ROC Analysis Identifies Baseline and Dynamic NLR and dNLR Cut-Offs to Predict ICI Outcome in 402 Advanced NSCLC Patients
Abstract
:1. Introduction
2. Methods and Materials
2.1. Patient Selection
2.2. Statistical Analysis
3. Results
3.1. Baseline Patients’ Characteristics
3.2. Disease Control and Survival in the Overall Study Population
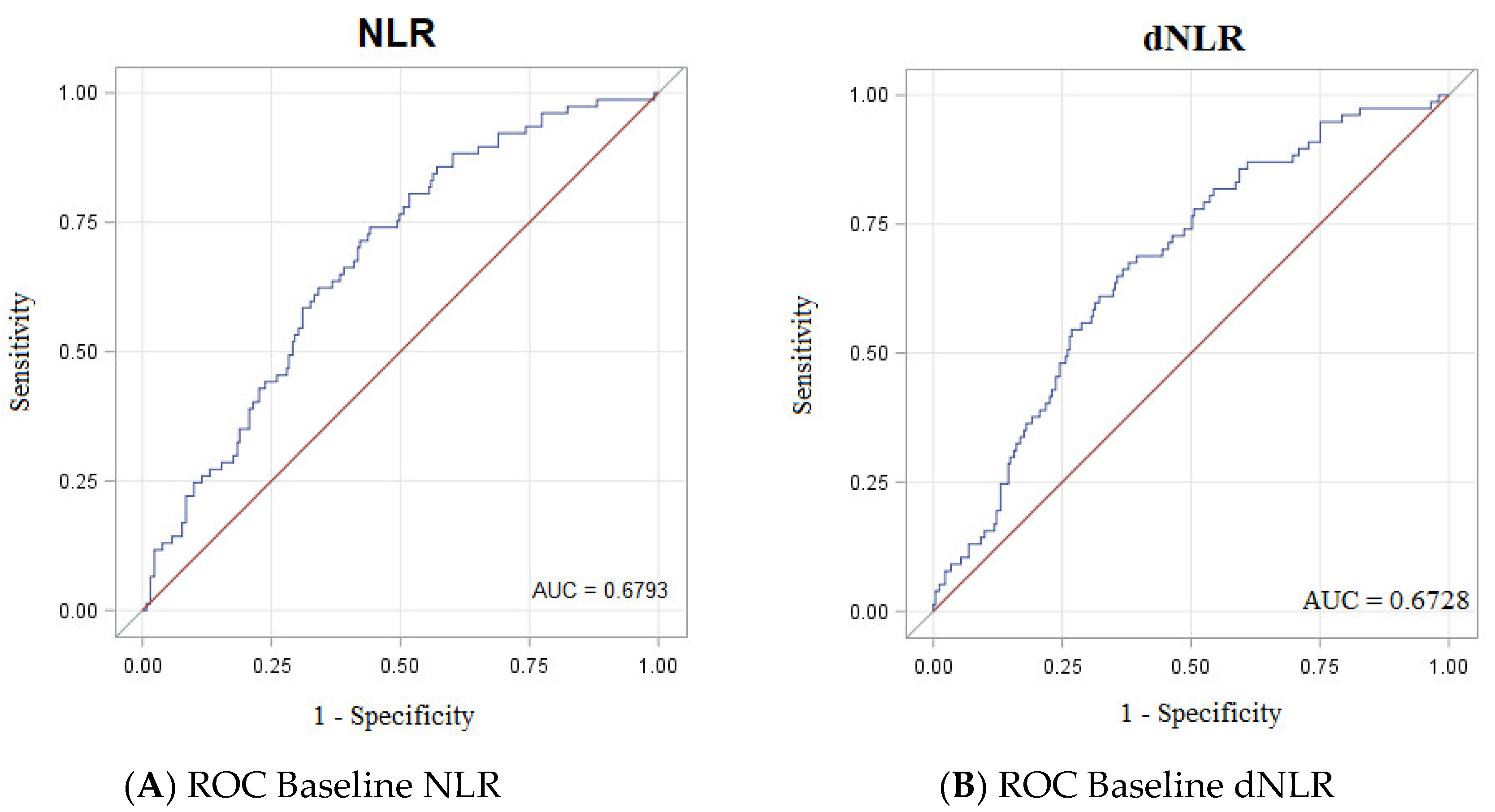
3.3. Accuracy of NLR, dNLR, PRL, Albumin and LDH as Biomarkers
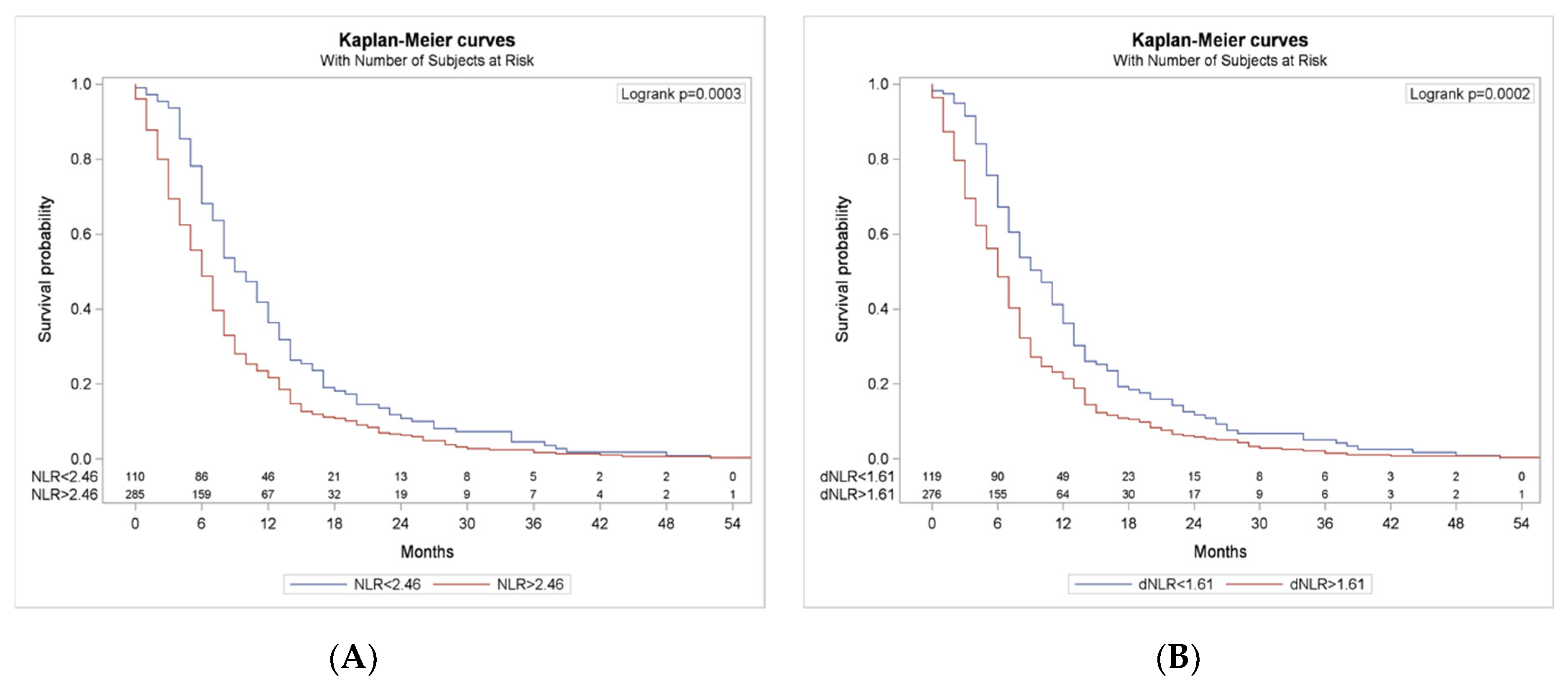
3.4. Survival According to NLR and dNLR Cut-Offs
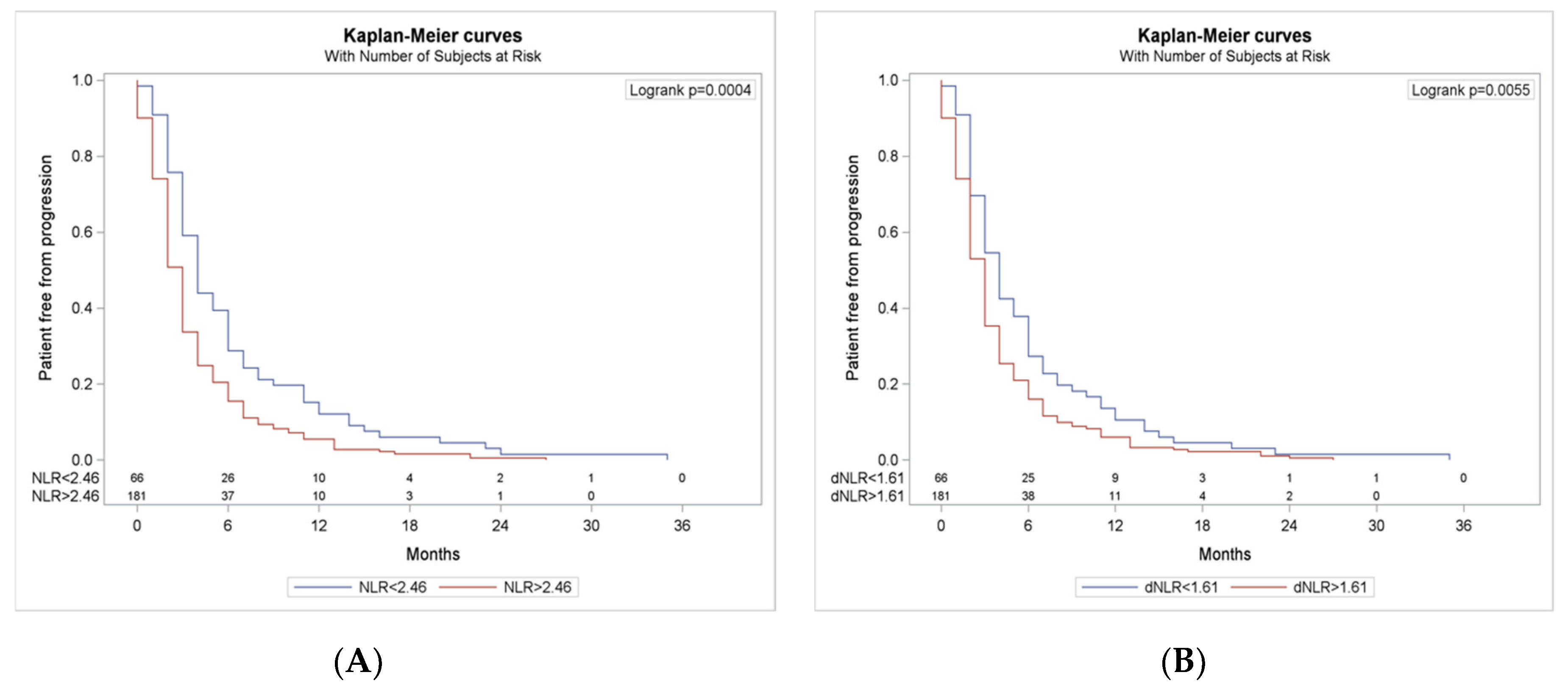
3.5. Progression Free Survival According to NLR and dNLR Cut-Offs
3.6. Cox Model for Survival and Response
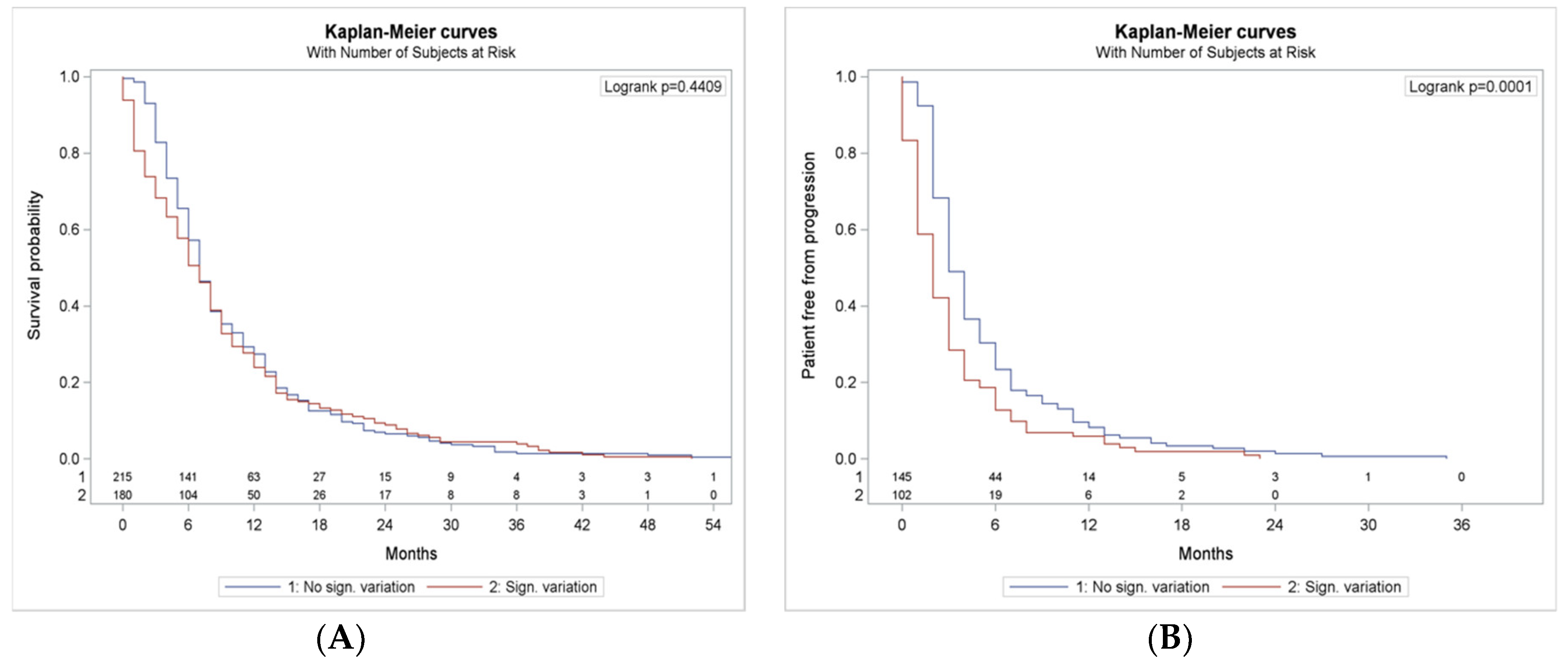
3.7. NLR and dNLR Changing During Treatment
4. Discussion
4.1. ROC Based Cut-Offs for NRL and dNLR
4.2. Other Circulating Markers
4.3. Independent Predictive and Prognostic Role of NLR and dNLR
4.4. NLR and dNLR Dynamics During Treatment
4.5. Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.; Faivre-Finn, C.; Mok, T.; Reck, M.; Van Schil, P.; Hellmann, M.; et al. Correction to: “Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up.”. Ann. Oncol. 2019, 30, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Attili, I.; Passaro, A.; Pavan, A.; Conte, P.; De Marinis, F.; Indraccolo, S. Combination immunotherapy strategies in advanced non-small cell lung cancer (NSCLC): Does biological rationale meet clinical needs? Crit. Rev. Oncol. 