Morphological Effects in SnO2 Chemiresistors for Ethanol Detection: A Systematic Statistical Analysis of Results Published in the Last 5 Years †
Abstract
:1. Introduction
2. Materials and Methods
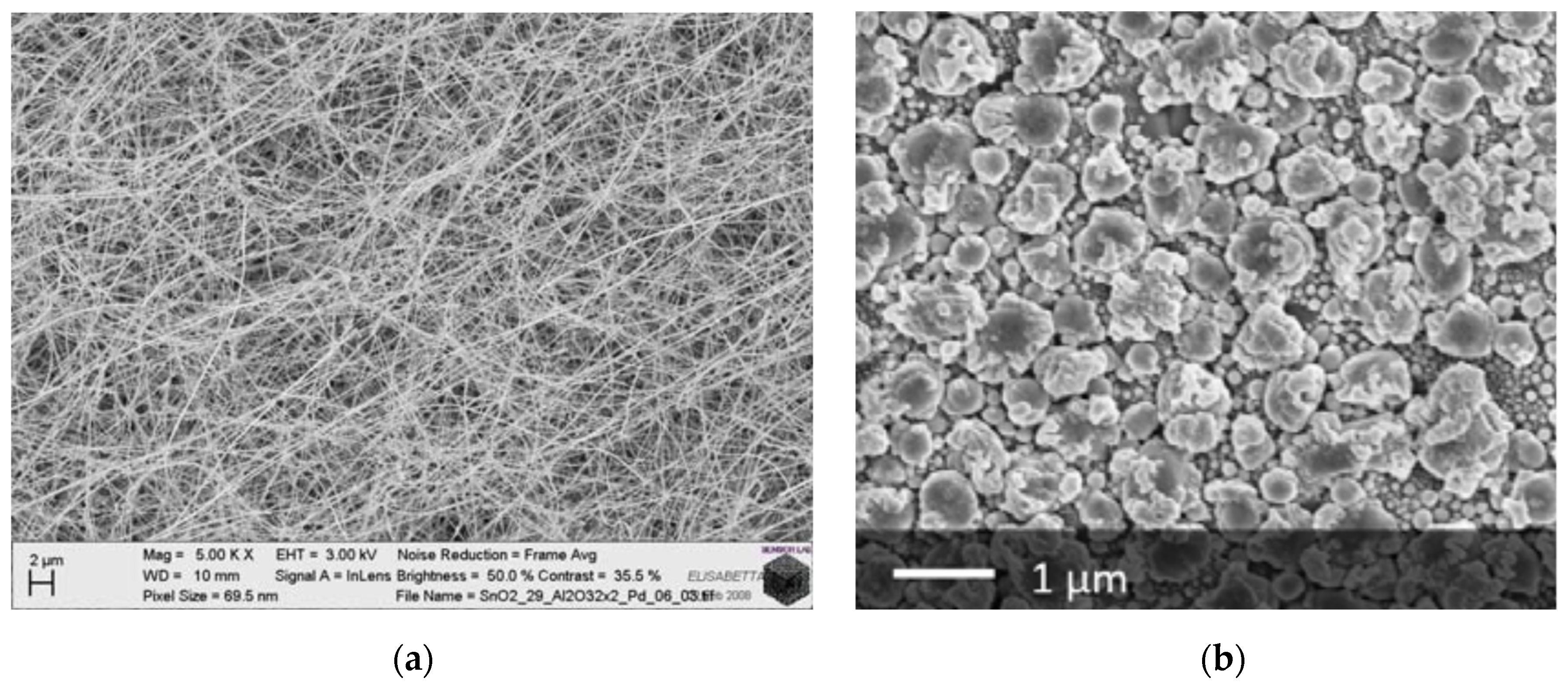
- Nanorods: elongated nanostructures with a high aspect-ratio, and surfaces identified by well-defined crystalline planes;
- Nanoparticles: spherical nanostructures, such as those used in thick films;
- Nanosheets: thin nanostructures extending in two dimensions.
