Abstract
Chitosan and its derivatives, known for their unique molecular structures and advantageous biological properties, have emerged as promising candidates for diverse applications, particularly in the realm of water treatment. This study investigated the effectiveness of chitosan hydrogel beads combined with activated carbon in removing lead from contaminated water sources. The overarching objective of this research endeavor is to develop a sustainable and cost-effective wastewater treatment system, aligning with Qatar Vision 2030’s emphasis on sustainable development goals. Experimental investigations were conducted to fabricate chitosan hydrogel beads and assess their characteristics through rigorous FTIR and ICP-OES analyses. Notably, the incorporation of activated carbon with chitosan significantly enhanced lead removal efficacy, achieving removal efficiencies ranging from 80.29% to 96.48% with various activated carbon mixtures, indicating promising opportunities for further optimization. The FTIR analysis showed that incorporating activated carbon into chitosan beads resulted in distinct changes in the IR spectra. AC-chitosan beads exhibited broad -OH peaks at 3272 cm−1 and a stretch at 1639 cm−1, which were less pronounced or absent in isolated chitosan beads. Both types showed a peak at 1376 cm−1, with higher intensity in regular chitosan beads. Beyond underscoring the importance of chitosan-based materials in water treatment, this study also provides insightful recommendations for future research endeavors aimed at fostering awareness and facilitating practical applications, thereby bolstering environmental conservation and sustainable water management initiatives.
1. Introduction
Water resources are indispensable for human survival and progress. However, the increasing activities associated with industry, agriculture, population growth, and urbanization have led to the contamination of water supplies [1]. This contamination poses risks to both humans and animals due to pollutants such as heavy metals, dyes, pesticides, and medications. Therefore, preserving and safeguarding water resources should be of paramount importance, necessitating the adoption of state-of-the-art water treatment technologies and effective pollution control methods. The contamination of water resources by harmful substances underscores the immediate requirement for efficient water management and pollution control strategies. To address this issue, research and development is paramount to discovering sustainable means of water treatment to prevent the release of pollutants into waterways [2]. Toxic metals are usually found in industrial, municipal, and urban runoff, which threatens humans and biotic life. Increased human activities (urbanization and industrialization) are to be blamed for an increased level of trace metals, especially heavy metals, in our water resources [3]. There are more than 50 elements that can be classified as heavy metals, some of them, around 17 of which, are considered to be very toxic and relatively accessible. Characteristically, anions also have an important effect on drinking water; results also showed that they affect human health (Figure 1). The toxicity level depends on the type of metal, its biological role, and the type of organisms that are exposed to it. Heavy metals that have a vital effect on the aquatic flora and fauna by biomagnification enter the food chain and ultimately affect the human body organs. They enter the food chain mainly through plants and fish. Plants are able to take up heavy metals dissolved in water, leading to accumulation at their roots and later to translocation to edible parts in plants such as vegetables and fruits [4]. Though a minimum concentration of these metals is required for the body, exceeding the limits can be poisonous and hazardous for humans. In addition, the heavy metals that accumulate in drinking water linked most often to human poisoning are lead, iron, cadmium (Cd) copper (Cu), zinc (Zn), chromium (Cr), etc. Heavy metals are usually required by the body in small amounts but can also be toxic in large doses. They constitute one important group of environmentally hazardous substances if present [1].
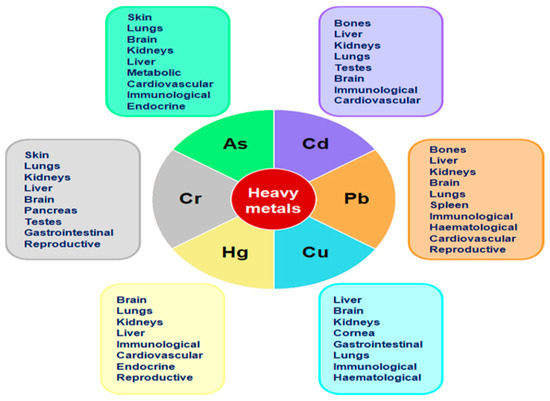
Figure 1.
