Activity of Wet-Spun Fibers Chemically Modified with Active Biomolecules against Gram-Positive and Gram-Negative Bacteria †
Abstract
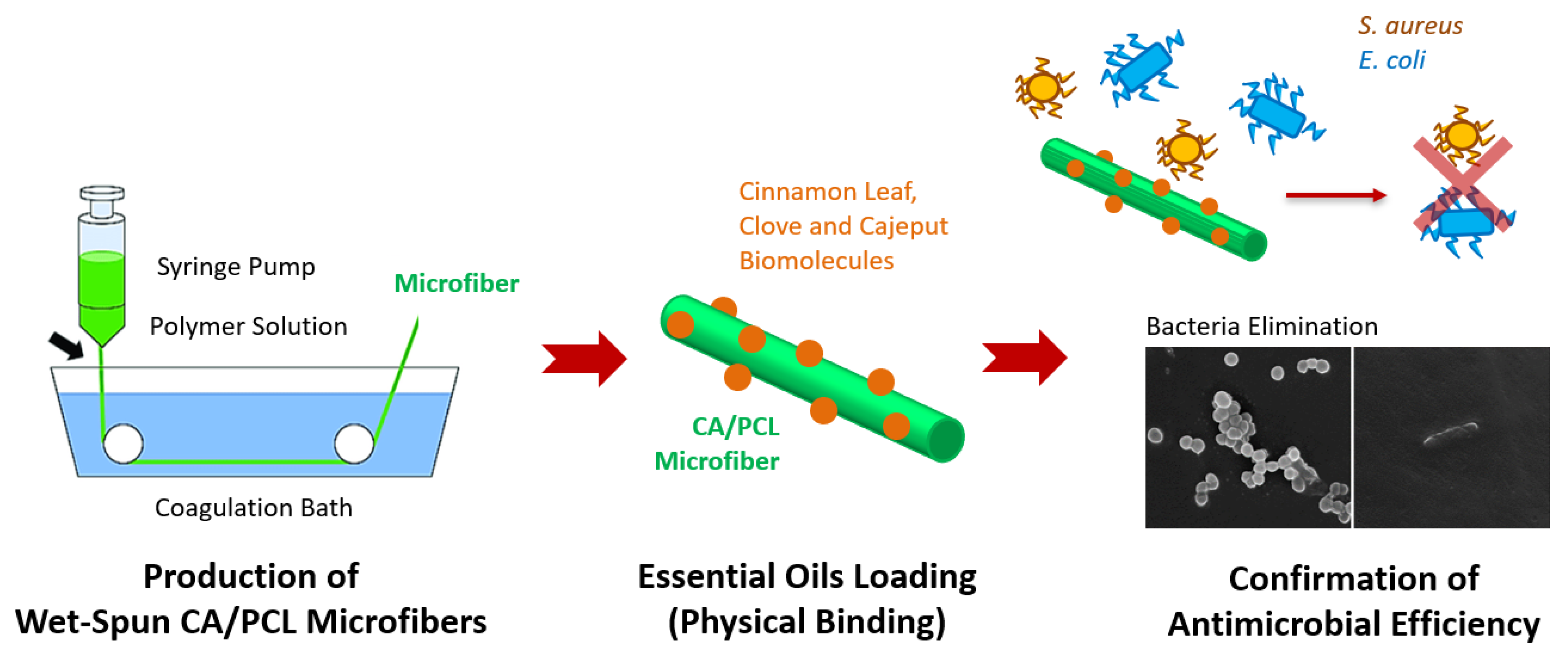
:1. Introduction
2. Materials and Methods
2.1. Polymeric Solution Preparation
2.2. Wet-Spinning Processing Conditions
2.3. Minimum Inhibitory Concentration Studies
2.4. Loading Efficiency
2.5. Chemical Characterization
2.6. Antimicrobial Testing
2.7. Membrane Permeabilization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- French, G.L. The continuing crisis in antibiotic resistance. Int. J. Antimicrob. Agents 2010, 36, S3–S7. [Google Scholar] [CrossRef]
- Felgueiras, H.P.; Teixeira, M.A.; Tavares, T.D.; Homem, N.C.; Zille, A.; Amorim, M.T.P. Antimicrobial action and clotting time of thin, hydrated poly (vinyl alcohol)/cellulose acetate films functionalized with LL37 for prospective wound-healing applications. J. Appl. Polym. Sci. 2020, 137, 48626. [Google Scholar] [CrossRef]
- Miranda, C.S.; Ribeiro, A.R.; Homem, N.C.; Felgueiras, H.P. Spun Biotextiles in Tissue Engineering and Biomolecules Delivery Systems. Antibiotics 2020, 9, 174. [Google Scholar] [CrossRef] [PubMed]
- Tavares, T.D.; Antunes, J.C.; Ferreira, F.; Felgueiras, H.P. Biofunctionalization of Natural Fiber-Reinforced Biocomposites for Biomedical Applications. Biomolecules 2020, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Omonijo, F.A.; Ni, L.; Gong, J.; Wang, Q.; Lahaye, L.; Yang, C. Essential oils as alternatives to antibiotics in swine production. Anim. Nutr. 2018, 4, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Felgueiras, H.P.; Tavares, T.D.; Amorim, M.T.P. Biodegradable, spun nanocomposite polymeric fibrous dressings loaded with bioactive biomolecules for an effective wound healing: A review. IOP Conf. Ser. Mater. Sci. Eng. 2019, 634, 012033. [Google Scholar] [CrossRef]
- Felgueiras, H.P.; Homem, N.C.; Teixeira, M.A.; Ribeiro, A.R.; Antunes, J.C.; Amorim, M.T.P. Physical, Thermal, and Antibacterial Effects of Active Essential Oils with Potential for Biomedical Applications Loaded onto Cellulose Acetate/Polycaprolactone Wet-Spun Microfibers. Biomolecules 2020, 10, 1129. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Kong, D.-X.; Wu, H. Analysis and evaluation of essential oil components of cinnamon barks using GC–MS and FTIR spectroscopy. Ind. Crops Prod. 2013, 41, 269–278. [Google Scholar] [CrossRef]
