Abstract
This study focuses on the design, production, and characterization of lipid-based nanosystems for the delivery of manganese-based compounds in diagnostic multimodal imaging techniques. Anionic liposomes, obtained by the direct hydration method and extrusion, were homogeneous, monodispersed, and negatively charged. The loading of manganese-based compounds was nearly quantitative, their magnetic properties were retained, and the in vitro cytotoxicity on human keratinocytes highlighted that liposomes loaded with hydrophilic manganese derivatives did not affect cell viability, while liposomes loaded with lipophilic manganese derivatives showed a dose-dependent antiproliferative effect. Further experiments need to be carried out to clarify both the type and concentration of manganese derivative to be used.
1. Introduction
Continuous research in pharmaceutical technology has led to the development of drug delivery systems capable of delivering different molecules within the body, exploiting different areas of application. Among these systems, liposomes have proved to be excellent candidates, able to modify the site and the release time of the substances loaded within them [1]. This preliminary study focused on the description and characterization of these vesicular systems as entities capable of carrying molecules with diagnostic activity.
The diagnostic application of liposomes was investigated by conveying complexes of manganese. This metal has both radioisotopes capable of emitting positrons, which can be used in Positron Emission Tomography (PET), and paramagnetic properties, in its Mn2+ and Mn3+ oxidation states, which allow it to be used as a contrast agent in Magnetic Resonance Imaging (MRI) [2]. PET/MRI is a multimodal imaging technique that combines the aforementioned diagnostic analyzes and is useful in providing anatomical, morphological, and at the same time, metabolic characterizations and currently employs two different contrast media that are injected into the body simultaneously.
Manganese (Mn) exists in several oxidation states: +2 (the most stable), +3, +4, +6, and +7. The transition metal cations have unpaired electrons, which gives paramagnetism. Specifically, Mn (II) is optimal for diagnostic use as it has a good number of unpaired electrons appropriate for use both as a PET radiotracer and a MRI paramagnetic substance. Therefore, it has been taken into consideration for the present study. However, manganese in its free form is toxic; consequently, the use of liposomes to encapsulate it has been considered to reduce its toxicity. In this regard, anionic liposomes were prepared by hydration of a thin layer and subsequent extrusion [3]. In particular, N-lauroylsarcosin sodium salt (NLS) and sodium lauroyl lactylate (SLL) have been used as anionic surfactants.
2. Methods
2.1. Production of Liposomes
Negatively charged liposomes were obtained by thin layer hydration followed by extrusion. Phosphatidylcholine (PC), cholesterol (CH), and the anionic surfactant (AS) in a 4:2:1 molar ratio were dissolved in dichloromethane: methanol (1:1 v/v), and the organic mixture was subjected to evaporation under vacuum (70 bar, 100 rpm for 40–45 min) using a Rotavapor R-200 (Buchi Italia, Cornaredo, Italy). Afterward, the hydration of the deposed film was performed with PBS (pH 7.4) and the mixture was subjected to ultrasound for 5 min. Mn-based compounds (500 μM) were added to the organic phase (manganese acetylacetonate, lipophilic, MnL) or to water (manganese chloride, hydrophilic, MnH) during the hydration step. Liposomes with a final PC content of 25 mg/mL, were then extruded (Extruder, Lipex Biomembranes, Vancouver, BC, Canada) through two stacked polycarbonate filters with a 0.2 µm pore size (Nucleopore Corp, Pleasanton, CA, USA) under 10–20 bar nitrogen pressure [4] and stored for further studies.
2.2. Liposomes Characterization
Liposomes were visualized using Cryo-Transmission Electron Microscopy (Cryo-TEM) after being vitrified and transferred to a cryo-holder (CT3500, Gatan Inc., Pleasanton, CA, USA) of a Zeiss EM922Omega instrument [5]. Images digitally recorded by a CCD camera (UltraScan 1000, Gatan Inc., Pleasanton, CA, USA) were processed by means of GMS 1.4 software (Gatan Inc., Pleasanton, CA, USA).
Zetasizer Nano S90 (Malvern Instr., Malvern, UK) equipped with a 5 mW helium-neon laser with a wavelength output of 633 nm was used to measure the size of the obtained vesicles on samples diluted with water (1:20 by volume). Plasticware was cleaned with detergent washing and rinsed twice with Milli-Q water. Measurements were taken at 25 °C at a 90° angle for approximately 180 seconds. Data were interpreted by the “CONTIN” method [6].