Main organs and systems affected by heavy metals [5].
Many and different methods have been developed for wastewater treatment (heavy metals) such as ion exchange [6], reverse osmosis, chemical precipitation, solvent extraction, and membrane processes [7]. Most of these methods are expensive, tedious, and cause secondary pollution, which limits their industrial application. However, one technique for water treatment from heavy metals that has gained much work and attention because of its low cost, simplicity, and effectiveness is adsorption [8]. By using biological materials as adsorbents for water treatment results in minimum to no secondary pollution; hence biosorption is preferred. Chitosan has excellent physical properties, e.g., mechanical properties, which not only accommodate a wide range of processing methods (such as casting, fiber spinning, supercritical fluid processing, and electrospinning), but also enable the product to be converted to many different dosage forms, from film to microparticles or nanofibers. In addition to that, chitosan has been explored as an alternative adsorbent material for wastewater treatment. As shown in Figure 2, it is an important polymer derived from the partial deacetylation of chitin that is the second most abundant natural polymer existing in carb shells, shrimp shells, and some fungi [9]. The main properties and characteristics of chitosan are summarized in the following Table 1 [10]. Some of the intrinsic properties of chitosan, such as its polycationic character in acid media and its ability to form hydrogen bonds, van der Walls, and electrostatic interactions, make it an efficient adsorbent material. Other characteristics, such as the degree of deacetylation, crystallinity, molecular weight, solubility, surface area, and particle size will all influence the properties of the final chitosan-based material and its adsorption potential effect. Therefore, these properties and their optimization are central to the formation of efficient adsorbent materials [10]. It also has been widely used in many different fields, such as the fields of chemistry, pharmacy, medicine, and environment, due to their specific characteristics and advantages, such as biocompatibility, low toxicity, biodegradability, and high adsorption capability. In the branch of environmental engineering, there have been successful attempts to remove all the heavy metal ions commonly found in wastewater with Chitosan and its derivatives [11]. It has been successfully used as an absorbent to remove other pollutants, including fluorides, pigments, phenols, etc. The preparation technique, adsorption mechanism, and other factors affect the adsorption performance of effective adsorbents for each type of heavy metal [11].
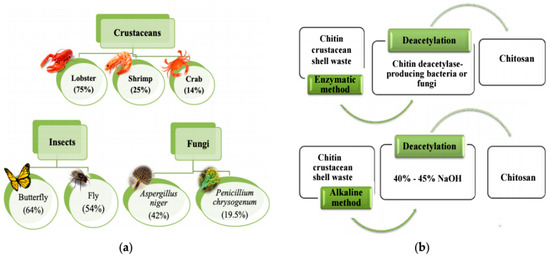
Figure 2.
(a) Examples of chitin extraction from different sources, (b) chitin deacetylation methods to produce chitosan [9].

Table 1.
Main chitosan properties, according to the information reported by Jiménez-Gómez and Cecilia.
However, accessing water in Qatar presents significant challenges. The escalating demand for water, driven by population expansion and rapid industrialization, poses a considerable hurdle. Consequently, Qatar’s water resources are under immense pressure. Moreover, Qatar exhibits one of the highest rates of household water consumption globally, further straining its water reserves [12]. Inefficient water utilization and network losses exacerbate the burden on water supplies. The widespread reliance on groundwater for agricultural purposes, coupled with inefficient conventional irrigation methods, contributes to water wastage. Therefore, addressing Qatar’s water demand necessitates the filtration of contaminated water to alleviate these challenges. These obstacles underscore Qatar’s significance in surmounting water scarcity issues and embracing sustainable water management practices. Consequently, wastewater treatment emerges as a crucial solution to this pressing issue [13].