- Rodríguez, J.D.W.; Peyron, S.; Rigou, P.; Chalier, P. Rapid quantification of clove (Syzygium aromaticum) and spearmint (Mentha spicata) essential oils encapsulated in a complex organic matrix using an ATR-FTIR spectroscopic method. PLoS ONE 2018, 13, e0207401. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, O.; Albu, L.; Loghin, M.C.; Gaidau, C.; Miu, L.; Coara, G. Selection and Characterization of Some Essential Oils for the Treatment of Medical Furs. Rev. Chim. 2019, 70, 498–502. [Google Scholar] [CrossRef]
- Matwijczuk, A.; Oniszczuk, T.; Matwijczuk, A.; Chruściel, E.; Kocira, A.; Niemczynowicz, A.; Wójtowicz, A.; Combrzyński, M.; Wiącek, D. Use of FTIR Spectroscopy and Chemometrics with Respect to Storage Conditions of Moldavian Dragonhead Oil. Sustainability 2019, 11, 6414. [Google Scholar] [CrossRef]
- Tavares, T.D.; Antunes, J.C.; Padrão, J.; Ribeiro, A.I.; Zille, A.; Amorim, M.T.P.; Ferreira, F.; Felgueiras, H.P. Activity of Specialized Biomolecules against Gram-Positive and Gram-Negative Bacteria. Antibiotics 2020, 9, 314. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felgueiras, H.P.; Homem, N.C.; Ribeiro, A.R.M.; Teixeira, M.O.; Teixeira, M.A.; Antunes, J.C.; Amorim, M.T.P. Activity of Wet-Spun Fibers Chemically Modified with Active Biomolecules against Gram-Positive and Gram-Negative Bacteria. Mater. Proc. 2021, 4, 85. https://doi.org/10.3390/IOCN2020-07935
Felgueiras HP, Homem NC, Ribeiro ARM, Teixeira MO, Teixeira MA, Antunes JC, Amorim MTP. Activity of Wet-Spun Fibers Chemically Modified with Active Biomolecules against Gram-Positive and Gram-Negative Bacteria. Materials Proceedings. 2021; 4(1):85. https://doi.org/10.3390/IOCN2020-07935
Chicago/Turabian StyleFelgueiras, Helena P., Natália C. Homem, Ana R. M. Ribeiro, Marta O. Teixeira, Marta A. Teixeira, Joana C. Antunes, and Maria Teresa P. Amorim. 2021. "Activity of Wet-Spun Fibers Chemically Modified with Active Biomolecules against Gram-Positive and Gram-Negative Bacteria" Materials Proceedings 4, no. 1: 85. https://doi.org/10.3390/IOCN2020-07935
APA StyleFelgueiras, H. P., Homem, N. C., Ribeiro, A. R. M., Teixeira, M. O., Teixeira, M. A., Antunes, J. C., & Amorim, M. T. P. (2021). Activity of Wet-Spun Fibers Chemically Modified with Active Biomolecules against Gram-Positive and Gram-Negative Bacteria. Materials Proceedings, 4(1), 85. https://doi.org/10.3390/IOCN2020-07935









