Development of Flexible Polycation-Based mRNA Delivery Systems for In Vivo Applications †
Abstract
1. Introduction
2. Results and Discussion
2.1. The Effect of Polymer Flexibility on the Stability and the Performance of mRNA/m
2.2. The Effect of Valency between Polycations and mRNA on the Stability and the Performance of mRNA/m
3. Conclusions
4. Materials and Methods
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Sahin, U.; Karikó, K.; Türeci, Ö. mRNA-based therapeutics—Developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef] [PubMed]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; Kataoka, K. Design concepts of polyplex micelles for in vivo therapeutic delivery of plasmid DNA and messenger RNA. J. Biomed. Mater. Res. Part A 2019, 107, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; Perche, F.; Pichon, C.; Cabral, H. Nanomedicine-Based Approaches for mRNA Delivery. Mol. Pharm. 2020, 17, 3654–3684. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, J.E.; Choi, C.H.J.; Han, H.; Davis, M.E. Polycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membrane. Proc. Natl. Acad. Sci. USA 2012, 109, 3137–3142. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; Kinoh, H.; Ishii, T.; Matsui, A.; Tockary, T.A.; Takeda, K.M.; Uchida, H.; Osada, K.; Itaka, K.; Kataoka, K. Systemic delivery of messenger RNA for the treatment of pancreatic cancer using polyplex nanomicelles with a cholesterol moiety. Biomaterials 2016, 82, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Dirisala, A.; Uchida, S.; Tockary, T.A.; Yoshinaga, N.; Li, J.; Osawa, S.; Gorantla, L.; Fukushima, S.; Osada, K.; Kataoka, K. Precise tuning of disulphide crosslinking in mRNA polyplex micelles for optimising extracellular and intracellular nuclease tolerability. J. Drug Target 2019, 27, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Grasso, G.; Deriu, M.A.; Patrulea, V.; Borchard, G.; Möller, M.; Danani, A. Free