3. Results
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ponzoni, A.; Comini, E.; Concina, I.; Ferroni, M.; Falasconi, M.; Gobbi, E.; Sberveglieri, V.; Sberveglieri, G. Nanostructured Metal Oxide Gas Sensors, a Survey of Applications Carried out at SENSOR Lab, Brescia (Italy) in the Security and Food Quality Fields. Sensors 2012, 12, 17023–17045. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H. Gas Sensors Using Hierarchical and Hollow Oxide Nanostructures: Overview. Sens. Actuators B Chem. 2009, 140, 319–336. [Google Scholar] [CrossRef]
- Palma, S.I.C.J.; Traguedo, A.P.; Porteira, A.R.; Frias, M.J.; Gamboa, H.; Roque, A.C.A. Machine Learning for the Meta-Analyses of Microbial Pathogens’ Volatile Signatures. Sci. Rep. 2018, 8, 3360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-H.; Galstyan, V.; Ponzoni, A.; Gonzalo-Juan, I.; Riedel, R.; Dourges, M.-A.; Nicolas, Y.; Toupance, T. Finely Tuned SnO2 Nanoparticles for Efficient Detection of Reducing and Oxidizing Gases: The Influence of Alkali Metal Cation on Gas-Sensing Properties. ACS Appl. Mater. Interfaces 2018, 10, 10173–10184. [Google Scholar] [CrossRef] [PubMed]
- Tricoli, A.; Pratsinis, S.E. Dispersed Nanoelectrode Devices. Nat. Nanotechnol. 2010, 5, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chu, S.; Ma, Q.; Li, H.; Che, Q.; Wang, J.; Wang, G.; Yang, P. Multilevel Effective Heterojunctions Based on SnO2/ZnO 1D Fibrous Hierarchical Structure with Unique Interface Electronic Effects. ACS Appl. Mater. Interfaces 2019, 11, 31551–31561. [Google Scholar] [CrossRef] [PubMed]
- Tofighi, G.; Degler, D.; Junker, B.; Müller, S.; Lichtenberg, H.; Wang, W.; Weimar, U.; Barsan, N.; Grunwaldt, J.-D. Microfluidically Synthesized Au, Pd and AuPd Nanoparticles Supported on SnO2 for Gas Sensing Applications. Sens. Actuators B Chem. 2019, 292, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Jiang, B.; Yu, Q.; Kou, X.; Sun, P.; Liu, F.; Lu, H.; Yan, X.; Lu, G. Realizing the Control of Electronic Energy Level Structure and Gas-Sensing Selectivity over Heteroatom-Doped In2O3 Spheres with an Inverse Opal Microstructure. ACS Appl. Mater. Interfaces 2019, 11, 9600–9611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, X.; Li, J.; Miao, Z.; Huang, F. Fabrication and Ethanol-Sensing Properties of Micro Gas Sensor Based on Electrospun SnO2 Nanofibers. Sens. Actuators B Chem. 2008, 132, 67–73. [Google Scholar] [CrossRef]
- Tricoli, A.; Righettoni, M.; Pratsinis, S.E. Minimal Cross-Sensitivity to Humidity during Ethanol Detection by SnO2–TiO2 Solid Solutions. Nanotechnology 2009, 20, 315502. [Google Scholar] [CrossRef]
- Kassem, O.; Saadaoui, M.; Rieu, M.; Viricelle, J.-P. A Novel Approach to a Fully Inkjet Printed SnO2-Based Gas Sensor on a Flexible Foil. J. Mater. Chem. C 2019, 7, 12343–12353. [Google Scholar] [CrossRef]
- Kotchasak, N.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S.; Yordsri, V.; Liewhiran, C. Highly Sensitive and Selective Detection of Ethanol Vapor Using Flame-Spray-Made CeOx-Doped SnO2 Nanoparticulate Thick Films. Sens. Actuators B Chem. 2018, 255, 8–21. [Google Scholar] [CrossRef]
- Li, X.; Peng, K.; Dou, Y.; Chen, J.; Zhang, Y.; An, G. Facile Synthesis of Wormhole-Like Mesoporous Tin Oxide via Evaporation-Induced Self-Assembly and the Enhanced Gas-Sensing Properties. Nanoscale Res. Lett. 2018, 13, 14. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Chen, S.; Lou, Z.; Li, L.; Huang, T.; Song, Y.; Chen, D.; Shen, G. Fabrication of Porous SnO2 Nanowires Gas Sensors with Enhanced Sensitivity. Sens. Actuators B Chem. 2017, 252, 79–85. [Google Scholar] [CrossRef]
