Geopolymers Based on Fly Ash from the Bełchatów Power Plant †
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Tests of Mechanical Properties—Flexural Strength Tests
- Rf—flexural strength (MPa)
- b—lateral length of the section (mm)
- Ff—maximum load (N)
- l—length between supports (mm).
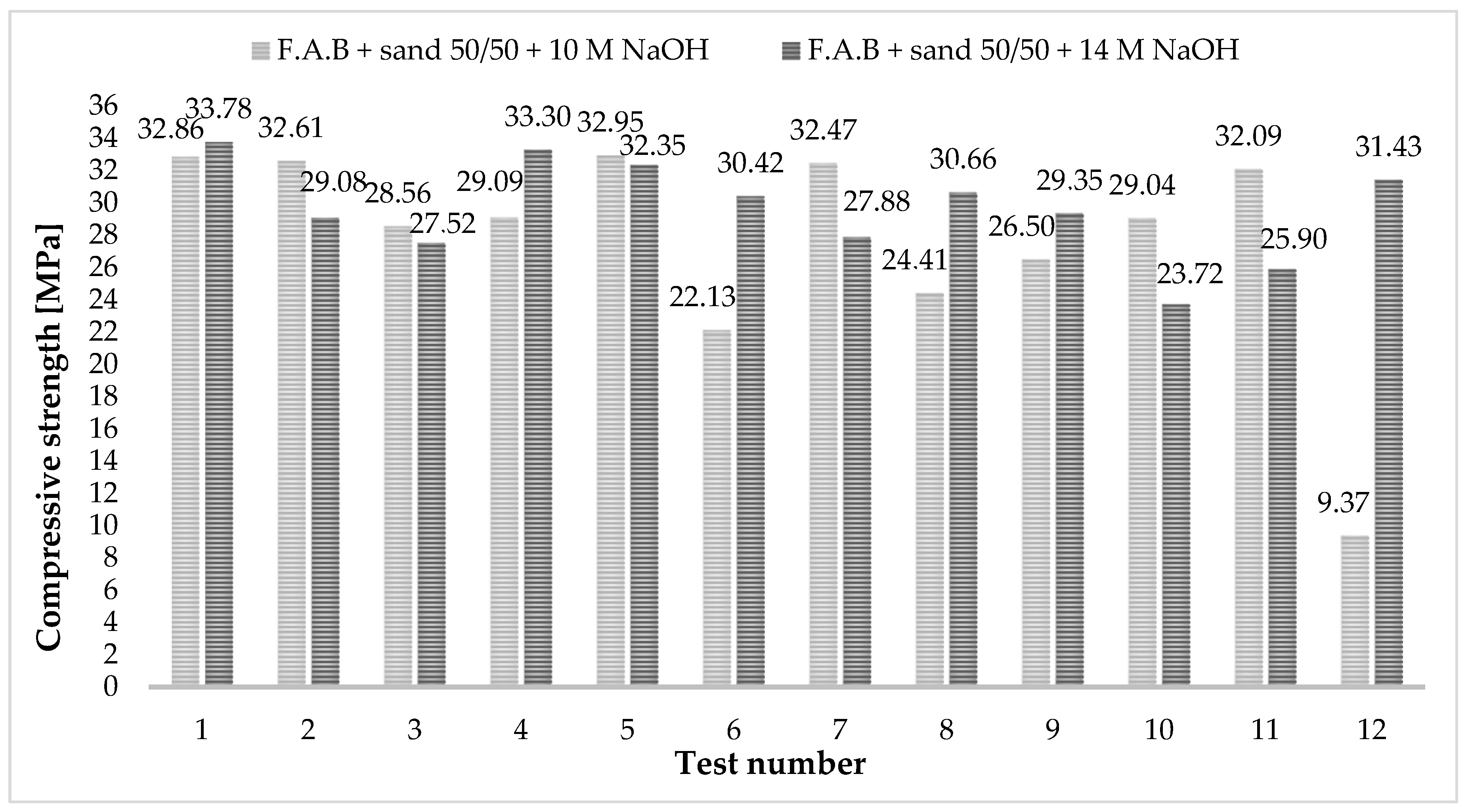
3.2. Tests of Mechanical Properties—Compressive Strength Tests
- Rc—compressive strength (MPa)
- 1600—surface of tiles (or auxiliary tiles) (mm2)
- Fc—maximum load (N).
