; Kita, K.; Kitai, H.; Kotani, H.; Sato, Y.; Inase, N.; Yano, S.; Ebi, H. Resistance mediated by alternative receptor tyrosine kinases in FGFR1-amplified lung cancer. Carcinogenesis 2017, 38, 1063–1072. [Google Scholar] [CrossRef]
- Desai, A.; Adjei, A.A. FGFR Signaling as a Target for Lung Cancer Therapy. J. Thorac. Oncol. 2016, 11, 9–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegab, A.E.; Ozaki, M.; Kameyama, N.; Gao, J.; Kagawa, S.; Yasuda, H.; Soejima, K.; Yin, Y.; Guzy, R.D.; Nakamura, Y.; et al. Effect of FGF/FGFR pathway blocking on lung adenocarcinoma and its cancer-associated fibroblasts. J. Pathol. 2019, 249, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Touat, M.; Ileana, E.; Postel-Vinay, S.; André, F.; Soria, J.-C. Targeting FGFR Signaling in Cancer. Clin. Cancer Res. 2015, 21, 2684–2694. [Google Scholar] [CrossRef] [Green Version]
- Weeden, C.; Solomon, B.; Asselin-Labat, M.-L. FGFR1 inhibition in lung squamous cell carcinoma: Questions and controversies. Cell Death Discov. 2015, 1, 15049. [Google Scholar] [CrossRef] [Green Version]
- Cheng, M.L.; Pectasides, E.; Hanna, G.J.; Parsons, H.A.; Choudhury, A.D.; Oxnard, G.R. Circulating tumor DNA in advanced solid tumors: Clinical relevance and future directions. CA A Cancer J. Clin. 2021, 71, 176–190. [Google Scholar] [CrossRef]
- Hofman, V.; Heeke, S.; Allegra, M.; Ilie, M.; Hofman, P. Liquid biopsy and genomic assessement for lung cancer: The role in clinical practice. In Oncogenomics: From Basic Research to Precision Medicine; Franco, D., Franco, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 11, pp. 165–180. [Google Scholar]
- Roncarati, R.; Lupini, L.; Miotto, E.; Saccenti, E.; Mascetti, S.; Morandi, L.; Bassi, C.; Rasio, D.; Callegari, E.; Conti, V.; et al. Molecular testing on bronchial washings for the diagnosis and predictive assessment of lung cancer. Mol. Oncol. 2020, 14, 2163–2175. [Google Scholar] [CrossRef] [PubMed]
- Satoh, Y.; Matsuo, Y.; Kuba, T.; Yamashita, K.; Sawano, M.; Tozaka, S.; Yamazaki, H.; Sonoda, D.; Mikubo, M.; Naito, M.; et al. EGFR mutation genotyping and ALK status determination in liquid-based cytology samples of non-small cell lung cancer. Virchows Arch. 2019, 476, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, S.; Emerson, R.E.; Wu, H.H.; Cramer, H.M.; Curless, K.; Chang, H.Y.; Zhang, S.; Randolph, M.L.; Cheng, L. Molecular Testing for EGFR Mutations and ALK Rearrangements in the Cytological Specimens From the Patients With Non–Small Cell Lung Cancer. Appl. Immunohistochem. Mol. Morphol. 2019, 27, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Kilgour, E.; Rothwell, D.G.; Brady, G.; Dive, C. Liquid Biopsy-Based Biomarkers of Treatment Response and Resistance. Cancer Cell 2020, 37, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, N.; Spencer, E.; Torkamani, A.; Nicholson, L. Liquid Biopsies for Cancer: Coming to a Patient near You. J. Clin. Med. 2017, 6, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makarem, M.; Leighl, N.B. Molecular testing for lung adenocarcinoma: Is it time to adopt a “plasma-first” approach? Cancer 2020, 126, 3176–3180. [Google Scholar] [CrossRef]