- Xue, N.; Zhang, Q.; Zhang, S.; Zong, P.; Yang, F. Highly Sensitive and Selective Hydrogen Gas Sensor Using the Mesoporous SnO2 Modified Layers. Sensors 2017, 17, 2351. [Google Scholar] [CrossRef] [Green Version]
- Zito, C.A.; Perfecto, T.M.; Volanti, D.P. Impact of Reduced Graphene Oxide on the Ethanol Sensing Performance of Hollow SnO2 Nanoparticles under Humid Atmosphere. Sens. Actuators B Chem. 2017, 244, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Li, S.-H.; Meng, F.-F.; Chu, Z.; Luo, T.; Peng, F.-M.; Jin, Z. Mesoporous SnO2 Nanowires: Synthesis and Ethanol Sensing Properties. Adv. Condens. Matter Phys. 2017, 2017, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Palla Papavlu, A.; Mattle, T.; Temmel, S.; Lehmann, U.; Hintennach, A.; Grisel, A.; Wokaun, A.; Lippert, T. Highly Sensitive SnO2 Sensor via Reactive Laser-Induced Transfer. Sci. Rep. 2016, 6, 25144. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Dai, M.; Wang, T.; Sun, P.; Liang, X.; Lu, G.; Shimanoe, K.; Yamazoe, N. Enhanced Gas Sensing Properties of SnO2 Hollow Spheres Decorated with CeO2 Nanoparticles Heterostructure Composite Materials. ACS Appl. Mater. Interfaces 2016, 8, 6669–6677. [Google Scholar] [CrossRef]
- Naik, A.; Parkin, I.; Binions, R. Gas Sensing Studies of an N-n Hetero-Junction Array Based on SnO2 and ZnO Composites. Chemosensors 2016, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Tan, W.; Yu, Q.; Ruan, X.; Huang, X. Design of SnO2-Based Highly Sensitive Ethanol Gas Sensor Based on Quasi Molecular-Cluster Imprinting Mechanism. Sens. Actuators B Chem. 2015, 212, 47–54. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, L.; Wang, R.; Fei, T.; Zhang, T. Synthesis and Ethanol Sensing Properties of SnO2 Nanosheets via a Simple Hydrothermal Route. Solid-State Electron. 2012, 76, 91–94. [Google Scholar] [CrossRef]
- Francioso, L.; De Pascali, C.; Creti, P.; Radogna, A.V.; Capone, S.; Taurino, A.; Epifani, M.; Baldacchini, C.; Bizzarri, A.R.; Siciliano, P.A. Nanogap Sensors Decorated with SnO2 Nanoparticles Enable Low-Temperature Detection of Volatile Organic Compounds. ACS Appl. Nano Mater. 2020, 3, 3337–3346. [Google Scholar] [CrossRef]
- Zhang, L.; Tong, R.; Ge, W.; Guo, R.; Shirsath, S.E.; Zhu, J. Facile One-Step Hydrothermal Synthesis of SnO2 Microspheres with Oxygen Vacancies for Superior Ethanol Sensor. J. Alloy Compd. 2020, 814, 152266. [Google Scholar] [CrossRef]
- Van Hieu, N.; Kim, H.-R.; Ju, B.-K.; Lee, J.-H. Enhanced Performance of SnO2 Nanowires Ethanol Sensor by Functionalizing with La2O3. Sens. Actuators B Chem. 2008, 133, 228–234. [Google Scholar] [CrossRef]
- Nguyen, K.; Hung, C.M.; Ngoc, T.M.; Thanh Le, D.T.; Nguyen, D.H.; Nguyen Van, D.; Nguyen Van, H. Low-Temperature Prototype Hydrogen Sensors Using Pd-Decorated SnO2 Nanowires for Exhaled Breath Applications. Sens. Actuators B Chem. 2017, 253, 156–163. [Google Scholar] [CrossRef]
- Choi, K.S.; Park, S.; Chang, S.-P. Enhanced Ethanol Sensing Properties Based on SnO2 Nanowires Coated with Fe2O3 Nanoparti-cles. Sens. Actuators B Chem. 2017, 238, 871–879. [Google Scholar] [CrossRef]
