Development of Au Nanostars/Graphene Oxide Paper for SERS †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
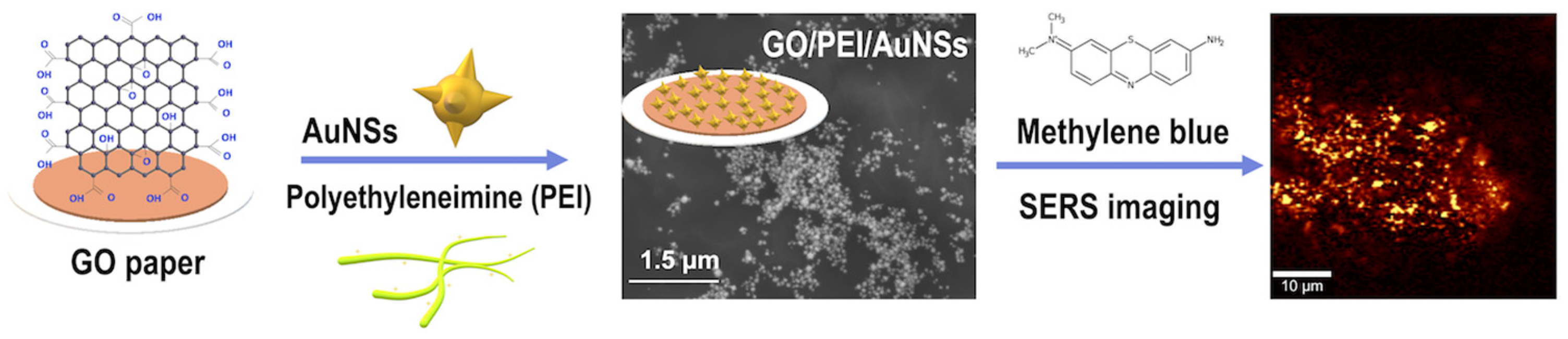
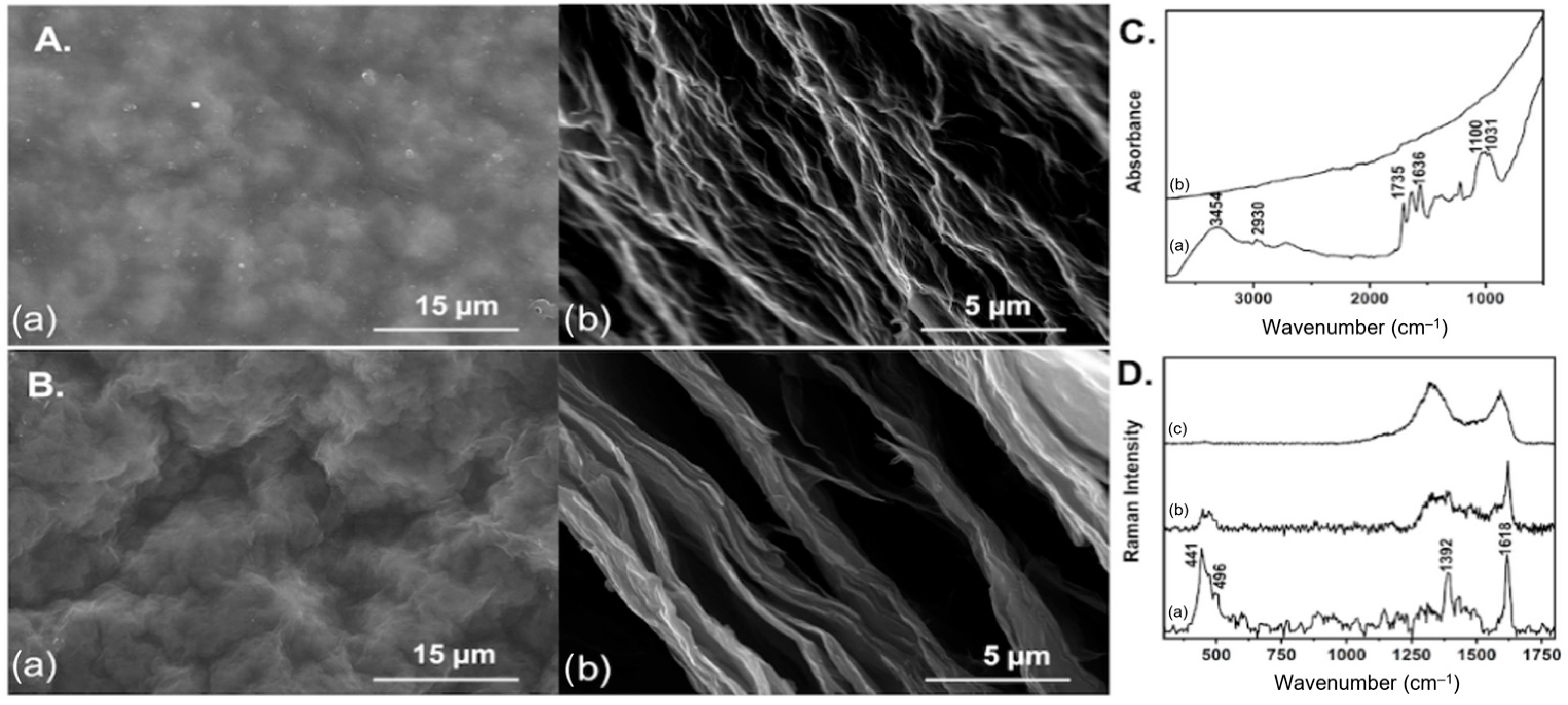
2.2. GO Paper Preparation
2.3. Au Nanostars (AuNSs) Preparation
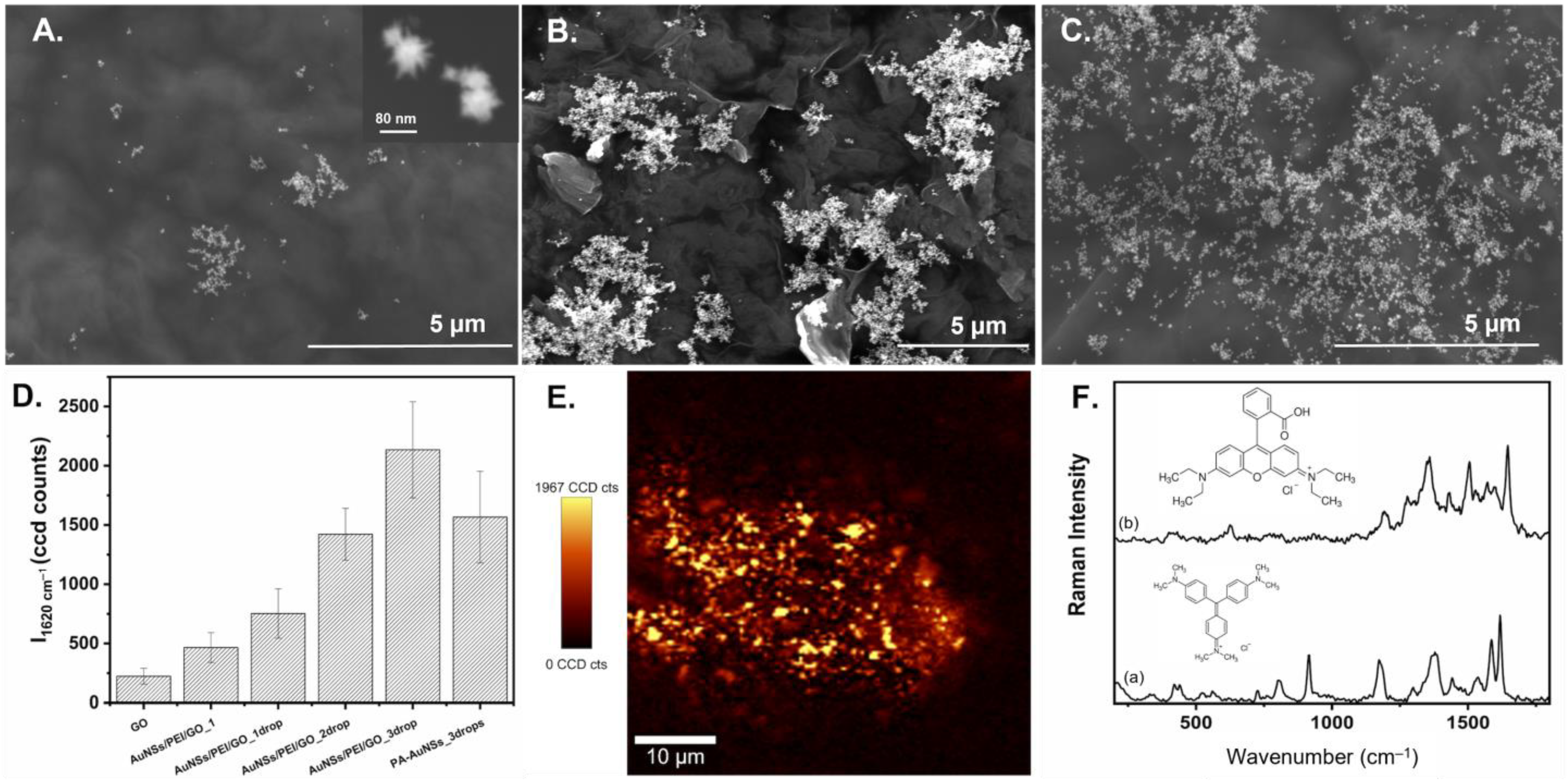
2.4. AuNSs/GO Hybrid Sensors Preparation
2.5. SERS Measurements
2.6. Instrumentation
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fateixa, S.; Nogueira, H.I.S.; Trindade, T. Hybrid Nanostructures for SERS: Materials Development and Chemical Detection. Phys. Chem. Chem. Phys. 2015, 17, 21046–21071. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.Y.; Yi, J.; Li, J.F.; Ren, B.; Wu, D.Y.; Panneerselvam, R.; Tian, Z.Q. Nanostructure-Based Plasmon-Enhanced Raman Spectroscopy for Surface Analysis of Materials. Nat. Rev. Mater. 2016, 1, 16021. [Google Scholar] [CrossRef]
- Lai, H.; Xu, F.; Zhang, Y.; Wang, L. Recent Progress on Graphene-Based Substrates for Surface-Enhanced Raman Scattering Applications. J. Mater. Chem. B 2018, 6, 4008–4028. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Mao, N.; Zhang, J. Graphene: A Platform for Surface-Enhanced Raman Spectroscopy. Small 2013, 9, 1206–1224. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Xie, L.; Fang, Y.; Xu, H.; Zhang, H.; Kong, J.; Dresselhaus, M.S.; Zhang, J.; Liu, Z. Can Graphene Be Used as a Substrate for Raman Enhancement? Nano Lett. 2010, 10, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Nurrohman, D.T.; Chiu, N.