- Aldea, M.; Hendriks, L.; Mezquita, L.; Jovelet, C.; Planchard, D.; Auclin, E.; Remon, J.; Howarth, K.; Benitez, J.C.; Gazzah, A.; et al. Circulating Tumor DNA Analysis for Patients with Oncogene-Addicted NSCLC With Isolated Central Nervous System Progression. J. Thorac. Oncol. 2020, 15, 383–391. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Bonanno, L.; Pavan, A.; Ferro, A.; Calvetti, L.; Frega, S.; Pasello, G.; Aprile, G.; Guarneri, V.; Conte, P.; Rete Oncologica Veneta (ROV). Clinical Impact of Plasma and Tissue Next-Generation Sequencing in Advanced Non-Small Cell Lung Cancer: A Real-World Experience. Oncologis 2020, 25, 1996–2005. [Google Scholar] [CrossRef]
- Heeke, S.; Hofman, P. Tumor mutational burden assessment as a predictive biomarker for immunotherapy in lung cancer patients: Getting ready for prime-time or not? Transl. Lung Cancer Res. 2018, 7, 631–638. [Google Scholar] [CrossRef]
- Aggarwal, C.; Thompson, J.C.; Chien, A.L.; Quinn, K.J.; Hwang, W.-T.; Black, T.A.; Yee, S.S.; Christensen, T.E.; Lariviere, M.J.; Silva, B.A.; et al. Baseline Plasma Tumor Mutation Burden Predicts Response to Pembrolizumab-based Therapy in Patients with Metastatic Non–Small Cell Lung Cancer. Clin. Cancer Res. 2020, 26, 2354–2361. [Google Scholar] [CrossRef] [Green Version]
- Gandara, D.R.; Paul, S.M.; Kowanetz, M.; Schleifman, E.; Zou, W.; Li, Y.; Rittmeyer, A.; Fehrenbacher, L.; Otto, G.; Malboeuf, C.; et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat. Med. 2018, 24, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Kuziora, M.; Quinn, K.J.; Helman, E.; Ye, J.; Liu, F.; Scheuring, U.; Peters, S.; Rizvi, N.A.; Brohawn, P.Z.; et al. A Blood-based Assay for Assessment of Tumor Mutational Burden in First-line Metastatic NSCLC Treatment: Results from the MYSTIC Study. Clin. Cancer Res. 2021, 27, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Pepe, F.; Pisapia, P.; Gristina, V.; Rocco, D.; Bs, M.M.; Micheli, P.; Iaccarino, A.; Tufano, R.; Bs, G.G.; De Luca, C.; et al. Tumor mutational burden on cytological samples: A pilot study. Cancer Cytopathol. 2020. [Google Scholar] [CrossRef]
- Berland, L.; Heeke, S.; Humbert, O.; Macocco, A.; Long-Mira, E.; Lassalle, S.; Lespinet-Fabre, V.; Lalvée, S.; Bordone, O.; Cohen, C.; et al. Current views on tumor mutational burden in patients with non-small cell lung cancer treated by immune checkpoint inhibitors. J. Thorac. Dis. 2019, 11, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, B.; West, H.J. Plasma vs Tissue Next-Generation Sequencing in Non–Small Cell Lung Cancer—Either, Both, or Neither? JAMA Oncol. 2019, 5, 148. [Google Scholar] [CrossRef]
