Abstract
Two types of core-shell composite particles, each comprising an aromatic poly(esteramide) (PEA) having amide and hydroxyl groups combined with silica (SiO2), were synthesized. This synthesis was performed by polymerizing two monomers in a mixture of acetone and N,N-dimethylacetamide in the presence of porous SiO2 particles. The surface morphologies of the resulting composite particles and the PEA loadings were determined to be highly dependent on the reaction solvent composition and the monomer used. Both types of particles exhibited unique adsorption properties depending on the dye being adsorbed and displayed differing adsorption efficiencies. In additional trials, carbon-SiO2 particles were obtained by heating the PEA-SiO2 particles to 700 °C under nitrogen.
1. Introduction
Polymer–ceramic composite particles with dimensions on the micrometer or nanometer scale are expected to have a wide range of applications as highly functional materials upon combining the unique properties of each material. Specific functional groups can also be introduced into these particles to provide stable dispersions in solution or promote interactions with other substances. However, it is often difficult to control the interface between polymers and ceramics, and so the development of composite particles with multiple functional groups is important.
One potential application for such particles is as adsorption-based carriers, and porous silica (SiO2) particles with high specific surface areas show promise in this regard [1]. Recently, composite particles combining aromatic polyamides combined with SiO2 were fabricated and found to exhibit adsorption properties [2]. In addition, the author has demonstrated a technique for producing porous poly(esteramide) (PEA) polymers with multiple functional groups [3]. In the present study, core–shell PEA-SiO2 composite particles in which the PEA incorporated both amino and hydroxyl groups were synthesized and characterized, including assessments of the dye adsorption capacities of these particles. Meanwhile, carbon is attracting attention because of unique properties such as excellent adsorption capacity and high thermal conductivity [4]. Thus, the PEA-SiO2 particles were converted into carbon-SiO2 composite particles via heat treatment.
2. Experimental Section
Porous SiO2 particles (Wakosil®5SIL) were purchased from the FUJIFILM Wako Pure Chemicals Co., Osaka, Japan, while m-phthalyl chloride (m-PC), 1,3,5-benzenetricarbonyl trichloride (tri-BC) and 4,4′-diamino-3,3′-dihydroxybiphenyl (DADH) were obtained from the Tokyo Kasei Co., Tokyo, Japan. Acetone, dimethylacetamide (DMAc) and pyridine were purchased from the Kishida Chemical Co., Ltd., Osaka, Japan.
In preparation for the syntheses, a 0.004 mol quantity of either m-PC or tri-BC was dissolved in acetone (400 mL) while 0.004 mol DADH was dissolved in a mixture of acetone (240 mL) and DMAc (160 mL). SiO2 particles (1 g) were subsequently dispersed in the DADH solution, after which pyridine (24 mL) was added with the subsequent addition of the entire m-PC or tri-BC solution. The resulting mixture was subjected to ultrasonication at 28 kHz in an ice water bath for 30 min, following which the product was separated by centrifugation and washed a total of five times with acetone and water. Finally, the sample was dried under vacuum at 100 °C for 2 h followed by drying at room temperature overnight. The reaction systems incorporating the m-PC and tri-BC are referred to herein as S1 and S2, respectively. Each batch is also named so as to reflect the acetone-to-DMAc ratio in the reaction solvent. As an example, the S1-82 specimen was prepared by polymerizing m-PC and DADH in a mixture with an acetone-to-DMAc ratio of 8:2.
Samples of each material were coated with gold via sputtering and their morphologies were assessed using field emission scanning electron microscopy (FE-SEM), employing an S-4700 instrument (Hitachi Ltd., Tokyo, Japan). The particle size distribution of each specimen as a dispersion in water was determined with an LS 13 320 XR laser diffraction particle size analyzer (Beckman Coulter Ltd., Tokyo, Japan). The thermal properties of the specimens were ascertained with thermogravimetry/differential thermal analysis (TG/DTA) using an STA7000 analyzer (Hitachi High-Tech Science Corp., Tokyo, Japan). Prior to each test, the sample was heated in air at 100 °C for 1 h and then heated to 1000 °C at a rate of 10 °C/min. The proportion of PEA in each sample was determined by subtracting the mass loss of pure SiO2 from the mass loss of the particular specimen at 1000 °C.