2017, 119, 30–39. [Google Scholar] [CrossRef]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017, 16, 2598–2608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowanetz, M.; Zou, W.; McCleland, M.; Gandara, D.; Gadgeel, S.; Rittmeyer, A.; Barlési, F.; Park, K.; Shames, D.; Koeppen, H.; et al. MA 05.09 Pre-Existing Immunity Measured by Teff Gene Expression in Tumor Tissue is Associated with Atezolizumad Efficacy in NSCLC. J. Thorac. Oncol. 2017, 12, S1817–S1818. [Google Scholar] [CrossRef] [Green Version]
- Meléndez, B.; Van Campenhout, C.; Rorive, S.; Remmelink, M.; Salmon, I.; D’Haene, N. Methods of measurement for tumor mutational burden in tumor tissue. Transl. Lung Cancer Res. 2018, 7, 661–667. [Google Scholar] [CrossRef]
- Bodor, J.N.; Boumber, Y.; Borghaei, H. Biomarkers for immune checkpoint inhibition in non-small cell lung cancer (NSCLC). Cancer 2020, 126, 260–270. [Google Scholar] [CrossRef]
- Feliciano, E.M.C.; Kroenke, C.H.; Meyerhardt, J.A.; Prado, C.M.; Bradshaw, P.T.; Kwan, M.L.; Xiao, J.; Alexeeff, S.; Corley, D.; Weltzien, E.; et al. Association of Systemic Inflammation and Sarcopenia With Survival in Nonmetastatic Colorectal Cancer. JAMA Oncol. 2017, 3, e172319. [Google Scholar] [CrossRef]
- Buttigliero, C.; Pisano, C.; Tucci, M.; Vignani, F.; Bertaglia, V.; Iaconis, D.; Guglielmini, P.; Numico, G.; Scagliotti, G.V.; Di Maio, M. Prognostic impact of pretreatment neutrophil-to-lymphocyte ratio in castration-resistant prostate cancer patients treated with first-line docetaxel. Acta Oncol. 2017, 56, 555–562. [Google Scholar] [CrossRef] [Green Version]