- Wang, Q.; Yao, N.; An, D.; Li, Y.; Zou, Y.; Lian, X.; Tong, X. Enhanced Gas Sensing Properties of Hierarchical SnO2 Nanoflower Assembled from Nanorods via a One-Pot Template-Free Hydrothermal Method. Ceram. Int. 2016, 42, 15889–15896. [Google Scholar] [CrossRef]
- Cai, Z.; Park, S. Enhancement Mechanisms of Ethanol-Sensing Properties Based on Cr2O3 Nanoparticle-Anchored SnO2 Nanowires. J. Mater. Res. Technol. 2020, 9, 271–281. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, M.; Li, X.; Wang, B.; Wang, R. Investigation on Synthesis and Excellent Gas-Sensing Properties of Hierarchical Au-Loaded SnO2 Nanoflowers. J. Mater. Res. 2019, 34, 2944–2954. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Sun, L.; Wang, Y. Template-Free Synthesis of Nanosheets-Assembled SnO2 Hollow Spheres for Enhanced Ethanol Gas Sensing. Mater. Lett. 2018, 218, 290–294. [Google Scholar] [CrossRef]
- Zito, C.A.; Perfecto, T.M.; Volanti, D.P. Palladium-Loaded Hierarchical Flower-like Tin Dioxide Structure as Chemosensor Exhibiting High Ethanol Response in Humid Conditions. Adv. Mater. Interfaces 2017, 4, 1700847. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, W.; Li, J.; Tang, C.; Zhang, H. Nanosheet-Assembled Flower-like SnO2 Hierarchical Structures with Enhanced Gas-Sensing Performance. Mater. Lett. 2015, 161, 499–502. [Google Scholar] [CrossRef]
- Xu, M.-H.; Cai, F.-S.; Yin, J.; Yuan, Z.-H.; Bie, L.-J. Facile Synthesis of Highly Ethanol-Sensitive SnO2 Nanosheets Using Homogeneous Precipitation Method. Sens. Actuators B Chem. 2010, 145, 875–878. [Google Scholar] [CrossRef]
- Cao, S.; Zeng, W.; Zhu, Z.; Peng, X. Synthesis of SnO2 Nanostructures from 1D to 3D via a Facile Hydrothermal Method and Their Gas Sensing Properties. J. Mater. Sci. Mater. Electron. 2015, 26, 1820–1826. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, H.; Li, Y.; Chen, W.; Wang, Z. Hydrothermal Synthesis of Hierarchical Flower-like SnO2 Nanostructures with Enhanced Ethanol Gas Sensing Properties. Mater. Res. Bull. 2014, 57, 91–96. [Google Scholar] [CrossRef]
- Zeng, W.; He, Q.; Pan, K.; Wang, Y. Synthesis of Multifarious Hierarchical Flower-like SnO2 and Their Gas-Sensing Properties. Phys. E Low-Dimens. Syst. Nanostructures 2013, 54, 313–318. [Google Scholar] [CrossRef]
- Firooz, A.A.; Mahjoub, A.R.; Khodadadi, A.A. Highly Sensitive CO and Ethanol Nanoflower-like SnO2 Sensor among Various Morphologies Obtained by Using Single and Mixed Ionic Surfactant Templates. Sens. Actuators B Chem. 2009, 141, 89–96. [Google Scholar] [CrossRef]
- Zhang, B.; Fu, W.; Meng, X.; Ruan, A.; Su, P.; Yang, H. Enhanced Ethanol Sensing Properties Based on Spherical-Coral-like SnO2 Nanorods Decorated with α-Fe2O3 Nanocrystallites. Sens. Actuators B Chem. 2018, 261, 505–514. [Google Scholar] [CrossRef]
- Hoa, L.T.; Cuong, N.D.; Hoa, T.T.; Khieu, D.Q.; Long, H.T.; Quang, D.T.; Hoa, N.D.; Hieu, N.V. Synthesis, Characterization, and Comparative Gas Sensing Properties of Tin Dioxide Nanoflowers and Porous Nanospheres. Ceram. Int. 2015, 41, 14819–14825. [Google Scholar] [CrossRef]
- Wang, X.; Han, X.; Xie, S.; Kuang, Q.; Jiang, Y.; Zhang, S.; Mu, X.; Chen, G.; Xie, Z.; Zheng, L. Controlled Synthesis and Enhanced Catalytic and Gas-Sensing Properties of Tin Dioxide Nanoparticles with Exposed High-Energy Facets. Chem. Eur. J. 2012, 18, 2283–2289. [Google Scholar] [CrossRef]
