Effect of Seasonal Environmental Changes on Leaf Anatomical Responses of Limoniastrum guyonianum in Sabkha Biotope †
Abstract
:1. Introduction
2. Materials and Methods
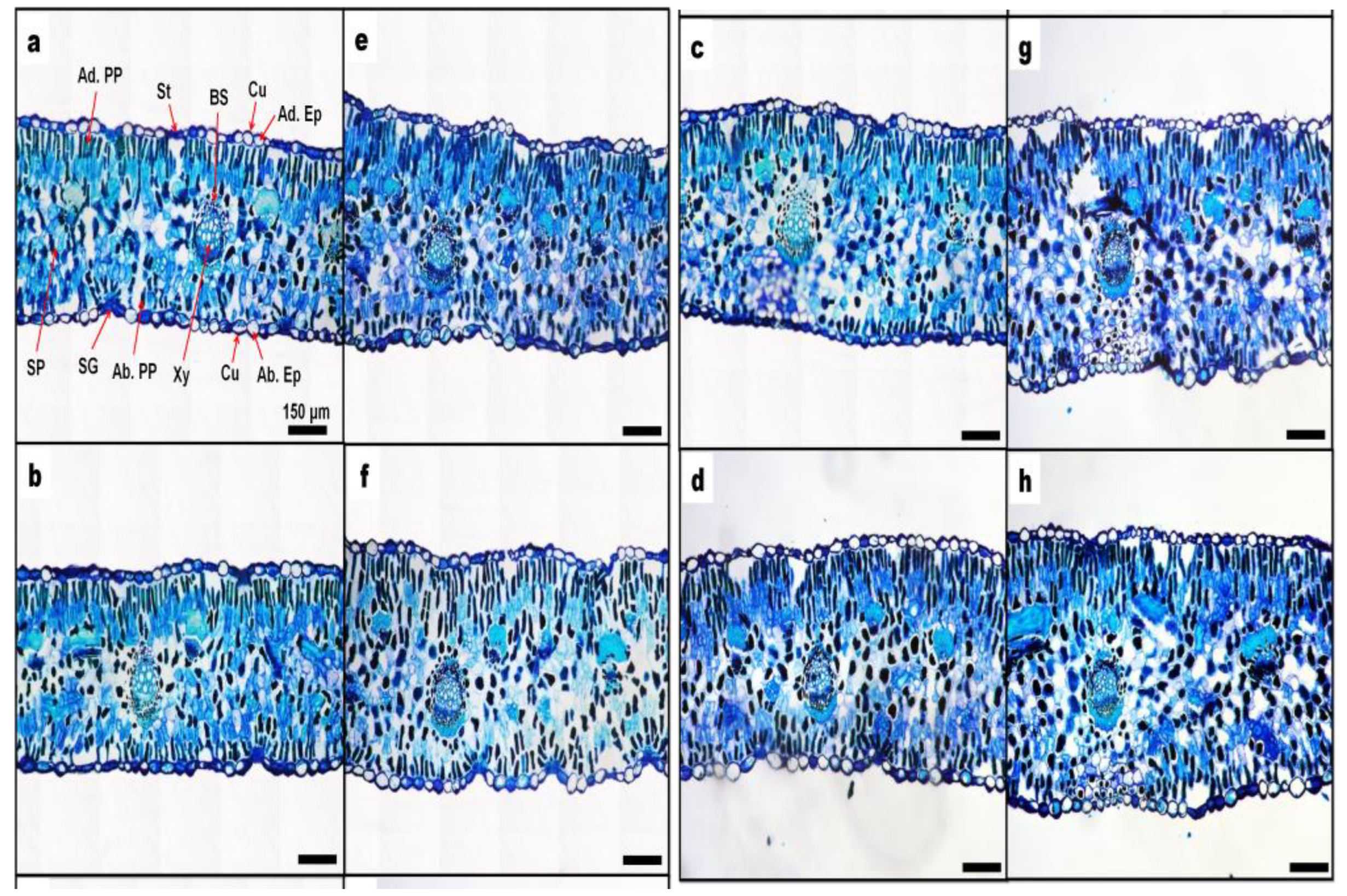
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quezel, P.; Santa, S. Nouvelle Flore de l’Algérie et des Régions Désertiques Méridionales; CNRS: Paris, France, 1963. [Google Scholar]
- Ding, F.; Chen, M.; Sui, N.; Wang, B.S. Ca2+ significantly enhanced development and salt-secretion rate of salt glands of Limonium bicolor under NaCl treatment. S. Afr. J. Bot. 2010, 76, 95–101. [Google Scholar] [CrossRef]
- Zouhaier, B.; Najla, T.; Abdallah, A.; Wahbi, D.; Wided, C.; Chedly, A.; Abderrazak, S. Salt stress response in the halophyte Limoniastrum guyonianum Boiss. Fun. Ecol. Plants 2015, 217, 1–9. [Google Scholar] [CrossRef]
- Telli, A.; Esnault, M.A.; Khelil, A.O.E.H. An ethnopharmacological survey of plants used in traditional diabetes treatment in south-eastern Algeria (Ouargla province). J. Arid Environ. 2016, 127, 82–92. [Google Scholar] [CrossRef]
- Krifa, M.; Mustapha, N.; Ghedira, Z.; Ghedira, K.; Pizzi, A.; Chekir-Ghedira, L. Limoniastrum guyonianum methanol extract induces immunomodulatory and anti-inflammatory effects by activating cellular anti-oxidant activity. Drug Chem. Toxicol. 2015, 38, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Al Hassan, M.; Chaura, J.; Donat-Torres, M.P.; Boscaiu, M.; Vicente, O. Antioxidant responses under salinity and drought in three closely related wild monocots with different ecological optima. AoB Plants 2017, 9, plx009. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.; Ashraf, M.; Naz, N. Anatomical adaptations to salinity in cogon grass (Imperatacylindrica (L.) Raeuschel) from the Salt Range, Pakistan. Plant Soil 2009, 322, 229–238. [Google Scholar] [CrossRef]
- Wankhade, S.D.; Comejo, M.J.; Mateu-Andre’s, I.; Sanz, A. Morphophysiological variations in response to NaCl stress during vegetative and reproductive development of rice. Acta Physiol. Plant 2013, 35, 323–333. [Google Scholar] [CrossRef]