The surface charge of the liposomes was measured by using the mean of the zeta potential (ζ) (Zetasizer Ultra, Malvern Panalytical Ltd., Malvern, UK) after sample dilution 1:20 by volume with deionized water and analyzed in triplicate at 25 °C.
2.3. Encapsulation Efficiency of Manganese
The manganese encapsulation efficiency (EE) in liposomes was established using Atomic Absorption Spectrophotometry (AAS) on ultracentrifuged samples. In particular, 500 μL of liposomes loaded on a Microcon centrifugal filter unit YM-10 membrane (NMWCO 10 kDa, Sigma-Aldrich, St. Louis, MO, USA) were centrifuged at 8000 rpm for 20 min (Spectrafuge™ 24D Digital Microcentrifuge, Woodbridge, NJ, USA) and both the lipid and aqueous phases were analyzed with an AAS device (Analyst 800, Perkin-Elmer, Shelton, CT, USA), together with liposomes before centrifugation, used as a control. The measurement was performed at 279.5 nm. Each sample, prepared twice, has been subjected to analysis. The EE was determined using Equation (1).
where MnL is the manganese concentration in the lipid phase, while MnA is the manganese concentration in the aqueous phase, both were analyzed using AAS.
EE = [MnL/(MnL + MnA)] × 100
2.4. Cell Viability Test
The colorimetric assay based on the use of 3-(4,5-dimethylthiazol-2- yl)-2,5-diphenyltetrazolium bromide or MTT [7] was conducted to evaluate cell viability. Cultured Human Keratinocyte (HaCaT) were grown in Dulbecco’s modified Eagle’s medium High Glucose (Lonza, Milan, Italy), supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM L-glutamine. Cells were incubated for 24 h at 37 °C in 95% air/5% CO2 until they reached 80% confluence. Different dilutions of each liposome formulation (namely 1:50, 1:100, 1:200, and 1:500, corresponding to liposome concentrations of 0.5, 0.25, 0.125, and 0.05 mg/mL, respectively) have been dispersed in cell culture medium. After exposing the seeded cells for 24 h to the selected formulations, the treatment was removed and 110 μL of MTT (0.5 mg/mL) was added and incubated for 4 h. The subsequent addition, incubation (15 min), and shaking of 100 μL of DMSO led to the conversion of MTT into a violet-colored formazan. The solution absorbance, proportional to the number of living cells, was measured using a spectrophotometer at 590 nm and converted into a percentage of viability.
The analysis of variance (ANOVA) and significance p-values < 0.05 were employed for the statistical study.
3. Results and Discussion
3.1. Liposomes Preparation and Characterization
This preliminary investigation was centered on the development of negatively charged liposomes for the transport of Mn2+ or Mn3+, with the purpose of controling ion toxicity and release into biological liquids [8,9].
A preformulatory study was previously conducted allowing the selection of two standard compositions containing anionic surfactants, NLS and SLL [10]. Afterward, Mn-loaded L-SLL and L-NLS were prepared for testing as model compounds, either hydrophilic (MnH) or lipophilic Mn-based compounds (MnL).
The extruded liposomes composed of PC, CH and SLL or NLS were subjected to size and charge analyses to obtain their average diameter, polydispersity index, and ζ potential. The obtained results are summarized in Table 1. It can be underlined that the presence of Mn-compound (either MnH or MnL) in the lipid bilayer has no effect on the size of the anionic vesicles, whose average diameter, never exceeds 200 nm. Moreover, the obtained ζ potential values confirmed a negative charge greater than 30 mV in absolute value on the surface of all the produced liposomal dispersions, therefore improving the stability of the dispersion by avoiding the coalescence of the vesicles [11].

Table 1.
Size and ζ potential values of extruded anionic liposomes.
After extrusion, the macroscopic aspect of the liposomal dispersions appears translucent. In-depth visualization: the morphology of all the prepared liposome dispersions was visualized using cryo-TEM, obtaining the images in Figure 1.
