Flotation of Sulphide Minerals Using Organosolv Lignin as Collector—Pilot-Scale Trials †
Abstract
1. Introduction
2. Materials and Methods
2.1. Ore Properties and Pretreatment
2.2. Lignin Production
- (i)
- Wood chip pretreatment through fractionation with ethanol to separate lignocellulosic biomass into the three main fractions of cellulose, hemicellulose and lignin from wood. Lignin and hemicellulose liquid was obtained through pressure filtration.
- (ii)
- Ethanol removal using evaporation and lignin recovery.
- (iii)
- Lignin dissolution in ethanol/water solution;
- (iv)
- Pressure homogenization of ethanol/water solution at 750 b;
- (v)
- Dilution of homogenized lignin with deionized water, causing nanoparticle development;
- (vi)
- Freeze-drying to obtain nanoparticles as a dry powder.
2.3. Flotation Conditions
3. Results and Discussion
4. Conclusions
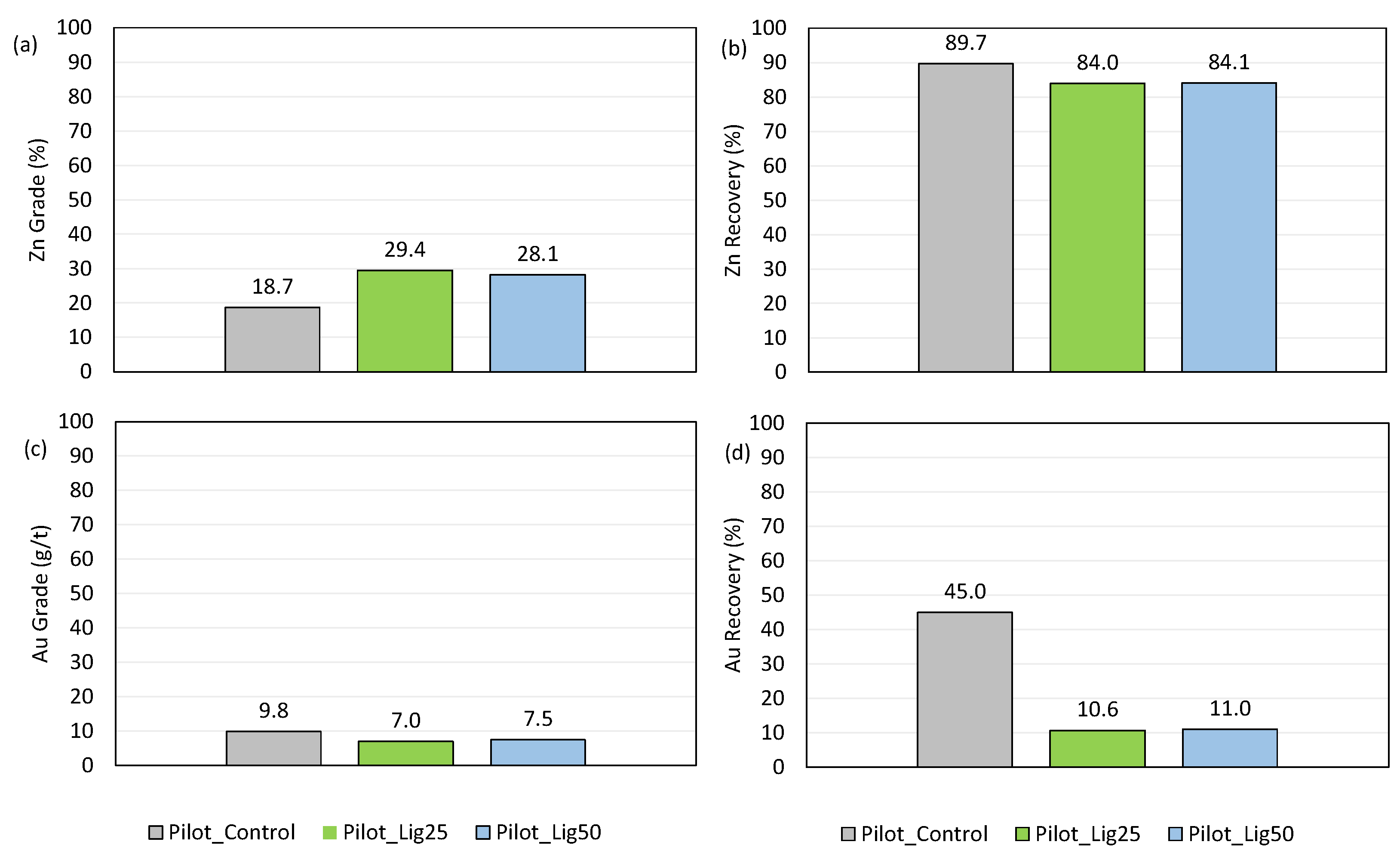
- A significant increase in Zn grade, as the Zn grade increased from 18.7% for pure SIPX to 29.4 and 28.1% for the 25 and 50% SIPX replacement with OLN. The Zn recovery reduced by approx. 5% when a mixed collector was applied, from 89.7% for pure SIPX to 84% and 84.1% for the 25 and 50% SIPX replacement with OLN, respectively.