In each adsorption trial, an 80 mg sample of the particles was added to an aqueous solution of Rhodamine B, Rhodamine 6G or Acid Red 87 (160 mL, 3.5 mg/L) with a pH of 7, followed by stirring at room temperature for 48 h. The solution was then filtered and the concentration of the dye in the filtrate was determined with ultraviolet–visible spectroscopy using a V-570 spectrometer (JASCO Corp., Tokyo, Japan). The proportional removal of the dye was calculated as (C0-C48H)/C0 × 100, where C0 and C48H are the initial dye concentration and the concentration after 48 h, respectively.
3. Results and Discussion
In both cases, the polymerization processes involved competing amidation and esterification reactions, resulting in the formation of crosslinked structures with amide and hydroxyl groups [3]. In this manner, composite particles were obtained in which SiO2 cores were coated with PEA incorporating these groups. SEM images of the original SiO2 and the resulting composite particles are presented in Figure 1a–f. In the case of the S1 specimens, the surface roughness evidently increased significantly as the proportion of acetone in the solvent mixture was increased (Figure 1b–d). In contrast, the surfaces of all the S2-type composite particles were relatively smooth and thus similar to the surfaces of the original SiO2 particles. The S1 specimens contained from 1.6 to 49.0 wt% PEA while the S2 specimens contained from 2.1 to 2.9 wt%. Therefore, both the surface morphology and the extent of PEA coating were greatly affected by the monomer that was used and the reaction solvent composition.
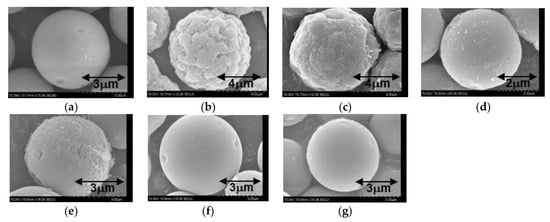
Figure 1.
SEM images of the (a) SiO2, (b) S1-82, (c) S1-73, (d) S1-64, (e) S2-82, (f) S2-73 and (g) S2-64 particles.
The effect of the PEA composition on adsorption capacity was evaluated by performing trials using the S1-64 and S2-73 particles, which had similar surface morphologies and PEA loadings (approximately 2 wt%). The particles’ size distributions determined for the original SiO2 and for the composite particles showed only a single sharp peak at approximately 5 μm, indicating a lack of aggregation and that pure PEA fragments were not present (Figure 2a–c). Based on these distributions, the median diameters were estimated to be 4.61 μm for the SiO2 particles, 4.73 μm for the S1-64 particles and 4.85 μm for the S2-73 particles. Hence, there was no correlation between the PEA loading and the average particle diameter.
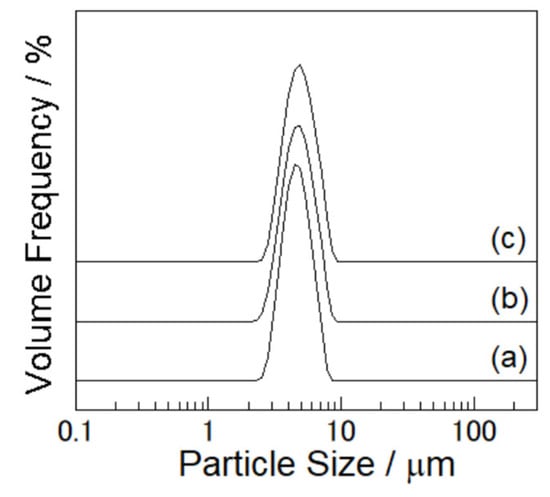
Figure 2.
Particle size distribution of the (a) SiO2, (b) S1-64 and (c) S2-73 particles.