-F. A Review of Graphene-Based Surface Plasmon Resonance and Surface-Enhanced Raman Scattering Biosensors: Current Status and Future Prospects. Nanomaterials 2021, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, G.; Marques, P.A.A.P.; Granadeiro, C.M.; Nogueira, H.I.S.; Singh, M.K.; Grácio, J. Surface Modification of Graphene Nanosheets with Gold Nanoparticles: The Role of Oxygen Moieties at Graphene Surface on Gold Nucleation and Growth. Chem. Mater. 2009, 21, 4796–4802. [Google Scholar] [CrossRef]
- Liu, J.; Liu, L.; Wu, X.; Zhang, X.; Li, T. Environmentally Friendly Synthesis of Graphene-Silver Composites with Surface-Enhanced Raman Scattering and Antibacterial Activity via Reduction with l-Ascorbic Acid/Water Vapor. New J. Chem. 2015, 39, 5272–5281. [Google Scholar] [CrossRef]
- Li, S.K.; Yan, Y.X.; Wang, J.L.; Yu, S.H. Bio-Inspired in Situ Growth of Monolayer Silver Nanoparticles on Graphene Oxide Paper as Multifunctional Substrate. Nanoscale 2013, 5, 12616–12623. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.; Borme, J.; Bdkin, I.; González-Mayorga, A.; Irurueta, G.; Nogueira, H.I.S.; Serrano, M.C.; Alpuim, P.; Marques, P.A.A.P. Reductive Nanometric Patterning of Graphene Oxide Paper Using Electron Beam Lithography. Carbon 2018, 129, 63–75. [Google Scholar] [CrossRef]