- Pinato, D.J.; Stavraka, C.; Flynn, M.J.; Forster, M.D.; O’Cathail, S.M.; Seckl, M.J.; Kristeleit, R.S.; Olmos, D.; Turnbull, S.J.; Blagden, S.P. An Inflammation Based Score Can Optimize the Selection of Patients with Advanced Cancer Considered for Early Phase Clinical Trials. PLoS ONE 2014, 9, e83279. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Geuna, E.; Michalarea, V.; Guardascione, M.; Naumann, U.; Lorente, D.; Kaye, S.B.; De Bono, J.S. The neutrophil-lymphocyte ratio and its utilisation for the management of cancer patients in early clinical trials. Br. J. Cancer 2015, 112, 1157–1165. [Google Scholar] [CrossRef]
- Ferrucci, P.F.; Ascierto, P.A.; Pigozzo, J.; Del Vecchio, M.; Maio, M.; Cappellini, G.C.A.; Guidoboni, M.; Queirolo, P.; Savoia, P.; Mandalà, M.; et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: Prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann. Oncol. 2016, 27, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, P.F.; Gandini, S.; Battaglia, A.; Alfieri, S.; Di Giacomo, A.M.; Giannarelli, D.; Cappellini, G.C.A.; De Galitiis, F.; Marchetti, P.; Amato, G.; et al. Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. Br. J. Cancer 2015, 112, 1904–1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martens, A.; Wistuba-Hamprecht, K.; Foppen, M.G.; Yuan, J.; Postow, M.A.; Wong, P.; Romano, E.; Khammari, A.; Dreno, B.; Capone, M.; et al. Baseline Peripheral Blood Biomarkers Associated with Clinical Outcome of Advanced Melanoma Patients Treated with Ipilimumab. Clin. Cancer Res. 2016, 22, 2908–2918. [Google Scholar] [CrossRef] [PubMed]
- Weide, B.; Martens, A.; Hassel, J.C.; Berking, C.; Postow, M.A.; Bisschop, K.; Simeone, E.; Mangana, J.; Schilling, B.; Di Giacomo, A.-M.; et al. Baseline Biomarkers for Outcome of Melanoma Patients Treated with Pembrolizumab. Clin. Cancer Res. 2016, 22, 5487–5496. [Google Scholar] [CrossRef] [Green Version]