Our research explores advancements in water treatment strategies using waste materials characterized by low cost, biodegradability, bioactivity, biocompatibility, and nontoxicity. As a result, we can keep water resources unpolluted and reuse wastewater after treatment to meet our daily needs. The expected benefits of harnessing chitosan and municipal waste residue in a novel composite biosorbent for effective heavy-metal removal from wastewater include high adsorption efficiency, low cost, and simple operation [13,14]. Chitosan is a hydrophilic, biodegradable, and biocompatible polymer with abundant -NH2 and -OH groups on its surface, which can chelate heavy metal ions to form stable chelates, making it an effective adsorbent for heavy metal ions in water bodies. The combination of chitosan with municipal waste residue could potentially enhance the adsorption capacity and facilitate the removal of heavy metals, such as Cr (VI), Cu (II), Pb (II), and Zn (II), from wastewater. However, for this research, only lead (Pb) was tested for removal efficiency due to its common presence and detrimental effects in wastewater. The use of such composite biosorbents is also environmentally friendly and can be modified to improve the separation effects and recyclability, which are important for practical applications in wastewater treatment [15,16,17]. The aim of our research was to develop a new method that is effective, low-cost, and sustainable for the treatment of wastewater contaminated with heavy metals. This research aligns with Qatar Vision 2030, which emphasizes the sustainable development and the preservation of water resources
2. Experiment
2.1. Procedure
Source and production: Chitosan is derived from chitin, usually obtained from natural sources in our environment such as from the residues of shrimp, crab and lobster, fungal mycelia, and green alga [15].
Stock preparation: The experimental methodology involved the preparation of stock solutions and subsequent steps for the synthesis of the chitosan–acetic acid mixture. First, a stock solution of acetic acid was prepared by adding 2 mL of acetic acid to a conical flask and diluting it with 98 mL of deionized water. A stock solution of 0.5 M sodium hydroxide (NaOH) was prepared by weighing 4 g of NaOH and diluting it with 100 mL of deionized water in another conical flask. In addition, 2 g of chitosan was weighed separately [15].
Beads formation: In the subsequent step, chitosan is partially added to the diluted acetic acid to achieve the desired viscosity. To ensure homogeneity, the chitosan–acetic acid mixture was further diluted with the acetic acid stock solution and stirred overnight. The chitosan and acetic acid mixture were added dropwise to NaOH to form small spherical hydrogel beads. A distance of 10 cm was used between the syringe and NaOH solution. The resulting mixture was stirred for 24 h to facilitate the reaction. The beads were filtered and dried at a temperature of 50 °C. One gram of four different biochars dried at different temperatures (550, 650, 750, and 850 °C) was ground, washed with deionized water, filtered, and left overnight to dry. An acetic acid stock was prepared and added to 2 g of chitosan powder to divide the solution into four different beakers, and each beaker had 25 mL of the solution. In addition, 0.2 g of each biochar was added to beakers containing the acetic acid–chitosan mix and placed on a stirrer for 15 min. The NaOH stock solution was prepared by dropping the previous mixture on the NaOH solution to form beads. The beads were then vacuum-filtered.
2.2. Analysis
ICP-OES (Inductively Coupled Plasma Optical Emission Spectrometry): This technique was used to quantitatively assess the concentration of lead in water before and after treatment with chitosan hydrogel beads, both with and without activated carbon (AC) enhancement. Samples were prepared from treated and untreated water and analyzed to determine the efficiency of lead removal.
FTIR (Fourier Transform Infrared Spectroscopy): FTIR analysis was conducted to characterize the chemical structure and functional groups present in the chitosan beads, including those mixed with activated carbon. This allowed for the assessment of any chemical changes or interactions that occurred during the adsorption process. Beads were analyzed at various stages, including pure chitosan beads and chitosan beads mixed with activated carbon treated at different temperatures (550, 650, 750, and 850 °C).
SEM (Scanning Electron Microscopy): SEM was utilized to visualize the morphology and surface characteristics of both the chitosan beads and the activated carbon, as well as the composite material formed by their combination. This method provided insight into the physical changes and the distribution of activated carbon within the beads. Images were captured for chitosan and activated carbon before mixing and for the final chitosan–AC beads.