- Yuan, H.; Khoury, C.G.; Hwang, H.; Wilson, C.M.; Grant, G.A.; Vo-Dinh, T. Gold Nanostars: Surfactant-Free Synthesis, 3D Modelling, and Two-Photon Photoluminescence Imaging. Nanotechnology 2012, 23, 75102–75111. [Google Scholar] [CrossRef] [PubMed]
- Feito, M.J.; Cicuéndez, M.; Casarrubios, L.; Diez-Orejas, R.; Fateixa, S.; Silva, D.; Barroca, N.; Marques, P.A.A.P.; Portolés, M.T. Effects of Graphene Oxide and Reduced Graphene Oxide Nanostructures on CD4+ Th2 Lymphocytes. Int. J. Mol. Sci. 2022, 23, 10625. [Google Scholar] [CrossRef] [PubMed]
- Jasim, D.A.; Lozano, N.; Kostarelos, K. Synthesis of Few-Layered, High-Purity Graphene Oxide Sheets from Different Graphite Sources for Biology. 2D Mater. 2016, 3, 014006. [Google Scholar] [CrossRef]
- Faniyi, I.O.; Fasakin, O.; Olofinjana, B.; Adekunle, A.S.; Oluwasusi, T.V.; Eleruja, M.A.; Ajayi, E.O.B. The Comparative Analyses of Reduced Graphene Oxide (RGO) Prepared via Green, Mild and Chemical Approaches. SN Appl. Sci. 2019, 1, 1181. [Google Scholar] [CrossRef]
- Fateixa, S.; Wilhelm, M.; Nogueira, H.I.S.; Trindade, T. SERS and Raman Imaging as a New Tool to Monitor Dyeing on Textile Fibres. J. Raman Spectrosc. 2016, 47, 1239–1246. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Env. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Ma, B.; Rodriguez, R.D.; Ruban, A.; Pavlov, S.; Sheremet, E. The Correlation between Electrical Conductivity and Second-Order Raman Modes of Laser-Reduced Graphene Oxide. Phys. Chem. Chem. Phys. 2019, 21, 10125–10134. [Google Scholar] [CrossRef] [PubMed]