- Diem, S.; Schmid, S.; Krapf, M.; Flatz, L.; Born, D.; Jochum, W.; Templeton, A.J.; Früh, M. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017, 111, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Mezquita, L.; Auclin, E.; Ferrara, R.; Charrier, M.; Remon, J.; Planchard, D.; Aix, S.P.; Ares, L.P.; Leroy, L.; Audigier-Valette, C.; et al. Association of the Lung Immune Prognostic Index With Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 351–357. [Google Scholar] [CrossRef]
- Delyon, J.; Mateus, C.; Lefeuvre, D.; Lanoy, E.; Zitvogel, L.; Chaput, N.; Roy, S.; Eggermont, A.M.M.; Routier, E.; Robert, C. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: An early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann. Oncol. 2013, 24, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Ku, G.; Yuan, J.; Page, D.B.; Schroeder, S.E.A.; Panageas, K.S.; Carvajal, R.D.; Chapman, P.B.; Schwartz, G.K.; Allison, J.; Wolchok, J.D. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting. Cancer 2010, 116, 1767–1775. [Google Scholar] [CrossRef]
- Simeone, E.; Gentilcore, G.; Giannarelli, D.; Grimaldi, A.M.; Caracó, C.; Curvietto, M.; Esposito, A.; Paone, M.; Palla, M.; Cavalcanti, E.; et al. Immunological and biological changes during ipilimumab treatment and their potential correlation with clinical response and survival in patients with advanced melanoma. Cancer Immunol. Immunother. 2014, 63, 675–683. [Google Scholar] [CrossRef]