TEM (Transmission Electron Microscopy): TEM imaging was performed to examine the nano-scale structure and detailed interaction between chitosan and activated carbon within the beads. This allowed for a deeper understanding of the composite material’s internal structure, crucial for assessing the homogeneity and efficacy of the adsorption sites. These methods collectively aimed to provide a comprehensive evaluation of the chitosan hydrogel beads’ ability to remove heavy metals from water, focusing on lead as the primary contaminant. The study also explored the impact of incorporating activated carbon into the beads, assessing potential enhancements or detriments to the adsorption process.
3. Results and Discussion
3.1. ICP-OES Results
The chitosan hydrogel beads demonstrated a remarkable adsorption efficiency for lead ions in water. The high surface area of the hydrogel beads, combined with the presence of amino groups in chitosan, facilitated strong interactions with lead ions, leading to effective removal from the aqueous solution. Evidently, the lead solution with an initial concentration of 100–110 ppm was significantly reduced to 3.7–3.8 ppm after incubation with chitosan hydrogel beads. This remarkable reduction indicates the high efficiency of chitosan hydrogel beads in adsorbing lead ions from the solution. The amino groups present in chitosan likely played a crucial role in forming strong bonds with lead ions, leading to effective removal. When chitosan beads were combined with activated carbon, the lead concentration further decreased, but not as significantly as with chitosan beads alone. The resulting concentration range of 14–20 ppm (specifically, 3.859, 11.489, 21.587, 12.723, and 12.485 ppm for chitosan beads, AC chitosan 550, AC chitosan 650, AC chitosan 750, and AC chitosan 850, respectively) suggests that the addition of activated carbon has influenced the overall adsorption capacity. Chitosan beads’ lead removal capacity with different activated carbon mixtures is shown in Table 2. Based on the provided data, the lead removal efficiency (calculated using initial and final lead concentration values) achieved with chitosan hydrogel beads ranged from 80.29% to 96.48% with the different mixtures. This signifies a highly effective reduction in lead concentration. However, activated carbon shows a negative impact on the beads’ lead removal efficiency, possibly due to inadequate binding with the chitosan bead. The results found are in tandem with other studies conducted worldwide. A lead removal efficiency of 99.88% was obtained by one study at pH 4 and a duration of 90 min [18]. Similar results were reported showcasing the excellent adsorption capacity of chitosan composites for heavy metal removal in wastewater [19,20,21,22,23,24]. In summary, the results obtained with chitosan hydrogel beads showcase their effectiveness in lead removal.

Table 2.
Results for lead removal using chitosan beads and activated carbon.
3.2. FTIR
The chitosan beads were characterized by FTIR (Fourier Transform Infra-Red). The characterized beads included the chitosan beads alone as well as chitosan beads that are mixed with AC (activated carbon from biochar heated at different temperature degrees 550, 650, 750, and 850 °C). The AC-mixed chitosan beads (Figure 3) all show similar IR spectra but with different intensities. However, the isolated chitosan beads showed some minor dissimilarities. Nevertheless, AC chitosan beads showed large broad -OH peaks at 3272 cm−1 that indicate water being within the beads [25], and the peak is also shown in chitosan beads. Also, AC chitosan beads show stretches at 1639 cm−1, which might indicate C=C stretching [26]. However, this stretch diminishes in the isolated chitosan beads. Moreover, both chitosan beads show stretching at 1376 cm−1 that indicates -OH bending, C-O-H in-plane bending, or -CH3 out-of-plane bending [27]. This peak is more intense in regular chitosan beads than in AC-mixed chitosan beads. Overall, these observations suggest that while the fundamental structure of chitosan remains relatively unchanged with the incorporation of activated carbon, the presence of AC does influence the chemical environment within the beads. This influence is manifested in changes in the intensity of certain IR peaks and the presence or absence of specific stretches, indicating differences in the interaction between functional groups within the chitosan matrix and the introduced AC.