- The produced concentrates contained much lower pyrite/arsenopyrite as revealed by their Au content. From an original 45% Au recovery using pure SIPX, it was reduced to 10.6% and 11.0% for the 25 and 50% SIPX replacement with OLN, respectively.
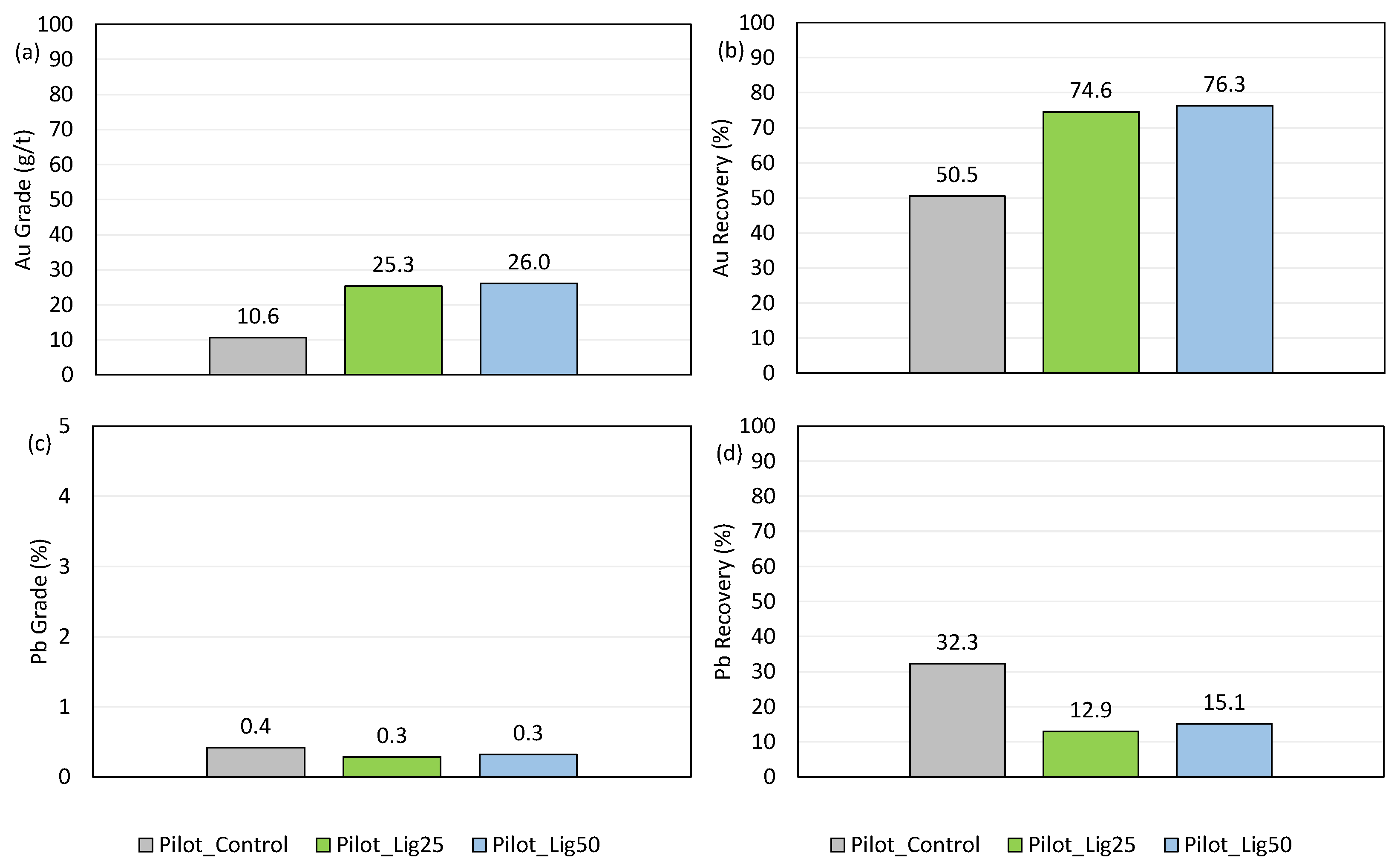
- A significant increase of Au grade in concentrates, from 10.5 g/t for pure SIPX to 25.3 g/t and 26 g/t Au (for of the 25 and 50% SIPX replacement with OLN, respectively) was identified by applying a mixed collector. A sharp increase in Au recovery was also identified, from 50.5% for pure SIPX to 74.6 and 76.3% Au recovery for of the 25 and 50% SIPX replacement with OLN, respectively.
- A significant reduction of Pb grade was identified, implying better selectivity, from a Pb recovery of 32.3% for pure SIPX to 12.9% and 15.12% for the 25 and 50% SIPX replacement with OLN, respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Wen, S.; Han, G.; Xu, L.; Feng, Q. Activation mechanism of lead ions in the flotation of sphalerite depressed with zinc sulfate. Miner. Eng. 2020, 146, 106132. [Google Scholar] [CrossRef]
- Wang, X.H.; Eric Forssberg, K.S. Mechanisms of pyrite flotation with xanthates. Int. J. Miner. Process. 1991, 33, 275–290. [Google Scholar] [CrossRef]
- Shen, Y.; Nagaraj, D.R.; Farinato, R.; Somasundaran, P. Study of xanthate decomposition in aqueous solutions. Miner. Eng. 2016, 93, 10–15. [Google Scholar] [CrossRef]
- Williams, C.; Peng, Y.; Dunne, R. Eucalyptus oils as green collectors in gold flotation. Miner. Eng. 2013, 42, 62–67. [Google Scholar] [CrossRef]
- Saim, A.K.; Darteh, F.K. Eco-Friendly and Biodegradable Depressants in Chalcopyrite Flotation: A Review. Miner. Process. Extr. Metall. Rev. 2022, 44, 492–510. [Google Scholar] [CrossRef]
- Slabov, V.; Jain, G.; Larsen, E.; Kota, H.R.; Chernyshova, I. Eco-Friendly Collectors for Flotation of Fine Hematite and Malachite Particles. In Mining, Metallurgy & Exploration; Springer International Publishing: Cham, Switzerland, 2023; pp. 475–492. [Google Scholar]