- Moschetta, M.; Uccello, M.; Kasenda, B.; Mak, G.; McClelland, A.; Boussios, S.; Forster, M.; Arkenau, H.-T. Dynamics of Neutrophils-to-Lymphocyte Ratio Predict Outcomes of PD-1/PD-L1 Blockade. BioMed Res. Int. 2017, 2017, 1–5. [Google Scholar] [CrossRef]
- Nakaya, A.; Kurata, T.; Yoshioka, H.; Takeyasu, Y.; Niki, M.; Kibata, K.; Satsutani, N.; Ogata, M.; Miyara, T.; Nomura, S. Neutrophil-to-lymphocyte ratio as an early marker of outcomes in patients with advanced non-small-cell lung cancer treated with nivolumab. Int. J. Clin. Oncol. 2018, 23, 634–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezquita, L.; Preeshagul, I.; Auclin, E.; Saravia, D.; Hendriks, L.; Rizvi, H.; Planchard, D.; Park, W.; Nadal, E.; Ruffinelli, J.C.; et al. Early change of dNLR is correlated with outcomes in advanced NSCLC patients treated with immunotherapy. Abstracts book. In Proceedings of the IASLC 2019 World Conference on Lung Cancer, Barcelona, Spain, 7–10 September 2019. [Google Scholar]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Bagley, S.J.; Kothari, S.; Aggarwal, C.; Bauml, J.M.; Alley, E.W.; Evans, T.L.; Kosteva, J.A.; Ciunci, C.A.; Gabriel, P.E.; Thompson, J.C.; et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 2017, 106, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Habibzadeh, F.; Habibzadeh, P.; Yadollahie, M. On determining the most appropriate test cut-off value: The case of tests with continuous results. Biochem. Medica 2016, 26, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Ruopp, M.D.; Perkins, N.J.; Whitcomb, B.W.; Schisterman, E.F. Youden Index and Optimal Cut-Point Estimated from Observations Affected by a Lower Limit of Detection. Biom. J. 2008, 50, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.