- Nawazish, S.; Hameed, M.; Naurin, S. Leaf anatomical adaptations of Oenchrusciliaris L. From the salt range, pakistan against drought stress. Pak. J. Bot. 2006, 38, 1723–1730. [Google Scholar]
- Stiller, V.; Lafitte, H.R.; Sperry, J.S. Hydraulic properties of rice and the response of gas exchange to water stress. Plant Physiol. 2003, 132, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef] [PubMed]
- Laajimi, N.O.; Boussadia, O.; Skhiri, F.H. Teixeira da Silva, J.A., Rezgui, S., Hellali, R. Anatomical Adaptations in Vegetative Structures of Apricot Tree (Prunus armeniaca L.) cv. ‘Amor El Euch’ Grown under Water Stress. FVCSB 2011, 5, 46–51. [Google Scholar]
- Barhoumi, Z.; Djebali, W.; Abdelly, C.; Chaïbi, W.; Smaoui, A. Ultrastructure of Aeluropus littoralis leaf salt glands under NaCl stress. Protoplasma 2008, 233, 195–202. [Google Scholar] [PubMed]
- Ioannidou-Akoumianaki, A.; Spentza, R.P.; Fasseas, C. Limoniastrum monopetalum (L.) Boiss, a candidate plant for use in urban and suburban areas with adverse conditions. An anatomical and histochemical study. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Hortic. 2015, 72, 438–440. [Google Scholar]

| Characters | Treatments | |||||||
|---|---|---|---|---|---|---|---|---|
| January | February | March | April | May | June | July | August | |
| Leaf thickness (µm) | 485.4 ± 3.98 F | 484.1 ± 7.66 F | 491.7 ± 6.98 F | 518.8 ± 7.18 E | 554.6 ± 9.66 D | 581.7 ± 6.45 C | 608.2 ± 3.29 B | 637.9 ± 6.98 A |
| Adaxial epidermis (µm) | 24.1 ± 0.35 B | 23.9 ± 0.40 B | 24.0 ± 0.32 B | 24.2 ± 0.38 B | 24.1 ± 0.42 B | 24.3 ± 0.30 B | 25.1 ± 0.71 B | 26.1 ± 0.26 A |
| Adaxial stomatal density (nb·mm−2) | 62.2 ± 1.01 C | 60.3 ± 1.52 C | 63.1 ± 1.05 C | 63.0 ± 2.64 C | 63.3 ± 1.55 C | 71.3 ± 4.02 B | 82.0 ± 1.18 A | 84.3 ± 0.69 A |
| Adaxial salt glandsdensity (nb·mm−2) | 8.23 ± 0.25 CD | 7.73 ± 0.61 D | 8.16 ± 0.15 CD | 8.32 ± 0.20 CD | 8.90 ± 0.36 C | 10.56 ± 0.40 B | 11.66 ± 0.76 A | 11.85 ± 0.33 A |
| Abaxial epidermis (µm) | 23.9 ± 0.15 BC | 23.2 ± 0.41 BC | 23.1 ± 0.30 C | 23.7 ± 0.35 BC | 23.9 ± 0.65 BC | 23.7 ± 0.40 BC | 24.3 ± 0.20 B | 25.1 ± 0.61 A |
| Abaxial stomatal density (nb·mm−2) | 58.7 ± 1.12 D | 57.9 ± 2.83 D | 58.8 ± 2.02 D | 60.4 ± 2.15 D | 58.5 ± 2.50 D | 66.7 ± 1.30 C | 75.6 ± 1.35 B | 82.1 ± 4.37 A |