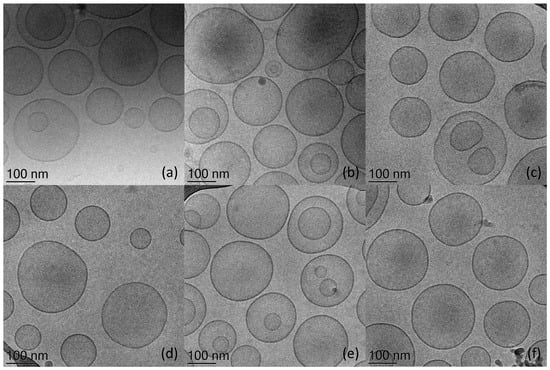
Figure 1.
Cryogenic Transmission Electron Microscopy images of unloaded and Mn-loaded anionic liposomes. Plain SLL (a), SLL-MnH (b), SLL-MnL (c), plain NLS (d), NLS-MnH (e), and NLS-MnL (f).
As expected, the extrusion process enabled us to obtain homogeneous sizes, as confirmed by the low polydispersity index (PdI) of size measurements (Table 1), mainly characterized by unilamellar liposomes [12]. Indeed, PdI values lower than 0.3 are evidence of a monomodal distribution of vesicles [13]. Moreover, it has to be emphasized that liposomal preparations with NLS and SLL in their composition were dimensionally stable over time [10].
The encapsulation efficiency of MnH and MnL in liposomes with NLS or SLL was evaluated by AAS analyses. The obtained results indicate that both Mn compounds were retained by the lipid portion of liposomes. In particular, the concentration of hydrophilic manganese compound is almost completely retained within liposomes (namely 99.83% in NLS liposomes and 99.72% in SLL vesicles), whilst the lipophilic manganese compound is loaded around 90% (e.g., 91.66% in NLS liposomes and 87.88% in SLL vesicles). Possibly, MnH could electrostatically interact with the cationic charge of Mn2+ ions and the negative phospholipid surface. Considering that the negative charge of the nanovesicular carriers is located either on the inner and/or outer surface, the electrostatic binding occurs mainly close to the phospholipid bilayer, thus allowing the retention of the MnH.
Therefore, anionic liposomes are able to carry manganese ions, possibly using an electrostatic binding with the surfactant negative charges on the surface of the lipid bilayer.
3.2. In Vitro Effect on HaCaT Cultured Cells
The anionic liposomal formulations were tested in vitro on the human keratinocyte cell line (HaCaT) by the MTT assay to get quick information on their effects on cell viability, proliferation, and cytotoxicity. However, as the MTT test is a sensitive and reliable indicator of cellular metabolic activity, in the future it will likely also be used for further experiments focusing on the negative effects on cell growth. HaCaT cells were employed as a model of a human non-tumor cell line.
Unloaded and Mn-loaded anionic liposomes were appropriately diluted and tested at different concentrations, comparing their effects to untreated cells taken as a control. The obtained results, summarized in Figure 2, were reported as cell viability percentage compared to that of untreated cells (100% viable).
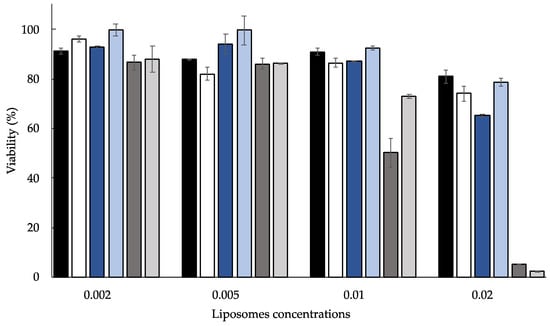
Figure 2.
MTT test results of in vitro effect on HaCaT cell proliferation of unloaded and loaded anionic liposomes appropriately diluted: NLS (black), SLL (white), NLS-MnH (dark blue), SLL-MnH (light blue), NLS-MnL (dark gray), and SLL-MnL (light gray). The data is the mean of three independent experiments conducted in triplicate, p-values are always <0.01 vs. untreated cells considered 100% viable.