-B.; Tian, T.; Tian, X.-J.; Zhang, X.-J. Prognostic significance of neutrophil-to-lymphocyte ratio in non-small cell lung cancer: A meta-analysis. Sci. Rep. 2015, 5, 12493. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Duan, J.; Bai, H.; Wang, Y.; Wan, R.; Wang, X.; Chen, S.; Tian, Y.; Wang, D.; Fei, K.; et al. TCR Repertoire Diversity of Peripheral PD-1+CD8+ T Cells Predicts Clinical Outcomes after Immunotherapy in Patients with Non-Small Cell Lung Cancer. Cancer Immunol. Res. 2019, 8, 146–154. [Google Scholar] [CrossRef]
| Age (Mean 65.8; Range 39–86) | N (%) |
|---|---|
| ≤60 y | 104 (26%) |
| 61–69 y | 134 (33%) |
| 70–75 y | 119 (30%) |
| 76–79 y | 27 (7%) |
| ≥80 y | 18 (4%) |
| Sex | |
| Male | 287 (71%) |
| Female | 155 (29%) |
| Smoking Status | |
| Smoker | 118 (29%) |
| Former smoker | 231 (57%) |
| Never smoker | 40 (10%) |
| Unknow | 13 (4%) |
| Histology | |
| Adenocarcinoma | 251 (62%) |
| Squamous | 135 (33%) |
| Others | 16 (5%) |
| ECOG PS | |
| 0 | 156 (39%) |
| 1 | 228 (57%) |
| 2 | 18 (4%) |
| Concomitant Steroids | |
| No | 259 (70%) |
| Yes (≤10 mg/day) | 111 (30%) |
| Number of Metastatic Sites | |
| 0–2 | 256 (64%) |
| 3–4 | 117 (29%) |
| 5–6 | 15 (4%) |
| >6 | 14 (3%) |
| Metastatic Sites | |
| Lung | 280 (70%) |
| Lymph Nodes | 252 (63%) |
| Pleura | 95 (24%) |
| Bone | 88 (22%) |
| Adrenal | 47 (12%) |
| Brain | 34 (8%) |
| Liver | 29 (7%) |
| Soft tissue | 9 (2%) |
| Pericardium | 6 (1%) |