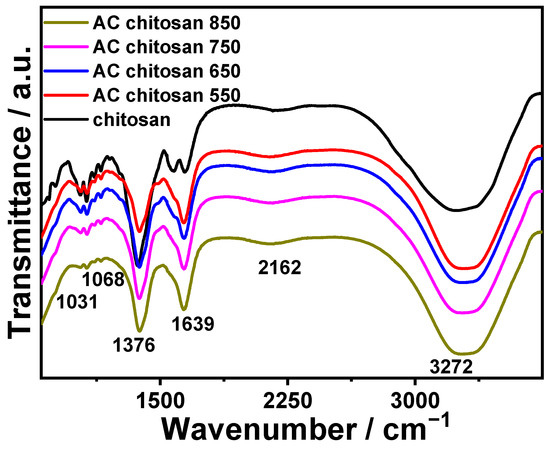
Figure 3.
FTIR spectrum for chitosan beads hydrogel doped with modified activated carbon complex.
3.3. SEM
The SEM images show all the starting materials and products for the chitosan beads, which are chitosan, activated carbon from biochar, chitosan beads, and chitosan beads with ACC. As shown in Figure 4, images for sample 1, show non-uniform conglomerations of chitosan, which is the starting material for the chitosan beads. Consequently, we can see images of sample 6, which is the product (chitosan beads). The image shows the final chitosan bead product having a spherical cluster structure, which is true in the sense of what was undertaken in the manufacturing process. Also, it is seen that grooves within the beads are uniform and parallel to each other in comparison to the starting material which is chitosan. This reflects the change that happened to sample 1. Images of samples 2 through 5 show the ACC from biochar before being added to the chitosan to make ACC chitosan beads. Images of samples 7 through 10 show the ACC chitosan beads. However, they show a tangential attachment of the ACC to the chitosan beads and not a homogenous mixture of the two components. Nevertheless, there is no demonstration of corrosion on any of the products within the SEM images.
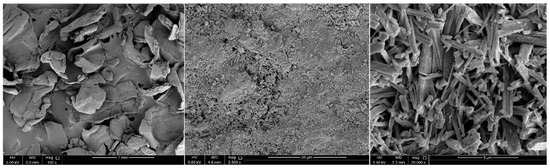
Figure 4.
SEM results from samples 1, 4, and 9.
3.4. TEM
The TEM image of sample 1 shows the chitosan starting material before being made into chitosan beads. The image of sample 5 shows the ACC from the biochar waste. Finally, the image of sample 8 shows the conglomeration of chitosan and activated carbon making the ACC chitosan beads (Figure 5).
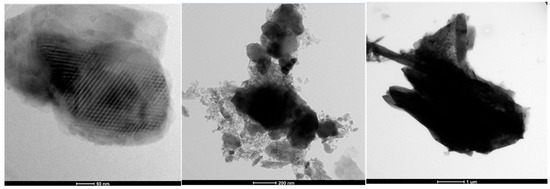
Figure 5.
TEM results from samples 1, 5, and 8.
4. Conclusions
This research effectively demonstrated the efficacy of chitosan hydrogel beads in removing heavy metals (lead) from contaminated water sources, in line with the overall objectives of sustainable water management, as stated in Qatar Vision 2030. The study demonstrates that incorporating activated carbon alongside chitosan significantly improves lead removal, achieving removal efficiencies ranging from 80.29% to 96.48% with various activated carbon mixtures. The FTIR analysis revealed distinct changes in the IR spectra upon incorporating activated carbon into chitosan beads. AC-chitosan beads exhibited prominent broad -OH peaks at 3272 cm−1 and a notable stretch at 1639 cm−1, characteristics that were less evident or absent in isolated chitosan beads. Both bead types displayed a peak at 1376 cm−1, with regular chitosan beads showing higher intensity. These findings underscore the potential for optimizing the effectiveness of chitosan-based materials in treating lead-contaminated environments. This study not only tackles urgent environmental issues, but also advances sustainable water pollution solutions, as well as highlighting the potential for researching other problematic heavy metals removal with chitosan, supporting the larger goals of Qatar Vision 2030. To enhance the effectiveness and practicality of chitosan-based hydrogel beads for water treatment, it is recommended to fine-tune the bead composition by exploring optimal bead sizes, chitosan concentrations, and the potential benefits of incorporating activated carbon. This optimization could lead to more cost-effective and efficient heavy metal removal. Additionally, scaling up production and performing field tests in actual water treatment settings are crucial steps. Such field testing will provide valuable insights into the beads’ real-world efficacy, ensuring their reliability and applicability in industrial-scale water purification efforts.