It was found that all the formulations indicated a dose-dependent effect on HaCaT cell proliferation. Precisely, the higher the liposome concentration, the lower the viability. However, both unloaded anionic liposome dispersions did not influence the HaCaT proliferation. Additionally, cell viability was not affected by anionic liposomes loaded with MnH as compared to the corresponding unloaded liposome dispersion. However, MnL-loaded anionic liposomes displayed a higher antiproliferative effect. Probably, the lipophilic character of MnL plays a part in enhancing the lipophilicity of the system, affecting the interaction with cells. Indeed, the higher the concentrations, the lower the viability, falling to 10%.
On the basis of the obtained results, further experiments, possibly using other cell lines, such as L929 fibroblasts and/or RAW 264.7 or J774 macrophages, must be performed to select the optimal concentration of Mn compound to be used.
4. Conclusions
Currently, this investigation is still in progress with the aim of determining the magnetic properties of the prepared liposome dispersions, which is a considerable factor for the potential application in diagnostic imaging.
However, this work underlined the importance of pharmaceutical technology in the design of negatively charged liposomes carrying Mn by employing a simple production method. In particular, the results obtained on morphology, size, loading capacity, and in vitro activity allowed the selection of SLL liposomes for future investigations.
Author Contributions
Conceptualization, R.C. and L.M.; methodology, R.C.; preparation of formulations, M.S. and W.P.; liposomal analyses, W.P., M.S. and M.D.; in vitro experiments and discussion M.S.; original draft preparation, R.C.; writing and editing, R.C. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Ferrara (FAR2019 and FAR2020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge Antonella Pagnoni of Ferrara University for AAS analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ta, T.; Porter, T.M. Thermosensitive Liposomes for Localized Delivery and Triggered Release of Chemotherapy. J. Control. Release 2013, 169, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Brandt, M.; Cardinale, J.; Rausch, I.; Mindt, T.L. Manganese in PET Imaging: Opportunities and Challenges. J. Label. Compd. Radiopharm. 2019, 62, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Aiello, M.; Cavaliere, C.; Marchitelli, R.; d’Albore, A.; De Vita, E.; Salvatore, M. Hybrid PET/MRI Methodology. Int. Rev. Neurobiol. 2018, 141, 97–128. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, R.; Romagnoli, R.; Drechsler, M.; Menegatti, E.; Zaid, A.N.; Ravani, L.; Esposito, E. Liposomes- and Ethosomes-Associated Distamycins: A Comparative Study. J. Liposome Res. 2010, 20, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Mariani, P.; Ravani, L.; Contado, C.; Volta, M.; Bido, S.; Drechsler, M.; Mazzoni, S.; Menegatti, E.; Morari, M.; et al. Nanoparticulate Lipid Dispersions for Bromocriptine Delivery: Characterization and in Vivo Study. Eur. J. Pharm. Biopharm. 2012, 80, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Pecora, R. Dynamic Light Scattering Measurement of Nanometer Particles in Liquids. J. Nanopart. Res. 2000, 2, 123–131. [Google Scholar] [CrossRef]
- Singh Hallan, S.; Sguizzato, M.; Pavoni, G.; Baldisserotto, A.; Drechsler, M.; Mariani, P.; Esposito, E.; Cortesi, R. Ellagic Acid Containing Nanostructured Lipid Carriers for Topical Application: A Preliminary Study. Molecules 2020, 25, 1449. [Google Scholar] [CrossRef] [PubMed]
- El-Hammadi, M.M.; Arias, J.L. An Update on Liposomes in Drug Delivery: A Patent Review (2014–2018). Expert Opin. Ther. Pat. 2019, 29, 891–907. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.R.; Imran, M.; Ullah, S. Liposomes. In Lipid-Based Nanocarriers for Drug Delivery and Diagnosis; Elsevier: Amsterdam, The Netherlands, 2017; pp. 63–110. ISBN 978-0-323-52729-3. [Google Scholar]
- Sguizzato, M.; Pula, W.; Bordin, A.; Pagnoni, A.; Drechsler, M.; Marvelli, L.; Cortesi, R. Manganese in Diagnostics: A Preformulatory Study. Pharmaceutics 2022, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Has, C.; Pan, S. Vesicle Formation Mechanisms: An Overview. J. Liposome Res. 2021, 31, 90–111. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.B.; Betageri, G.V.; Singh, M. Factors Affecting Microencapsulation of Drugs in Liposomes. J. Microencapsul. 1995, 12, 229–246. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).