Adsorption capacity tests were performed using all three dyes. It should be noted that these dyes are all based on xanthene and exhibit intense light absorption and emission properties, such that they are often used as optical materials and medical test dyes. Rhodamine B and Rhodamine 6G are cationic dyes whereas Acid Red 87 is anionic in aqueous solution. The proportional removals of these dyes are summarized in Figure 3a–c. In trials with the Rhodamine B and Rhodamine 6G, the S2-73 particles showed a higher adsorption capacity than the S1-64, while neither particle type adsorbed the Acid Red 87. Hence, there was also a significant difference between the uptakes of the cationic and anionic dyes. Overall, these data demonstrate that the present composite particles were able to adsorb certain dyes and a close correlation was observed between adsorption capacity and the chemical structure of the PEA.
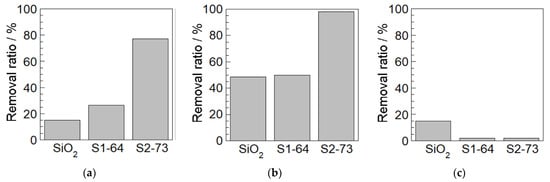
Figure 3.
Adsorption capacity for (a) Rhodamine B, (b) Rhodamine 6G and (c) Acid Red 87.
In subsequent experiments, the S1-82 particles (with the highest PEA loading of 49.0 wt%) were heated to 700 °C under nitrogen and held at that temperature for 30 min. During this heat treatment, the PEA was changed to pure carbon and so carbon-SiO2 composite particles were obtained. An SEM image of the resulting carbon-SiO2 composite particles is shown in Figure 4 and indicates that the particle surfaces became smoother and also that there was no aggregation between particles. The carbon loading of these particles was determined to be 34.8 wt% from mass losses at 1000 °C of pure SiO2 and carbon-SiO2 composite particles under air. Hence, it was confirmed that carbon-SiO2 composite particles could be obtained through heat treatment of the PEA-SiO2 composite particles.
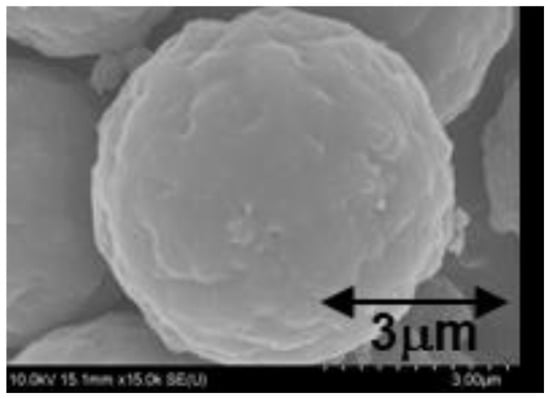
Figure 4.
SEM image of the carbon-SiO2 composite particles.
4. Conclusions
Composite particles made of SiO2 coated with PEA were obtained by polymerizing dual monomers in a mixture of acetone and DMAc containing porous SiO2 particles. The resulting PEA incorporated both amide and hydroxyl groups and had a cross-linked structure. The particle surface morphology and proportion of PEA were both greatly affected by the monomer that was used and the reaction solvent composition. The two types of composite particles exhibited unique dye adsorption characteristics and showed different adsorption capacities. These composite particles with high adsorption selectivity are expected to have applications as adsorption carriers in environmental remediation. Carbon-SiO2 particles obtained by heating PEA-SiO2 particles are expected to be applied not only as adsorption carriers, but also as highly functional materials such as fillers with high thermal conductivity.
Funding
This study was partly supported by JSPS KAKENHI, grant number JP21K12317.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available because they are part of the study which will be published in a full-length paper later.
Conflicts of Interest
The author declares no conflict of interest.
References
- Hikosaka, R.; Nagata, F.; Tomita, M.; Kato, K. Adsorption and desorption characteristics of DNA onto the surface of amino functional mesoporous silica with various particle morphologies. Colloid Surface B. 2016, 140, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Ehiro, T. Synthesis of aromatic polyamide-silica composite particles and the effect of incorporating silane coupling agent. e-J. Surf. Sci. Nanotechnol. 2022, 20, 161–166. [Google Scholar] [CrossRef]
- Yoshioka, Y. Synthesis of Hydrophilic Aromatic Polyesteramide Porous Bodies Having Controlled Structures and Characteristics from Submicron-Sized Particles. ChemistrySelect 2020, 5, 7867–7872. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).