- Punginsang, M.; Wisitsoraat, A.; Sriprachuabwong, C.; Phokharatkul, D.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. Roles of Cobalt Doping on Ethanol-Sensing Mechanisms of Flame-Spray-Made SnO2 Nanoparticles−electrolytically Exfoliated Graphene Interfaces. Appl. Surf. Sci. 2017, 425, 351–366. [Google Scholar] [CrossRef]
- Asgari, M.; Saboor, F.H.; Mortazavi, Y.; Khodadadi, A.A. SnO2 Decorated SiO2 Chemical Sensors: Enhanced Sensing Performance toward Ethanol and Acetone. Mater. Sci. Semicond. Process. 2017, 68, 87–96. [Google Scholar] [CrossRef]
- Li, M.; Zhu, H.; Cheng, J.; Zhao, M.; Yan, W. Synthesis and Improved Ethanol Sensing Performance of CuO/SnO2 Based Hollow Microspheres. J. Porous Mater. 2017, 24, 507–518. [Google Scholar] [CrossRef]
- NaderiNasrabadi, M.; Mortazavi, Y.; Khodadadi, A.A. Highly Sensitive and Selective Gd2O3-Doped SnO2 Ethanol Sensors Synthesized by a High Temperature and Pressure Solvothermal Method in a Microreactor. Sens. Actuators B Chem. 2016, 230, 130–139. [Google Scholar] [CrossRef]
- Ponzoni, A. Morphological Effects in SnO2 Chemiresistors for Ethanol Detection: A Review in Terms of Central Performances and Outliers. Sensors 2021, 21, 29. [Google Scholar] [CrossRef]

| Nanoparticles | Nanorods | Nanosheets | |
|---|---|---|---|
| Number of samples [Refs.] | 30 [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24] | 5 [25,26,27,28,29] | 7 [22,30,31,32,33,34] |
| Number of outliers | 4 | 0 | 1 |
| Ggas/Gair, Q1 | 3 | 2.075 | 4.175 |
| Ggas/Gair, Q2 (median) | 4.55 | 2.3 | 10 |
| Ggas/Gair, Q3 | 14 | 5.825 | 16.75 |
| Ggas/Gair, whisker low | 1.8 | 2 | 2.4 |
| Ggas/Gair, whisker up | 30 | 8 | 18 |
| p-value median test, nanorods | NaN | 0.68 | 0.18 |
| p-value median test, nanoparticles | 0.68 | NaN | 0.08 |
| p-value median test, nanosheets | 0.18 | 0.08 | NaN |
| Nanosheets | Nanorods | Nanoparticles | |
|---|---|---|---|
| Number of samples [Refs.] | 5 [30,35,36,37,38] | 12 [35,36,37,38,39,40,41] | 7 [35,37,40,42,43,44,45] |
| Number of outliers | 1 | 2 | 1 |
| Ggas/Gair, Q1 | 58.25 | 23.5 | 14.375 |
| Ggas/Gair, Q2 (median) | 71 | 52 | 38 |
| Ggas/Gair, Q3 | 193 | 115 | 85 |
| Ggas/Gair, whisker low | 29 | 3.4 | 2.9 |
| Ggas/Gair, whisker up | 93 | 135 | 100 |
| p-value median test, nanorods | NaN | 0.5 | 0.08 |
| p-value median test, nanoparticles | 0.5 | NaN | 0.21 |
| p-value median test, nanosheets | 0.08 | 0.21 | NaN |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponzoni, A. Morphological Effects in SnO2 Chemiresistors for Ethanol Detection: A Systematic Statistical Analysis of Results Published in the Last 5 Years. Chem. Proc. 2021, 5, 75. https://doi.org/10.3390/CSAC2021-10474
Ponzoni A. Morphological Effects in SnO2 Chemiresistors for Ethanol Detection: A Systematic Statistical Analysis of Results Published in the Last 5 Years. Chemistry Proceedings. 2021; 5(1):75. https://doi.org/10.3390/CSAC2021-10474
Chicago/Turabian StylePonzoni, Andrea. 2021. "Morphological Effects in SnO2 Chemiresistors for Ethanol Detection: A Systematic Statistical Analysis of Results Published in the Last 5 Years" Chemistry Proceedings 5, no. 1: 75. https://doi.org/10.3390/CSAC2021-10474
APA StylePonzoni, A. (2021). Morphological Effects in SnO2 Chemiresistors for Ethanol Detection: A Systematic Statistical Analysis of Results Published in the Last 5 Years. Chemistry Proceedings, 5(1), 75. https://doi.org/10.3390/CSAC2021-10474