| PD-L1 Status (n = 224) | |
| <1% | 62 (27%) |
| 1–49% | 39 (17%) |
| >50% | 96 (43%) |
| ICI Treatment Line | |
| 1st | 84 (21%) |
| ≥2nd | 318 (79%) |
| Type of Prior Therapies | |
| Chemotherapy | 307 (96%) |
| Targeted therapy | 6 (2%) |
| Chemo + antiangiogenics | 4 (1%) |
| Vaccines | 1 |
| ICI Agent | |
| Nivolumab | 239 (60%) |
| Pembrolizumab | 128 (32%) |
| Atezolizumab | 17 (4%) |
| Durvalumab | 13 (3%) |
| Durvalumab + tremelimumab | 5 (1%) |
| Variable | HR | 95% CI | p |
|---|---|---|---|
| A | |||
| Age > 75 years | 1.032 | 0.749–1.422 | 0.85 |
| Male | 1.016 | 0.778–1.327 | 0.91 |
| Smokers | 1.442 | 0.988–2.104 | 0.06 |
| ECOG 1–2 * | 1.377 | 1.073–1.765 | 0.01 |
| PD-L1 > 50% | 1.372 | 1.061–1.774 | 0.02 |
| NLR ≥ 2.56 | 1.552 | 1.178–2.046 | 0.002 |
| B | |||
| Age > 75 years | 1.016 | 0.738–1.399 | 0.92 |
| Male | 1.008 | 0.772–0.317 | 0.95 |
| Smokers | 1.407 | 0.964–2.053 | 0.08 |
| ECOG 1–2* | 1.363 | 1.061–1.752 | 0.01 |
| PD-L1 > 50% | 1.392 | 1.078–1.798 | 0.01 |
| dNLR ≥ 1.61 | 1.522 | 1.162–1.994 | 0.002 |
| Variable | HR | 95% CI | p |
|---|---|---|---|
| A | |||
| Age > 75 years | 0.827 | 0.520–1.317 | 0.42 |
| Male | 0.866 | 0.608–1.233 | 0.42 |
| Smokers | 1.157 | 0.676–1.980 | 0.59 |
| ECOG 1–2 * | 2.033 | 1.414–2.922 | <0.000 |
| PD-L1 > 50% | 1.010 | 0.695–1.468 | 0.96 |
| NLR ≥ 2.56 | 1.675 | 1.131–2.480 | 0.01 |
| B | |||
| Age >75 years | 0.0814 | 0.512–1.295 | 0.38 |
| Male | 0.858 | 0.602–1.222 | 0.4 |
| Smokers | 1.124 | 0.656–1.924 | 0.67 |
| ECOG 1–2 * | 1.969 | 1.368–2.835 | <0.000 |
| PDL1 > 50% | 1.021 | 0.704–1.483 | 0.9 |