energy landscape of siRNA-polycation complexation: Elucidating the effect of molecular geometry, polymer flexibility, and charge neutralization. PLoS ONE 2017, 12, e0186816. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Uchida, S.; Nagatoishi, S.; Koji, K.; Hong, T.; Fukushima, S.; Tsumoto, K.; Ishihara, K.; Kataoka, K.; Cabral, H. Polymeric Nanocarriers with Controlled Chain Flexibility Boost mRNA Delivery In Vivo through Enhanced Structural Fastening. Adv. Healthc. Mater. 2020, 9, 2000538. [Google Scholar] [CrossRef] [PubMed]
- Carlson, P.M.; Schellinger, J.G.; Pahang, J.A.; Johnson, R.N.; Pun, S.H. Comparative study of guanidine-based and lysine-based brush copolymers for plasmid delivery. Biomater. Sci. 2013, 1, 736–744. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hori, M.; Cabral, H.; Toh, K.; Kishimura, A.; Kataoka, K. Robust Polyion Complex Vesicles (PICsomes) under Physiological Conditions Reinforced by Multiple Hydrogen Bond Formation Derived by Guanidinium Groups. Biomacromolecules 2018, 19, 4113–4121. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Uchida, S.; Hatano, H.; Miyahara, Y.; Matsumoto, A.; Cabral, H. Guanidine-phosphate interactions stabilize polyion complex micelles based on flexible catiomers to improve mRNA delivery. Eur. Polym. J. 2020, 140, 110028. [Google Scholar] [CrossRef]
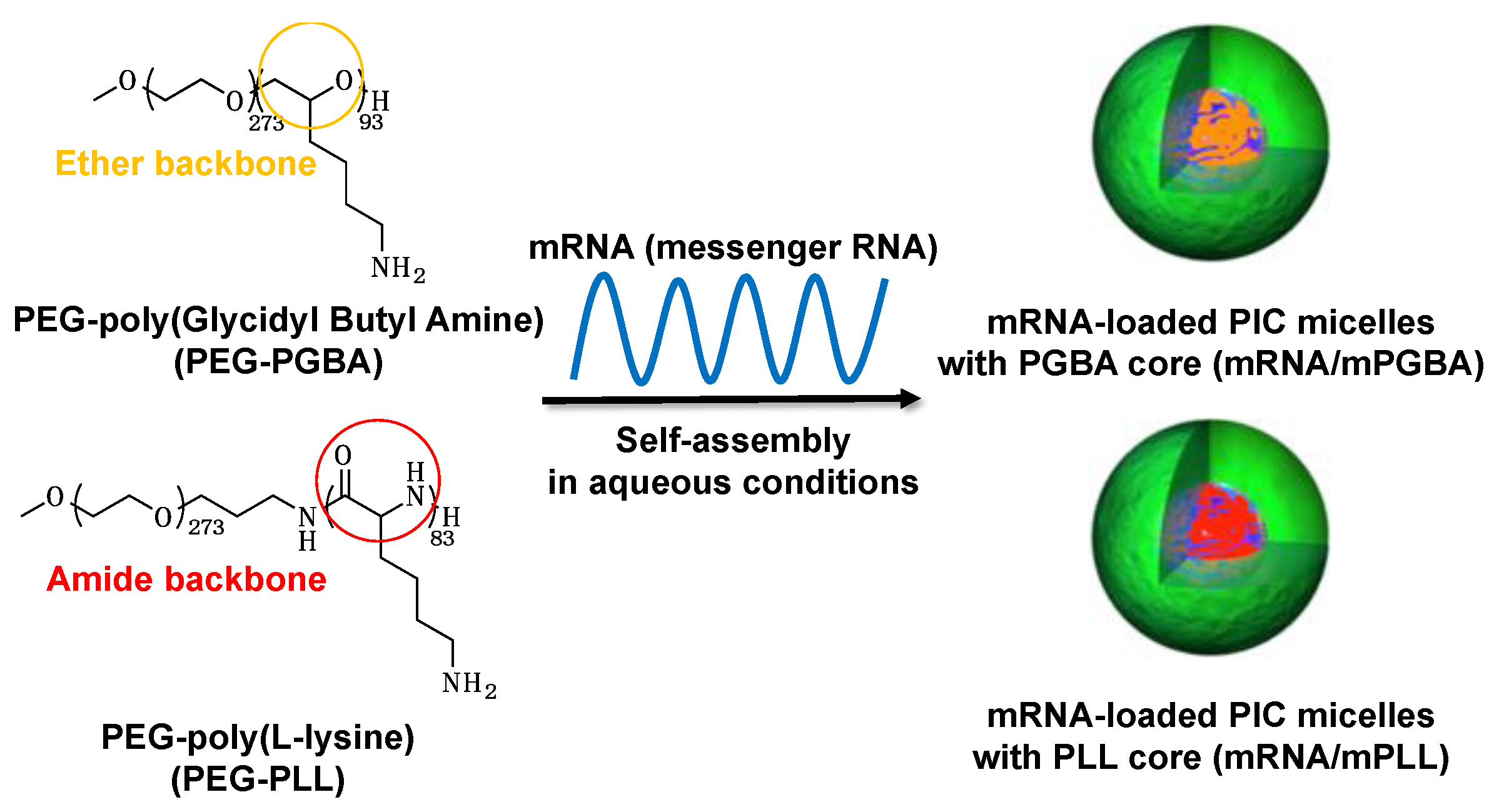
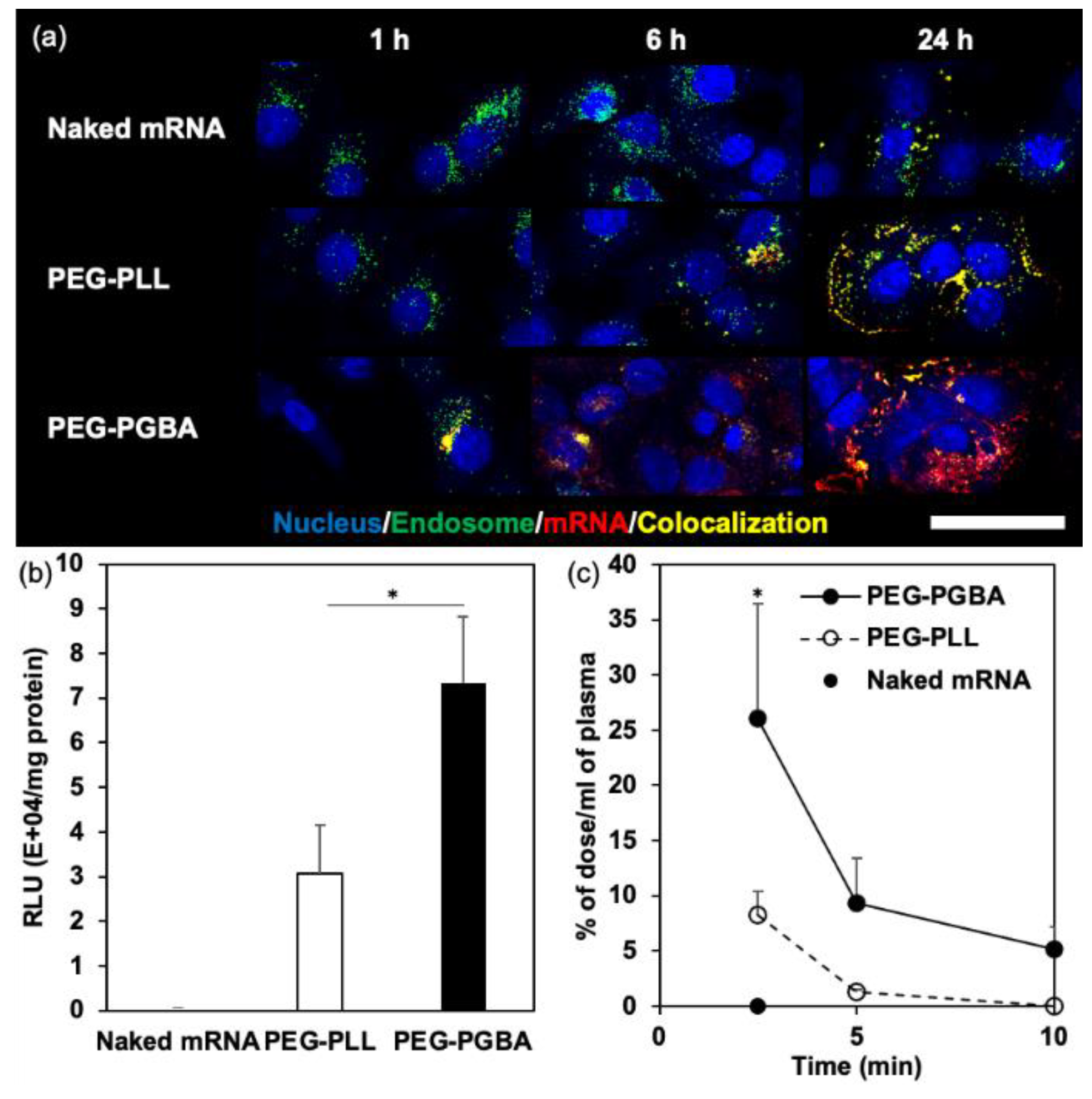
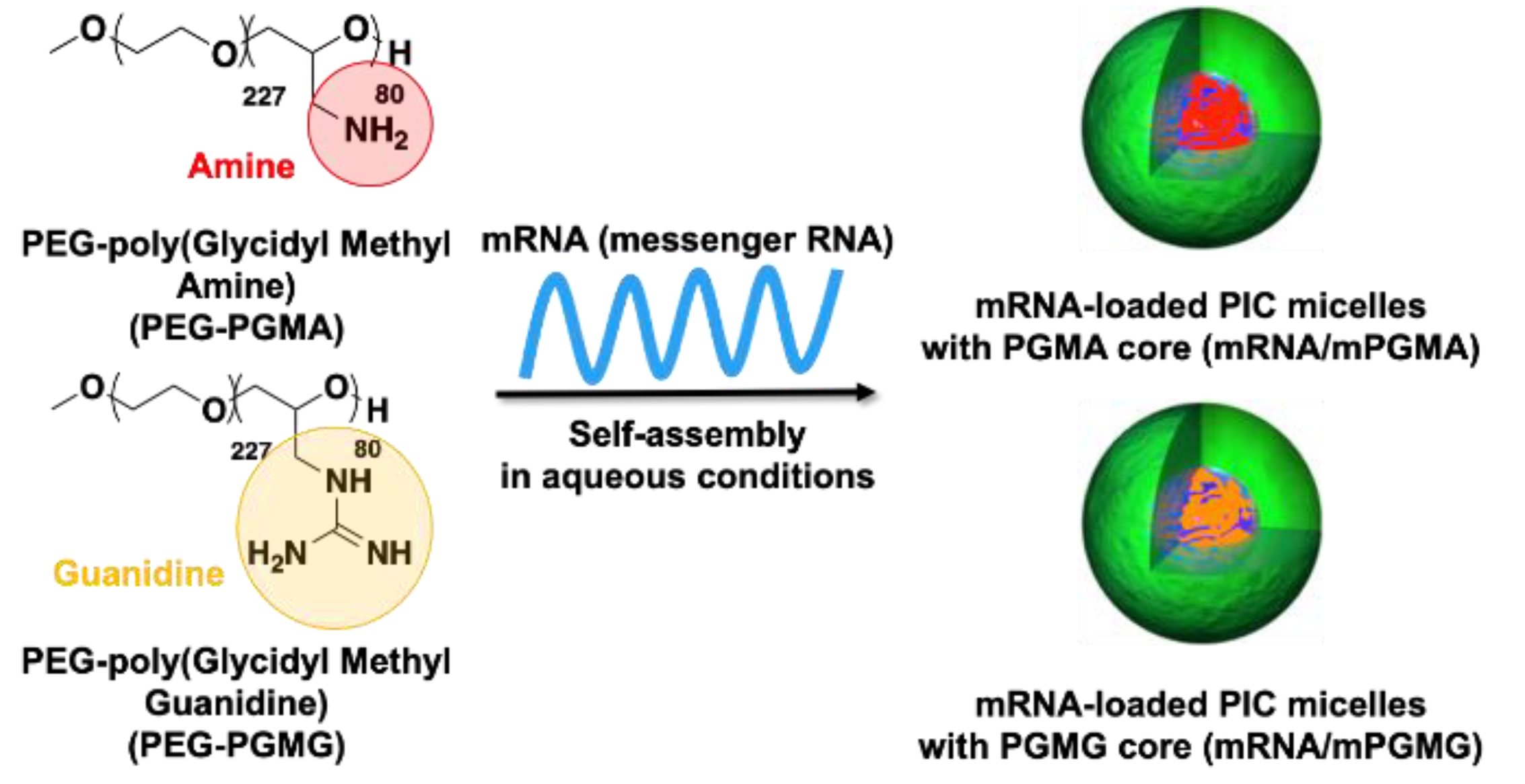
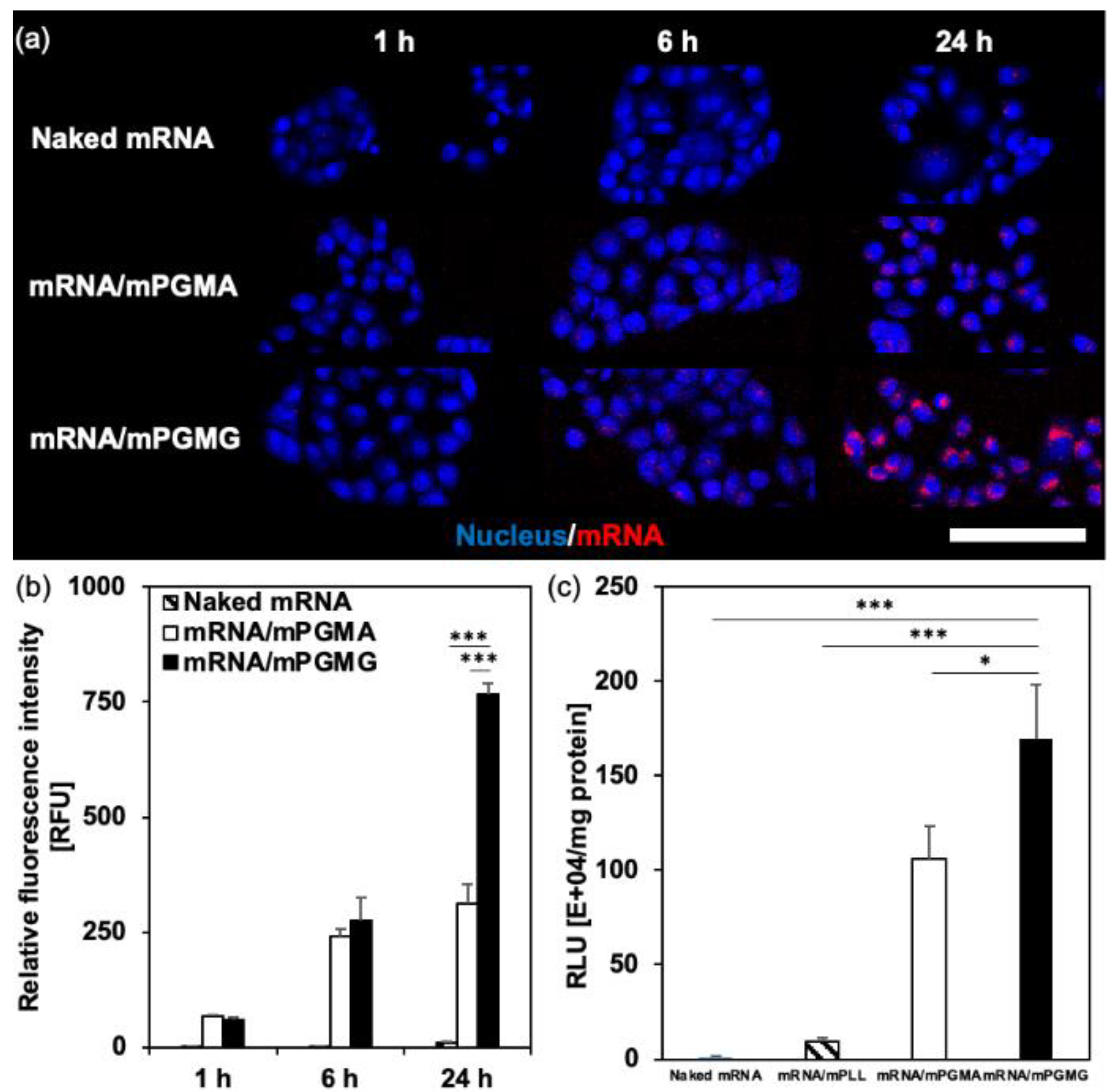
| Micelles | ΔH [kcal/mol] a | ΔS [cal/(mol·K)] a | ΔG [kcal/mol] a | KA [M−1] a |
|---|---|---|---|---|
| mRNA/mPGBA | 6.07 ± 0.06 | 57.7 ± 0.5 | −10.5 ± 0.2 | (1.40 ± 0.51) × 108 |
| mRNA/mPLL | 7.00 ± 0.19 | 52.8 ± 0.2 | −8.27 ± 0.12 | (2.59 ± 0.54) × 108 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyazaki, T.; Uchida, S.; Miyahara, Y.; Matsumoto, A.; Cabral, H. Development of Flexible Polycation-Based mRNA Delivery Systems for In Vivo Applications. Mater. Proc. 2021, 4, 5. https://doi.org/10.3390/IOCN2020-07857
Miyazaki T, Uchida S, Miyahara Y, Matsumoto A, Cabral H. Development of Flexible Polycation-Based mRNA Delivery Systems for In Vivo Applications. Materials Proceedings. 2021; 4(1):5. https://doi.org/10.3390/IOCN2020-07857
Chicago/Turabian StyleMiyazaki, Takuya, Satoshi Uchida, Yuji Miyahara, Akira Matsumoto, and Horacio Cabral. 2021. "Development of Flexible Polycation-Based mRNA Delivery Systems for In Vivo Applications" Materials Proceedings 4, no. 1: 5. https://doi.org/10.3390/IOCN2020-07857
APA StyleMiyazaki, T., Uchida, S., Miyahara, Y., Matsumoto, A., & Cabral, H. (2021). Development of Flexible Polycation-Based mRNA Delivery Systems for In Vivo Applications. Materials Proceedings, 4(1), 5. https://doi.org/10.3390/IOCN2020-07857