- Hrůzová, K.; Matsakas, L.; Sand, A.; Rova, U.; Christakopoulos, P. Organosolv lignin hydrophobic micro- and nanoparticles as a low-carbon footprint biodegradable flotation collector in mineral flotation. Bioresour. Technol. 2020, 306, 123235. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulos, P.M.; Kountouris, N.; Anastassakis, G.; Taxiarchou, M.; Engineering, M. Partial replacement of xanthates by organosolv lignin on pyrite/arsenopyrite flotation. In Proceedings of the XV International Mineral Processing and Recycling Conference, Belgrade, Serbia, 17–19 May 2023. [Google Scholar]
- Angelopoulos, P.M.; Anastassakis, G.; Kountouris, N.; Koukoulis, N.; Taxiarchou, M. Combined use of organosolv lignin and xanthates on sphalerite flotation from mixed sulphides. In Proceedings of the XV International Mineral Processing and Recycling Conference, Belgrade, Serbia, 17–19 May 2023. [Google Scholar]
- Kalogiannis, K.G.; Matsakas, L.; Aspden, J.; Lappas, A.A.; Rova, U.; Christakopoulos, P. Acid assisted organosolv delignification of beechwood and pulp conversion towards high concentrated cellulosic ethanol via high gravity enzymatic hydrolysis and fermentation. Molecules 2018, 23, 1647. [Google Scholar] [CrossRef] [PubMed]
- Matsakas, L.; Gerber, M.; Yu, L.; Rova, U.; Christakopoulos, P. Preparation of low carbon impact lignin nanoparticles with controllable size by using different strategies for particles recovery. Ind. Crop. Prod. 2020, 147, 112243. [Google Scholar] [CrossRef]
- Leja, J. Surface Chemistry of Froth Flotation; Plenum Press: New York, NY, USA, 1982. [Google Scholar]

| Oxide | Content (wt.%) |
|---|---|
| PbO | 0.69 |
| ZnO | 4.33 |
| Fe2O3 | 17.81 |
| As2O3 | 5.25 |
| S | 16.26 |
| Sb | 0.03 |
| CuO | 0.05 |
| C | 4.02 |
| MgO | 2.04 |
| Al2O3 | 5.25 |
| SiO2 | 22.12 |
| K2O | 1.14 |
| CaO | 16.27 |
| MnO | 1.15 |
| Other | 3.59 |
| Ore | Content (wt.%) |
|---|---|
| Galena (PbS) | 0.74 |
| Sphalerite (ZnS) | 5.18 |