- Lópezlópez-Díaz, D.; López, M.; Holgado, L.; García-Fierro, J.J.; Mercedes Velázquezvelázquez, M. Evolution of the Raman Spectrum with the Chemical Composition of Graphene Oxide. J. Phys. Chem. C 2017, 121, 20489–20497. [Google Scholar] [CrossRef]
- Xiao, G.N.; Man, S.Q. Surface-Enhanced Raman Scattering of Methylene Blue Adsorbed on Cap-Shaped Silver Nanoparticles. Chem. Phys. Lett. 2007, 447, 305–309. [Google Scholar] [CrossRef]
- Ederer, J.; Ecorchard, P.; Slušná, M.Š.; Tolasz, J.; Smržová, D.; Lupínková, S.; Janoš, P. A Study of Methylene Blue Dye Interaction and Adsorption by Monolayer Graphene Oxide. Adsorpt. Sci. Technol. 2022, 2022, 7385541. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, E.F.; Gonçalves, G.; Fateixa, S. Development of Au Nanostars/Graphene Oxide Paper for SERS. Mater. Proc. 2023, 14, 75. https://doi.org/10.3390/IOCN2023-14538
Silva EF, Gonçalves G, Fateixa S. Development of Au Nanostars/Graphene Oxide Paper for SERS. Materials Proceedings. 2023; 14(1):75. https://doi.org/10.3390/IOCN2023-14538
Chicago/Turabian StyleSilva, Eduarda F., Gil Gonçalves, and Sara Fateixa. 2023. "Development of Au Nanostars/Graphene Oxide Paper for SERS" Materials Proceedings 14, no. 1: 75. https://doi.org/10.3390/IOCN2023-14538
APA StyleSilva, E. F., Gonçalves, G., & Fateixa, S. (2023). Development of Au Nanostars/Graphene Oxide Paper for SERS. Materials Proceedings, 14(1), 75. https://doi.org/10.3390/IOCN2023-14538