Author Contributions
Conceptualization, methodology, software, G.A., M.H., M.A.F.A., A.H.S.A., R.A.M.K., M.Y.F. and J.H.A.A.-M.; validation, N.A.-Q.; formal analysis, J.H.A.A.-M.; investigation, G.A. and M.H.; resources, M.A.F.A., A.H.S.A. and R.A.M.K.; data curation, J.H.A.A.-M. and M.H.R.S.; writing—original draft preparation, G.A. and M.H.; writing—review and editing, A.M. and N.A.-Q.; visualization, M.A.F.A.; supervision, project administration, funding acquisition, N.A.-Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Qatar National Research Fund, a member of Qatar Foundation, and is recognized for providing the UREP award [UREP30-105-1-020] that made this research possible.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original data and findings presented in this study are available within the article; for any additional questions, please contact the corresponding author.
Acknowledgments
The authors express profound gratitude to the Central Laboratories Unit (CLU) at Qatar University for their invaluable guidance throughout this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ali, I.; Alharbi, O.M.; Tkachev, A.G.; Galunin, E.; Burakov, A.E.; Grachev, V.A. Water treatment by new-generation graphene materials: Hope for bright future. Environ. Sci. Pollut. Res. 2018, 25, 7315–7329. [Google Scholar] [CrossRef] [PubMed]
- Basheer, A.A. New generation nano-adsorbents for the removal of emerging contaminants in water. J. Mol. Liq. 2018, 261, 583–593. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.Z.; Salleh, W.N.; Ismail, A.F.; Yusof, N.; Yusop, M.Z.; Aziz, F. Adsorptive removal of heavy metal ions using graphene-based nanomaterials: Toxicity, roles of functional groups and mechanisms. Chemosphere 2020, 248, 126008. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Qasem, N.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. npj Clean Water 2021, 4, 1–5. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Cui, Y.; Chen, N. Removal of copper ions from wastewater: A review. Int. J. Environ. Res. Public Health 2023, 20, 3885. [Google Scholar] [CrossRef]
- El Batouti, M.; Al-Harby, N.F.; Elewa, M.M. A review on promising membrane technology approaches for heavy metal removal from water and wastewater to solve water crisis. Water 2021, 13, 3241. [Google Scholar] [CrossRef]
- Pal, K.; Sarkar, P.; Anis, A.; Wiszumirska, K.; Jarzębski, M. Polysaccharide-Based Nanocomposites for Food Packaging Applications. Materials 2021, 14, 5549. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A natural biopolymer with a wide and varied range of applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Ajaj, Y.; Mahmoud, Z.H.; Ghadir, G.K.; Alani, Z.K.; Hussein, M.M.; Hussein, S.A.; Karim, M.M.; Al-khalidi, A.; Abbas, J.K.; et al. Adsorption of heavy metal ions use chitosan/graphene nanocomposites: A review study. Results Chem. 2024, 7, 101332. [Google Scholar] [CrossRef]
- Baalousha, H.M.; Ouda, O.K. Domestic water demand challenges in Qatar. Arab. J. Geosci. 2017, 10, 537. [Google Scholar] [CrossRef]
- He, Y.; Zhang, P.; Wang, L. Adsorption and Removal of Cr6+, Cu2+, Pb2+, and Zn2+ from Aqueous Solution by Magnetic Nano-Chitosan. Molecules 2023, 28, 2607. [Google Scholar] [CrossRef]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood waste: A source for preparation of commercially employable chitin/chitosan materials. Bioresour. Bioprocess. 2019, 6, 1–20. [Google Scholar] [CrossRef]
