Abstract
Plasmonic nanostructures represent a suitable platform for the detection of biomolecule interactions. Their surface functionalization can be performed through different strategies. Optimal thickness, homogeneity, and hydrophilicity of the functional layer can play a crucial role in defining the sensing capabilities required to perform bioassays. In this framework, a combination of tetraethylorthosilicate (TEOS) and a commercial polymer (MCP) was evaluated to improve these features. In our more recent studies, we focused on plasmon-enhanced fluorescence for the detection of a microbial-derived synthetic oligonucleotide. An effective improvement of the fluorescence signal was detected for the combined TEOS and MCP coating.
1. Introduction
Biosensors represent a class of promising analytical devices where physical phenomena are coupled with biological elements to enable the acquisition of information inherent in chemical processes. Beyond this definition, biosensors are a wide class of devices characterized by different sensitivity, robustness, and area of application. In particular, sensors based on surface plasmon resonance (SPR) are especially convenient in terms of application spectrum, as these platforms can be adapted for many different types of analytes [1].
The feasibility of shaping the surface chemistry by different strategies enables the development of interfaces with specific characteristics, such as thickness, homogeneity, and hydrophilicity, which are critical in determining the sensing capabilities required to perform bioassays. In this context, one of the more fascinating fields of application is represented by plasmonic-enhanced fluorescence (PEF). PEF occurs when plasmons can be coupled with the fluorescence features of specific fluorophores, thereby changing their signals. It is a well-described effect depending on several elements, but primarily the dependency between the fluorophore and the metal distance, an increase in the excitation field and the modulation in photon emission mediated by the metal [2].
From an implementation perspective, there is an unrealized potential for a wide range of methodologies that are routinely performed in many laboratories, particularly for methodologies affected by the low sensitivity and reliability underlying the properties of some common fluorophores [2].
From this perspective, starting from our nanoplasmonic grating (NPG) [3] we built up a feasible approach suitable for PEF application. First, the plasmonic nanostructure was covered with a thin layer of tetraethyl orthosilicate (TEOS) [4], which improves the chemical homogeneity of the surface. Then a commercial Lucidant MCP polymer [5] was coated on the TEOS layer. The polymer decreases the contact angle of the surface, preventing the aggregation of biomolecules during immobilization. Since the overall thickness of the functionalization bi-layer is in the order of 10 nm, we had the advantage of being able to keep the fluorophore close to the sensing surface, as is crucial for exploiting the PEF modality of detection. To verify the capabilities in PEF detection, we employed a DNA microarray, where, in building up the assay, the detector probe was labeled with the dye Alexa Fluor 750 (AF750; AAT Bioquest, Pleasanton, CA, USA), which was selected thanks to the spectral overlap with the plasmonic features of our grating. Several surfaces were examined and the corresponding images compared by measuring the fluorescence intensity by means of a microarray scanner in the so-called front–front configuration (Figure 1).
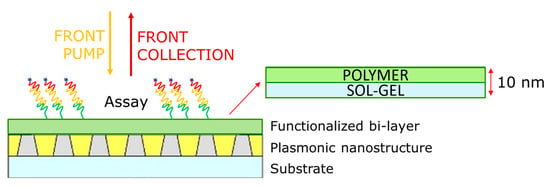
Figure 1.
Diagram of the functionalized plasmonic nanostructure and measurement scheme.
2. Materials and Methods
2.1. Nanoplasmonic Grating Fabrication
Standard NPGs are produced by following an established fabrication protocol based on colloidal lithography. A monolayer of colloidal nanoparticles acting as a lithographic mask is deposited on top of a dielectric layer of a few hundred nanometers. This is followed by a dry etching step that produces an ordered pattern of nanospheres on top of pillars at well-defined distances and geometries. After this etching step, the substrate is coated with a thin gold film with controlled thickness, and the colloidal mask is removed through a lift-off step to create a final hexagonal lattice crystal geometry [3].
2.2. Functionalized Bi-Layer
Sol-gel preparation using tetraethoxysilane (TEOS) as a silica monomer has been previously established [4]. The sol precursor was prepared by mixing TEOS (8 mL), ethanol (EtOH) (20 mL), and water (H2O) (3.75 mL) under stirring at room temperature (RT) for 24 h. Subsequently, to combine EtOH and methyl cyanide (MeCN), a 6 mL volume of EtOH/MeCN (v/v = 1:1), and chloride acid (HCl) (up to pH 4) was added to 9 mL of the sol. The mixture was then maintained under stirring at 50 °C for 1 h. The resulting TEOS solution was gently mixed in a closed vessel at RT. Before the coating process, both the flat gold and nanoplasmonic substrates were extensively cleaned. The sol-gel films were then deposited by dip coating using an automatized device, setting the withdrawal speed at 20 mm/s. After deposition, all the films were dried at RT for 48 h [4].