3.3. Evaluation of the Microstructure and Analysis of the Oxide Chemical Composition of the Resulting Geopolymers
4. Short Discussion
5. Conclusions
- Limestone fly ash contains quartz, gelenite, anorthite, hematite, anhydrite, mullite and calcium oxide, as well as typical cement clinker phases, i.e., C12A7, C3A and C4A3Ŝ.
- The following oxides can be identified in the base material: SiO2, CaO, Al2O3, Fe2O3, SO3, CaO free, MgO, K2O and Na2O.
- The flexural strength for samples activated with the two solutions is at a similar level and is no more than 3 MPa. Compressive strength for both activators is similar, with results not exceeding 35 MPa.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baran, T.; Drożdż, W. Evaluation of properties of domestic calcareous fly ash and its processing methods. Roads Bridges 2013, 12, 5–15. [Google Scholar]
- Gibas, K.; Glinicki, M.A.; Nowowiejski, G. Evaluation of impermeability of concrete containing calcareous fly ash in respect to environmental media. Roads Bridges 2013, 12, 159–171. [Google Scholar]
- Giergiczny, Z.; Garbacik, A.; Ostrowski, M. Pozzolanic and hydraulic activity of calcareous fly ash. Roads Bridges 2013, 12, 71–81. [Google Scholar]
- PN-EN 197-1:2012; Cement—Część 1: Skład, Wymagania i Kryteria Zgodności Dotyczące Cementów Powszechnego Użytku. Polish Version. Available online: https://sklep.pkn.pl/pn-en-197-1-2012p.html (accessed on 5 December 2022).
- PN-EN 206:2014-04; Beton—Wymagania, Właściwości, Produkcja i Zgodność. Polish Version. Available online: https://sklep.pkn.pl/pn-en-206-2014-04p.html (accessed on 5 December 2022).
- Giergiczny, Z. The role of calcium and silica fly ashes in shaping the properties of modern construction binders and cementitious plastics. Bull. Tadeusz Kosciuszko Crac. Univ. Technol. 2006, 1–50. [Google Scholar]
- Figiela, B.; Brudny, K.; Lin, W.T.; Korniejenko, K. Investigation of mechanical properties and microstructure of construction—And demolition—Waste—Based geopolymers. J. Compos. Sci. 2022, 6, 191. [Google Scholar] [CrossRef]
- Kurdowski, W. Cement and Concrete Chemistry; Polish Cement Publishing, PWN Scientific Publishers: Warsaw, Poland, 2010. [Google Scholar]
- Pachowski, J. Development of technologies for the formation of power plant by-products and their characteristics and potential applications in road construction technologies. Roads Bridges 2002, 1, 59–99. [Google Scholar]
- PN-EN 196-1:2016-07; Metody Badania Cementu—Część 1: Oznaczanie Wytrzymałości. Polish Version. Available online: https://sklep.pkn.pl/pn-en-196-1-2016-07p.html (accessed on 5 December 2022).
