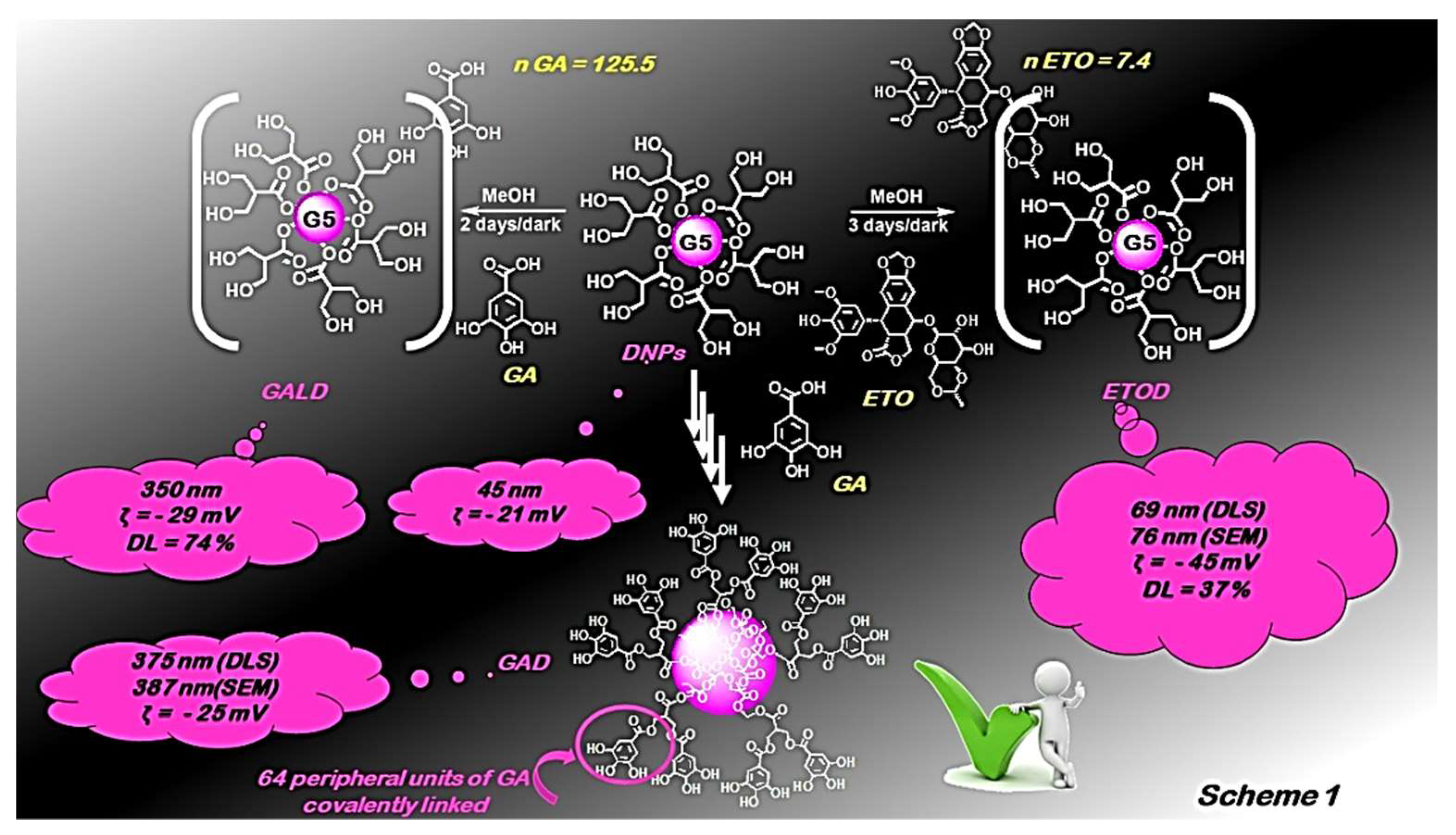
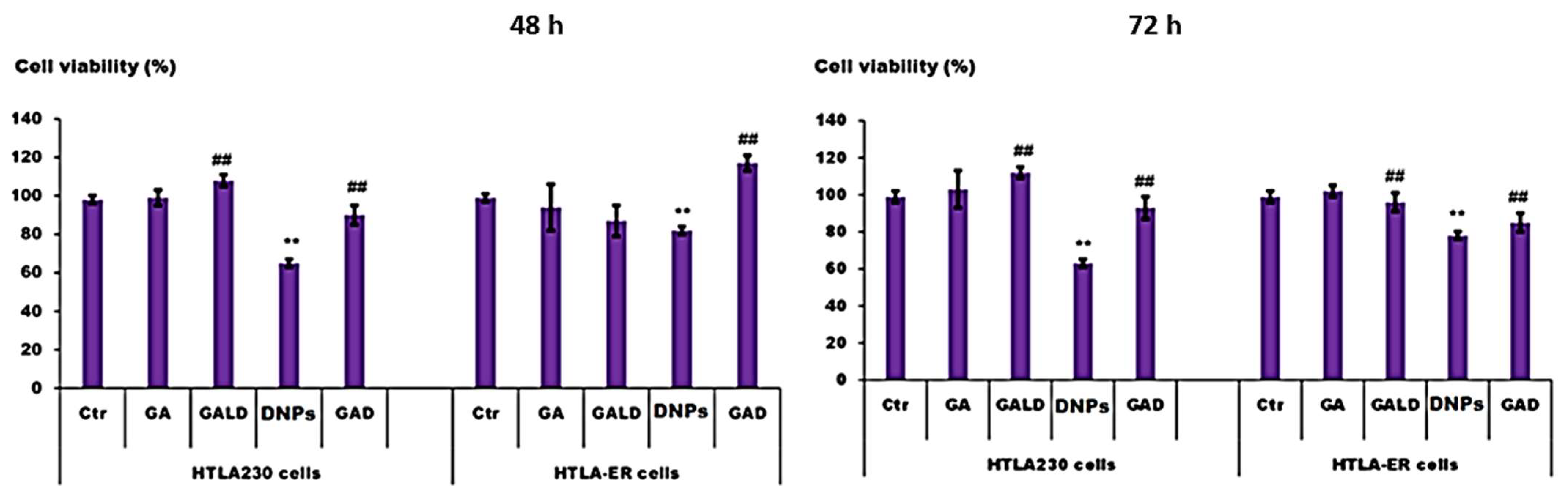
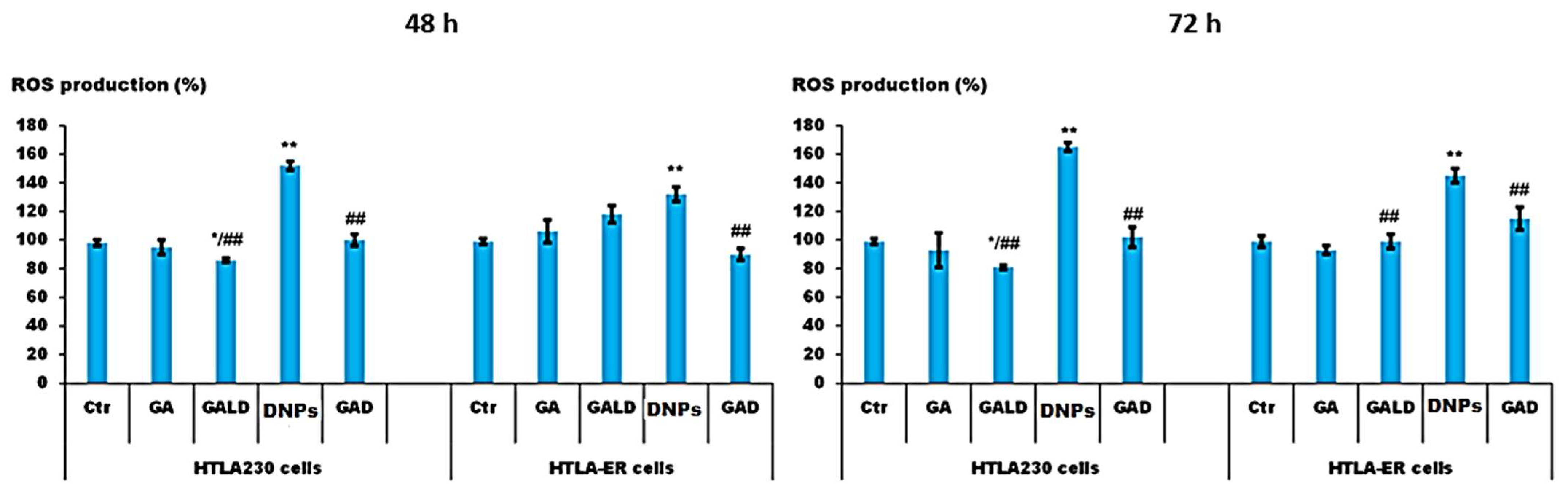
Cytotoxic Activity of Dendrimer Nanoparticles and Dendrimer Drugs Formulations on Human Neuroblastoma Cells: Our Recent Update †
Data Availability Statement
References
- Alfei, S.; Marengo, B.; Zuccari, G.; Turrini, F.; Domenicotti, C. Dendrimer Nanodevices and Gallic Acid as Novel Strategies to Fight Chemoresistance in Neuroblastoma Cells. Nanomaterials 2020, 10, 1243. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Domenicotti, C. Polyester-Based Dendrimer Nanoparticles Combined with Etoposide Have an Improved Cytotoxic and Pro-Oxidant Effect on Human Neuroblastoma Cells. Antioxidants 2020, 9, 50. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfei, S.; Marengo, B.; Valenti, G.E.; Zuccari, G.; Domenicotti, C. Cytotoxic Activity of Dendrimer Nanoparticles and Dendrimer Drugs Formulations on Human Neuroblastoma Cells: Our Recent Update. Mater. Proc. 2021, 4, 48. https://doi.org/10.3390/IOCN2020-07970
Alfei S, Marengo B, Valenti GE, Zuccari G, Domenicotti C. Cytotoxic Activity of Dendrimer Nanoparticles and Dendrimer Drugs Formulations on Human Neuroblastoma Cells: Our Recent Update. Materials Proceedings. 2021; 4(1):48. https://doi.org/10.3390/IOCN2020-07970
Chicago/Turabian StyleAlfei, Silvana, Barbara Marengo, Giulia Elda Valenti, Guendalina Zuccari, and Cinzia Domenicotti. 2021. "Cytotoxic Activity of Dendrimer Nanoparticles and Dendrimer Drugs Formulations on Human Neuroblastoma Cells: Our Recent Update" Materials Proceedings 4, no. 1: 48. https://doi.org/10.3390/IOCN2020-07970
APA StyleAlfei, S., Marengo, B., Valenti, G. E., Zuccari, G., & Domenicotti, C. (2021). Cytotoxic Activity of Dendrimer Nanoparticles and Dendrimer Drugs Formulations on Human Neuroblastoma Cells: Our Recent Update. Materials Proceedings, 4(1), 48. https://doi.org/10.3390/IOCN2020-07970