Lucidant MCP polymers, as well as TEOS coating, are consolidated systems employed in our laboratories [6]. The polymer was coated by drop-casting on the surfaces and after 30 min, it was roughly washed in water. This was followed by a curing step at 80 °C for 15 min, after which each platform (NPG, flat gold and glass) was ready for biomolecule immobilization.
2.3. Assay
The assay for the detection of DNA was designed in a three-component format.
A capture oligonucleotide was printed on derivatized substrates to form an array using a piezoelectric spotter (SciFLEXARRAYER S3; Scienion, Berlin, Germany). After an overnight incubation, all residual reactive groups of the coating polymer were blocked and then the surfaces were rinsed with a warmed buffer (50 °C) as a pre-conditioning step before the DNA hybridization.
The target oligonucleotide designed on E. coli bacteria was then diluted to 250 nM in a total volume of 4 µL in the hybridization buffer (SSC 2X, Saline Sodium Citrate 2X + SDS 0.1% + BSA 0.2 mg/mL) and immediately applied to the microarrays. The surfaces were transferred to a humidified incubator at a temperature of 65 °C for 2 h to allow DNA hybridization to occur. Since the target sequence was then present on the microarray, the oligonucleotide detector labeled with Alexa Fluor 750 dye (AF750) [7] at 1 µM could be finally applied on the surface, and similar to the previous molecule, it was hybridized for 2 h at 40 °C. The surfaces were then again extensively washed with SSC buffer at different ionic strengths (SSC 4X + SDS 0.1%, two times; SSC 0.2X + SDS 0.1%, two times; SSC 0.1X + SDS 0.1%, two times) and dried before measurement.
2.4. Optical Measurement
The different functionalized platforms were designed to be analyzed on a commercial microarray scanner. This gave us the advantage of being able to characterize the fluorescence signal with a quick, simple, user-accessible, and robust method.
The fluorescence images were collected using an InnoScan 710-IR (Innopsys, Carbonne, France) device [6]. The fluorophore AF750 linked to a detector oligonucleotide was excited through a laser at 785 nm at low laser power (5 mW), and the collection was performed at 1% of PMT. From the resulting scans, regions of interest (ROIs) matching the spots grid were defined and the total fluorescence intensity for each ROI was generated by subtracting the background signal.
3. Results and Discussion
Four samples were fabricated employing the same techniques. Their characteristics are reported in Table 1 and the corresponding fluorescence images are reported in Figure 2.

Table 1.
Summary of the sample treatments.
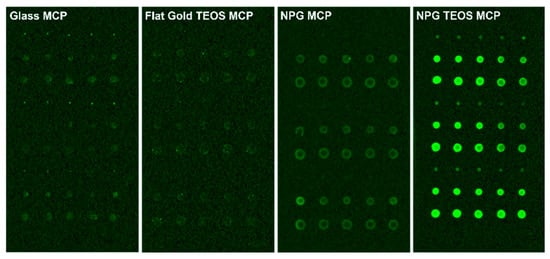
Figure 2.
Fluorescence emission images were collected with an Innopsys microarray scanner.
The images reported in Figure 2 show the compatibility of our platform with PEF detection in imaging mode using a benchmark instrument.
It is evident that the fluorescence intensity is higher (perceived as brighter) on NPG with the combination of the bi-layer TEOS + MCP with respect to all the other platforms.
Since the ratio of oligonucleotides hybridization is 1 to 1, the number of drops of a single spot is directly correlated to the number of fluorophores present on that region of the surface. Thus, starting from the total intensity values given by the microarray scanner, we calculated the average of the spots with the same number of drops for each platform (Figure 3). This result resembles the observations obtained with the imaging scanner but also reveals a different behaviour in the signals between non-nanostructured and nanostructured surfaces.