- Nath, S.K.; Maitra, S.; Mukherjee, S.; Kumar, S. Microstructural and morphological evolution of fly ash based geopolymers. Constr. Build. Mater. 2016, 111, 758–765. [Google Scholar] [CrossRef]
- Kumar, S.; Mucsi, G.; Kristály, F.; Pekker, P. Mechanical activation of fly ash and its influence on micro and nano-structural behaviour of resulting geopolymers. Adv. Powder Technol. 2017, 28, 805–813. [Google Scholar] [CrossRef]
- Baran, T.; Garbacik, A.; Synowiec, K.; Żak, A. Use of calcareous fly ash as an active constituent of hydraulic road binders. Roads Bridges 2013, 12, 17–29. [Google Scholar]


| Precursor | Oxide Composition (wt%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LOI * | SiO2 | CaO | Al2O3 | Fe2O3 | SO3 | CaO Free | MgO | K2O | Na2O | |
| F.A. Bełchatów ** | 2.1 | 42.8 | 22.5 | 20.5 | 4.4 | 4.3 | 4.1 | 0.9 | 0.2 | 0.1 |
| Precursor | Oxide Composition (wt%) | |||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | K2O | CaO | MgO | TiO2 | Na2O | |
| F.A. Skawina *** | 55.9 | 23.49 | 5.92 | 3.55 | 2.72 | 2.61 | 1.09 | 0.59 |
| Fly Ash from Bełchatów |
|---|
| Mineral components listed in the decreasing order of content |
| Quartz Gehlenite Anhydrite Hematite AnorthiteLarnite Ye’elemit C4A3 Ŝ C12A7 C3A Free lime Mullite |
| Amorphous phase |
| Calcium aluminosilicate glass |
| Fly Ash from Skawina |
|---|
| Mineral components listed in the decreasing order of content |
| Mullite Quartz Hematite Magnetite |
| Amorphous phase |
| Aluminosilicate glass |
| Fly Ash from Bełchatów | |||
|---|---|---|---|
| Identified Phase | Chemical Formula | Percentage Share [wt.%] | Data Sheet Number |
| Quartz | SiO2 | 1.7 | 01-074-1811 |
| Gehlenite | Ca2Al2SiO7 | 31.3 | 04-015-3030 |
| Anhydrite | CaSO4 | 15.9 | 00-006-0226 |
| Hematite | Fe2O3 | 9.8 | 04-006-2616 |
| Anorthite | CaAl2Si2O8 | 15.1 | 00-041-1486 |
| Ye’elimite | Ca4Al6(SO4) | 5.9 | 04-009-7268 |
| Chlormayenite (C12A7) | C12A14O33 | 3.4 | 00-048-1882 |
| Lime | CaO | 3.2 | 04-005-4757 |
| Mullite | Al6Si2O13 | 13.7 | 00-015-0776 |
| Material | D10 [μm] | D50 [μm] | D90 [μm] | Mean Size [μm] |
|---|---|---|---|---|
| Fly ash from Bełchatów | 3.29 | 20.74 | 37.24 | 21.46 |
| 3.35 | 20.80 | 37.06 | 21.45 | |
| 3.46 | 20.83 | 36.81 | 21.43 | |
| 3.87 | 21.88 | 37.30 | 22.21 | |
| 3.88 | 21.42 | 37.23 | 21.91 |
| Index | Base Materials (S) [Weight Ratio] | Alkaline Activator (L) | Liquid/Solid Ratio [Weight Ratio] | |
|---|---|---|---|---|
| Fly Ash | Sand | |||
| R10 | 1 | 1 | 10 M NaOH + sodium water glass (weight ratio: 1:2.5) | 1:0.30 |
| R14 | 1 | 1 | 14 M NaOH + sodium water glass (weight ratio: 1:2.5) | 1:0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bąk, A.; Bazan, P.; Pławecka, K.; Łach, M. Geopolymers Based on Fly Ash from the Bełchatów Power Plant. Mater. Proc. 2023, 13, 17. https://doi.org/10.3390/materproc2023013017
Bąk A, Bazan P, Pławecka K, Łach M. Geopolymers Based on Fly Ash from the Bełchatów Power Plant. Materials Proceedings. 2023; 13(1):17. https://doi.org/10.3390/materproc2023013017
Chicago/Turabian StyleBąk, Agnieszka, Patrycja Bazan, Kinga Pławecka, and Michał Łach. 2023. "Geopolymers Based on Fly Ash from the Bełchatów Power Plant" Materials Proceedings 13, no. 1: 17. https://doi.org/10.3390/materproc2023013017
APA StyleBąk, A., Bazan, P., Pławecka, K., & Łach, M. (2023). Geopolymers Based on Fly Ash from the Bełchatów Power Plant. Materials Proceedings, 13(1), 17. https://doi.org/10.3390/materproc2023013017