- Ignatiadis, M.; Sledge, G.W.; Jeffrey, S.S. Liquid biopsy enters the clinic—Implementation issues and future challenges. Nat. Rev. Clin. Oncol. 2021, 18, 297–312. [Google Scholar] [CrossRef]
- Clery, E.; Pisapia, P.; Vigliar, E.; Malapelle, U.; Bellevicine, C.; Troncone, G.; Schmitt, F.C. Role of Cytomorphology in the Era of Liquid Biopsy. Acta Cytol. 2019, 63, 497–505. [Google Scholar] [CrossRef]
- Liu, H.E.; Vuppalapaty, M.; Wilkerson, C.; Renier, C.; Chiu, M.; Lemaire, C.; Che, J.; Matsumoto, M.; Carroll, J.; Crouse, S.; et al. Detection of EGFR Mutations in cfDNA and CTCs, and Comparison to Tumor Tissue in Non-Small-Cell-Lung-Cancer (NSCLC) Patients. Front. Oncol. 2020, 10, 572895. [Google Scholar] [CrossRef]
- Leone, G.; Passiglia, F.; Bironzo, P.; Bertaglia, V.; Novello, S. Is there any place for immune-checkpoint inhibitors in the treatment algorithm of fusion-driven non-small cell lung cancer?—A literature review. Transl. Lung Cancer Res. 2020, 9, 2674–2685. [Google Scholar] [CrossRef]
- Mazieres, J.; Drilon, A.; Lusque, A.; Mhanna, L.; Cortot, A.; Mezquita, L.; Thai, A.; Mascaux, C.; Couraud, S.; Veillon, R.; et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the IMMUNOTARGET registry. Ann. Oncol. 2019, 30, 1321–1328. [Google Scholar] [CrossRef]
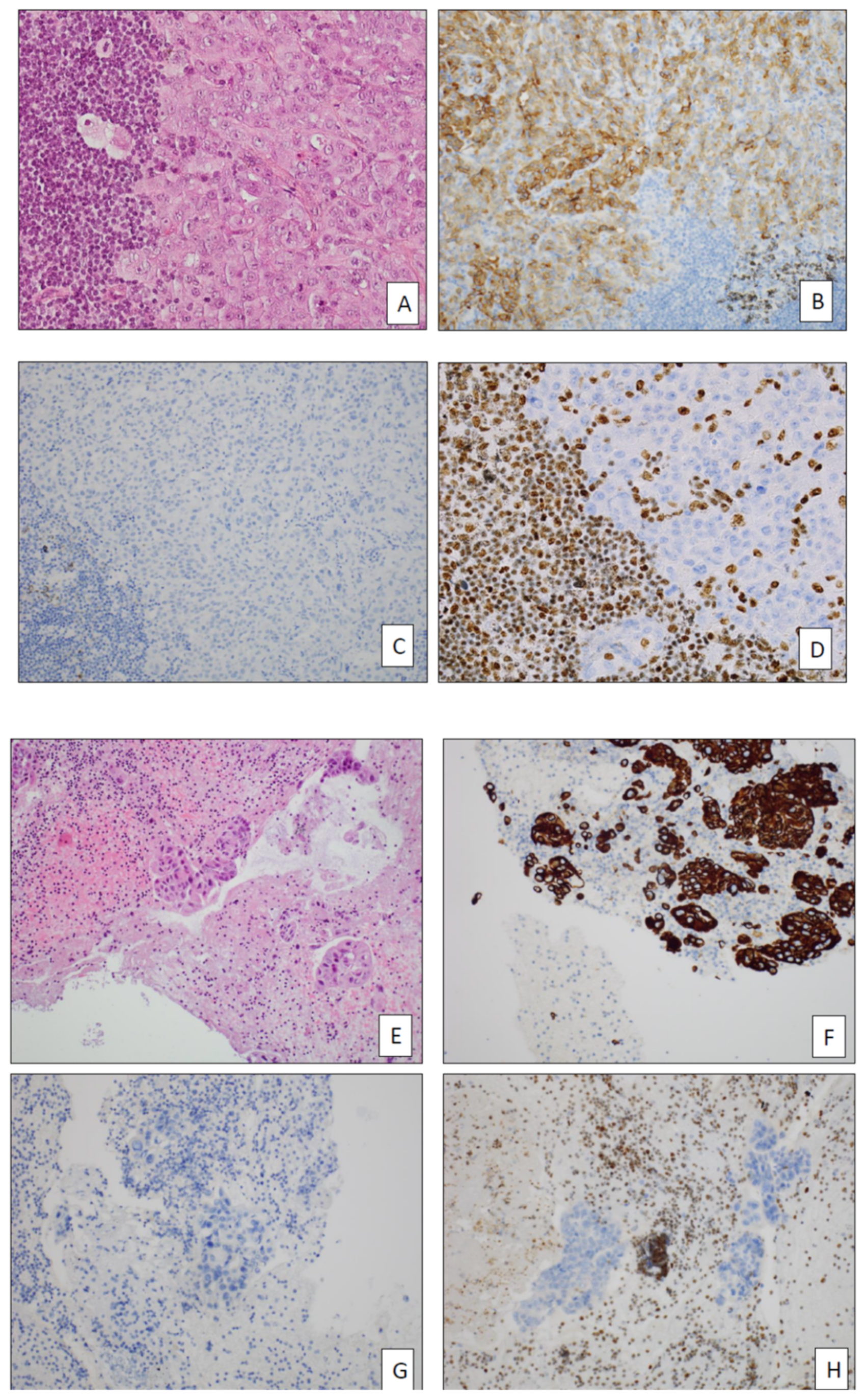


| EMA Approved | Coming Soon | Investigational |
|---|---|---|
| EGFR | RET | NRG1 |
| ALK | MET | FGFR1 |
| ROS1 | KRAS G12C | SMARCA4 |
| BRAFV600 | HER2 | NUT |
| NTRK | Others (MEK, PI3KCA, STK11, etc.) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofman, P. What Is New in Biomarker Testing at Diagnosis of Advanced Non-Squamous Non-Small Cell Lung Carcinoma? Implications for Cytology and Liquid Biopsy. J. Mol. Pathol. 2021, 2, 147-172. https://doi.org/10.3390/jmp2020015
Hofman P. What Is New in Biomarker Testing at Diagnosis of Advanced Non-Squamous Non-Small Cell Lung Carcinoma? Implications for Cytology and Liquid Biopsy. Journal of Molecular Pathology. 2021; 2(2):147-172. https://doi.org/10.3390/jmp2020015
Chicago/Turabian StyleHofman, Paul. 2021. "What Is New in Biomarker Testing at Diagnosis of Advanced Non-Squamous Non-Small Cell Lung Carcinoma? Implications for Cytology and Liquid Biopsy" Journal of Molecular Pathology 2, no. 2: 147-172. https://doi.org/10.3390/jmp2020015
APA StyleHofman, P. (2021). What Is New in Biomarker Testing at Diagnosis of Advanced Non-Squamous Non-Small Cell Lung Carcinoma? Implications for Cytology and Liquid Biopsy. Journal of Molecular Pathology, 2(2), 147-172. https://doi.org/10.3390/jmp2020015