| dNLR ≥ 1.61 | 1.843 | 1.244–2.730 | 0.002 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnio, S.; Mariniello, A.; Pizzutilo, P.; Numico, G.; Borra, G.; Lunghi, A.; Soto Parra, H.; Buosi, R.; Vavalà, T.; Stura, I.; et al. ROC Analysis Identifies Baseline and Dynamic NLR and dNLR Cut-Offs to Predict ICI Outcome in 402 Advanced NSCLC Patients. J. Mol. Pathol. 2020, 1, 19-31. https://doi.org/10.3390/jmp1010004
Carnio S, Mariniello A, Pizzutilo P, Numico G, Borra G, Lunghi A, Soto Parra H, Buosi R, Vavalà T, Stura I, et al. ROC Analysis Identifies Baseline and Dynamic NLR and dNLR Cut-Offs to Predict ICI Outcome in 402 Advanced NSCLC Patients. Journal of Molecular Pathology. 2020; 1(1):19-31. https://doi.org/10.3390/jmp1010004
Chicago/Turabian StyleCarnio, Simona, Annapaola Mariniello, Pamela Pizzutilo, Gianmauro Numico, Gloria Borra, Alice Lunghi, Hector Soto Parra, Roberta Buosi, Tiziana Vavalà, Ilaria Stura, and et al. 2020. "ROC Analysis Identifies Baseline and Dynamic NLR and dNLR Cut-Offs to Predict ICI Outcome in 402 Advanced NSCLC Patients" Journal of Molecular Pathology 1, no. 1: 19-31. https://doi.org/10.3390/jmp1010004
APA StyleCarnio, S., Mariniello, A., Pizzutilo, P., Numico, G., Borra, G., Lunghi, A., Soto Parra, H., Buosi, R., Vavalà, T., Stura, I., Genestroni, S., Alemanni, A., Arizio, F., Catino, A., Montrone, M., Tabbò, F., Galetta, D., Migliaretti, G., & Novello, S. (2020). ROC Analysis Identifies Baseline and Dynamic NLR and dNLR Cut-Offs to Predict ICI Outcome in 402 Advanced NSCLC Patients. Journal of Molecular Pathology, 1(1), 19-31. https://doi.org/10.3390/jmp1010004