- da Silva Alves, D.C.; Healy, B.; Pinto, L.A.; Cadaval, T.R., Jr.; Breslin, C.B. Recent developments in chitosan-based adsorbents for the removal of pollutants from aqueous environments. Molecules 2021, 26, 594. [Google Scholar] [CrossRef]
- Harugade, A.; Sherje, A.P.; Pethe, A.M. Chitosan: A review on properties, biological activities and recent progress in biomedical applications. React. Funct. Polym. 2023, 191, 105634. [Google Scholar] [CrossRef]
- Kumar, N.; Chandan, N.K.; Bhushan, S.; Singh, D.K.; Kumar, S. Health risk assessment and metal contamination in fish, water and soil sediments in the East Kolkata Wetlands, India, Ramsar site. Sci. Rep. 2023, 13, 1546. [Google Scholar] [CrossRef]
- Pratiwi, R.; Prinajati, P.D. Adsorption for lead removal by chitosan from shrimp shells. Indones. J. Urban Environ. Technol. 2018, 2, 35–46. [Google Scholar] [CrossRef]
- Mohammad, A.M.; Eldin TA, S.; Hassan, M.A.; El-Anadouli, B.E. Efficient treatment of lead-containing wastewater by hydroxyapatite/chitosan nanostructures. Arab. J. Chem. 2017, 10, 683–690. [Google Scholar] [CrossRef]
- Zhang, C.J.; Hu, M.; Ke, Q.F.; Guo, C.X.; Guo, Y.J.; Guo, Y.P. Nacre-inspired hydroxyapatite/chitosan layered composites effectively remove lead ions in continuous-flow wastewater. J. Hazard. Mater. 2020, 386, 121999. [Google Scholar] [CrossRef]
- Karim, M.R.; Aijaz, M.O.; Alharth, N.H.; Alharbi, H.F.; Al-Mubaddel, F.S.; Awual, M.R. Composite nanofibers membranes of poly (vinyl alcohol)/chitosan for selective lead (II) and cadmium (II) ions removal from wastewater. Ecotoxicol. Environ. Saf. 2019, 169, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Sheth, Y.; Dharaskar, S.; Khalid, M.; Sonawane, S. An environment friendly approach for heavy metal removal from industrial wastewater using chitosan based biosorbent: A review. Sustain. Energy Technol. Assess. 2021, 43, 100951. [Google Scholar] [CrossRef]
- Rahman, A. Promising and Environmentally Friendly Removal of Copper, Zinc, Cadmium, and Lead from Wastewater Using Modified Shrimp-Based Chitosan. Water 2024, 16, 184. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Gao, M.; Hu, X.; Zhang, X.; Li, Y.; Xu, X.; Hu, J.; Tang, C.; Hu, X. Chitosan and biochar synergize the efficient elimination of lead from wastewater by sulfidised nano-zero-valent iron. J. Environ. Chem. Eng. 2022, 10, 107101. [Google Scholar] [CrossRef]
- Frost, R.L.; López, A.; Theiss, F.L.; Aarão, G.M.; Scholz, R. A vibrational spectroscopic study of the phosphate mineral rimkorolgite (Mg, Mn2+) 5 (Ba, Sr)(PO4)4· 8H2O from Kovdor massif, Kola Peninsula, Russia. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 762–766. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anjeh, A.M.; Nabavi, S.R. Preparation, characterization and properties of a novel electrospun polyamide-6/chitosan/graphene oxide composite nanofiber. J. Polym. Environ. 2022, 30, 3934–3948. [Google Scholar] [CrossRef]
- Atangana, E.; Chiweshe, T.T.; Roberts, H. Modification of novel chitosan-starch cross-linked derivatives polymers: Synthesis and characterization. J. Polym. Environ. 2019, 27, 979–995. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).