| Pyrite (FeS) | 14.09 |
| Arsenopyrite (FeAsS) | 6.04 |
| Stage | Reagents in g/t | Time (min) | pH | Air (L/min) | ||||
|---|---|---|---|---|---|---|---|---|
| CuSO4 | Collector SIPX:OLN Ratio= 100:0, 75:25, 50:50 | DF250 | CaO | H2SO4 | ||||
| ZnS Conditioning | 200 | 25:0 18.75:6.25 12.5:12.5 | + | + | 5 | 10.5–11 | 0 | |
| ZnS Rougher | - | + | 3 | 15 | ||||
| ZnS Scavenger 1 | 2.5:0 1.9:0.6 1.25:1.25 | + | 2 | |||||
| ZnS Scavenger 2 | 1.5:0 1.1:0.4 0.75:0.75 | + | 1 | |||||
| AsPy Conditioning | 200 | 100:0 75:25 50:50 | + | + | 10 | 6.5–7 | 0 | |
| AsPy Rougher | + | 3 | 15 | |||||
| AsPy Scavenger | 40 | 30:0 22.5:7.5 15:15 | + | 2 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelopoulos, P.M.; Anastassakis, G.; Kountouris, N.; Taxiarchou, M.; Koutsotheodorou, E.; Pefkos, T.; Klepkos, V.; Samara, C.; Mprokos, G. Flotation of Sulphide Minerals Using Organosolv Lignin as Collector—Pilot-Scale Trials. Mater. Proc. 2023, 15, 81. https://doi.org/10.3390/materproc2023015081
Angelopoulos PM, Anastassakis G, Kountouris N, Taxiarchou M, Koutsotheodorou E, Pefkos T, Klepkos V, Samara C, Mprokos G. Flotation of Sulphide Minerals Using Organosolv Lignin as Collector—Pilot-Scale Trials. Materials Proceedings. 2023; 15(1):81. https://doi.org/10.3390/materproc2023015081
Chicago/Turabian StyleAngelopoulos, Panagiotis M., Georgios Anastassakis, Nikolaos Kountouris, Maria Taxiarchou, Effrosyni Koutsotheodorou, Tilemachos Pefkos, Vasileios Klepkos, Christina Samara, and Giorgos Mprokos. 2023. "Flotation of Sulphide Minerals Using Organosolv Lignin as Collector—Pilot-Scale Trials" Materials Proceedings 15, no. 1: 81. https://doi.org/10.3390/materproc2023015081
APA StyleAngelopoulos, P. M., Anastassakis, G., Kountouris, N., Taxiarchou, M., Koutsotheodorou, E., Pefkos, T., Klepkos, V., Samara, C., & Mprokos, G. (2023). Flotation of Sulphide Minerals Using Organosolv Lignin as Collector—Pilot-Scale Trials. Materials Proceedings, 15(1), 81. https://doi.org/10.3390/materproc2023015081