| Abaxial salt glandsdensity (nb·mm−2) | 10.4 ± 0.64 D | 10.5 ± 0.71 D | 11.7 ± 0.27 D | 12.3 ± 0.36 D | 13.0 ± 0.30 D | 15.7 ± 0.37 C | 23.8 ± 2.41 B | 25.7 ± 2.83 A |
| Adaxial palisadeparenchyma (µm) | 95.3 ± 3.51 F | 92.6 ± 2.08 F | 96.3 ± 1.52 F | 102.7 ± 2.10 E | 113.4 ± 4.49 D | 127.1 ± 2.68 C | 138.0 ± 3.10 B | 144.9 ± 2.57 A |
| Abaxial palisadeparenchyma (µm) | 62.0 ± 1.01 CD | 60.3 ± 1.52 D | 63.7 ± 2.05 CD | 64.6 ± 1.50 CD | 68.0 ± 2.04 C | 75.3 ± 4.85 B | 82.8 ± 1.25 A | 85.3 ± 3.51 A |
| Spongyparenchyma (µm) | 269.3 ± 6.94 D | 273.0 ± 9.58 D | 273.7 ± 8.61 D | 292.8 ± 6.25 C | 313.8 ± 6.27 B | 319.4 ± 3.87 B | 326.5 ± 4.11 B | 344.6 ± 3.81 A |
| Xylem vessel diameter (µm) | 14.7 ± 0.21 A | 14.9 ± 0.15 A | 14.1 ± 0.10 B | 12.0 ± 0.10 C | 11.4 ± 0.25 D | 11.0 ± 0.05 D | 9.4 ± 0.10 F | 9.4 ± 0.06 F |
| Xylem density (nb·mm−2) | 1934.3 ± 15.1 E | 1909.0 ± 10.6 E | 1930.6 ± 24.1 E | 2149.5 ± 54.3 D | 2662.1 ± 53.8 C | 2852.0 ± 50.1 B | 3246.2 ± 77.4 A | 3317.0 ± 43.8 A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boughalleb, F.; Maaloul, S.; Abdellaoui, R. Effect of Seasonal Environmental Changes on Leaf Anatomical Responses of Limoniastrum guyonianum in Sabkha Biotope. Environ. Sci. Proc. 2022, 16, 12. https://doi.org/10.3390/environsciproc2022016012
Boughalleb F, Maaloul S, Abdellaoui R. Effect of Seasonal Environmental Changes on Leaf Anatomical Responses of Limoniastrum guyonianum in Sabkha Biotope. Environmental Sciences Proceedings. 2022; 16(1):12. https://doi.org/10.3390/environsciproc2022016012
Chicago/Turabian StyleBoughalleb, Fayçal, Sameh Maaloul, and Raoudha Abdellaoui. 2022. "Effect of Seasonal Environmental Changes on Leaf Anatomical Responses of Limoniastrum guyonianum in Sabkha Biotope" Environmental Sciences Proceedings 16, no. 1: 12. https://doi.org/10.3390/environsciproc2022016012
APA StyleBoughalleb, F., Maaloul, S., & Abdellaoui, R. (2022). Effect of Seasonal Environmental Changes on Leaf Anatomical Responses of Limoniastrum guyonianum in Sabkha Biotope. Environmental Sciences Proceedings, 16(1), 12. https://doi.org/10.3390/environsciproc2022016012