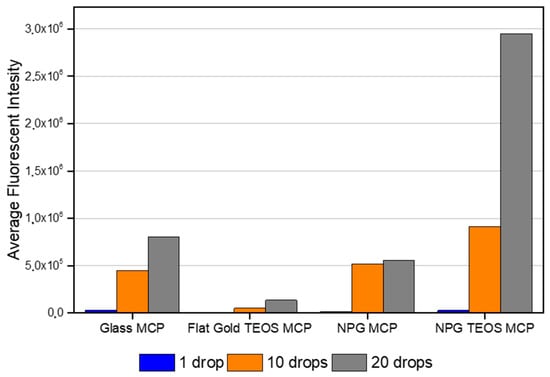
Figure 3.
Fluorescence intensity average collected with the Innopsys microarray scanner.
For this reason, in a final analysis, we decided to plot a trend of each sample in terms of average fluorescence intensities versus the number of drops (Figure 4). Although it shows a linear or semi-linear trend for most of the surfaces, in the case of the NPG TEOS + MCP, a supralinear behaviour can be observed. In any case, the role of the bi-layer in improving the fluorescence intensity is evident.
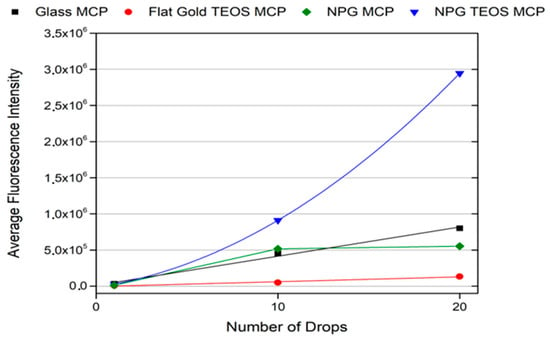
Figure 4.
Fluorescence intensity variation trends in function of the number of drops.
4. Conclusions
Enhancing a fluorescence signal using a plasmonic platform may be complex, and many parameters can be involved. Despite this, in the present work, we present a well-established protocol to place a fluorophore into an optimal configuration to exploit part of this phenomenon. Our nanostructured plasmonic surfaces have also been shown to perform suitably for this purpose, so further studies will be conducted to corroborate our achievements.
Author Contributions
Conceptualization, F.F., L.L.-S. and E.M.; methodology, L.L.-S. and E.M.; validation, F.F., L.L.-S. and E.M.; formal analysis, L.L.-S. and E.M.; investigation, F.F. and F.M.; resources, P.P., V.T. and E.M.; data curation, F.F. and E.M.; writing—original draft preparation, F.F. and E.M.; writing—review and editing, F.F., F.M., L.L.-S., E.M. and V.T.; supervision, F.F. and F.M.; project administration, F.M.; funding acquisition, F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-funded by the European Union’s Horizon 2020 project h-ALO (photonic system for Adaptable multiple-analyte monitoring of food quality), grant agreement No. 101016706.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sadana, A. Biosensors: Kinetics of Binding and Dissociation Using Fractals; Elsevier: Amsterdam, The Netherlands, 2003; pp. 295–300. [Google Scholar]
- Geddes, C.D.; Lakowicz, J.R. Metal-Enhanced Fluorescence. J. Fluoresc. 2002, 12, 121–129. [Google Scholar] [CrossRef]
- Giudicatti, S.; Marabelli, F.; Valsesia, A.; Pellacani, P.; Colpo, P.; Rossi, F. Interaction among plasmonic resonances in a gold film embedding a two-dimensional array of polymeric nanopillars. J. Opt. Soc. Am. B 2012, 29, 1641–1647. [Google Scholar] [CrossRef]
- Floris, F.; Figus, C.; Fornasari, L.; Patrini, M.; Pellacani, P.; Marchesini, G.; Valsesia, A.; Artizzu, F.; Marongiu, D.; Saba, M.; et al. Optical Sensitivity Gain in Silica-Coated Plasmonic Nanostructures. J. Phys. Chem. Lett. 2014, 5, 2935–2940. [Google Scholar] [CrossRef] [PubMed]
- Sola, L.; Damin, F.; Cretich, M.; Chiari, M. Novel polymeric coatings with tailored hydrophobicity to control spot size and morphology in DNA microarray. Sens. Actuators B Chem. 2016, 231, 412–422. [Google Scholar] [CrossRef]
- InnoScan® 710-IR. Available online: https://www.innopsys.com/wp-content/uploads/2020/01/PInnoScan-710-IR.pdf (accessed on 10 December 2022).
- DNA Oligo Synthesis from PCR Primers to GMP|IDT. Available online: https://eu.idtdna.com/pages/products/custom-dna-rna/dna-oligosdtdna.com (accessed on 10 December 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).