Nanostructured Bismuth Electrodes for Non-Enzymatic Paracetamol Sensing: Development, Testing, and Computational Approach †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Material Synthesis and Modification of SPCEs
2.2.2. Electrochemical Measurements
2.2.3. Modelling
3. Results and Discussion
3.1. Electrochemical Measurements
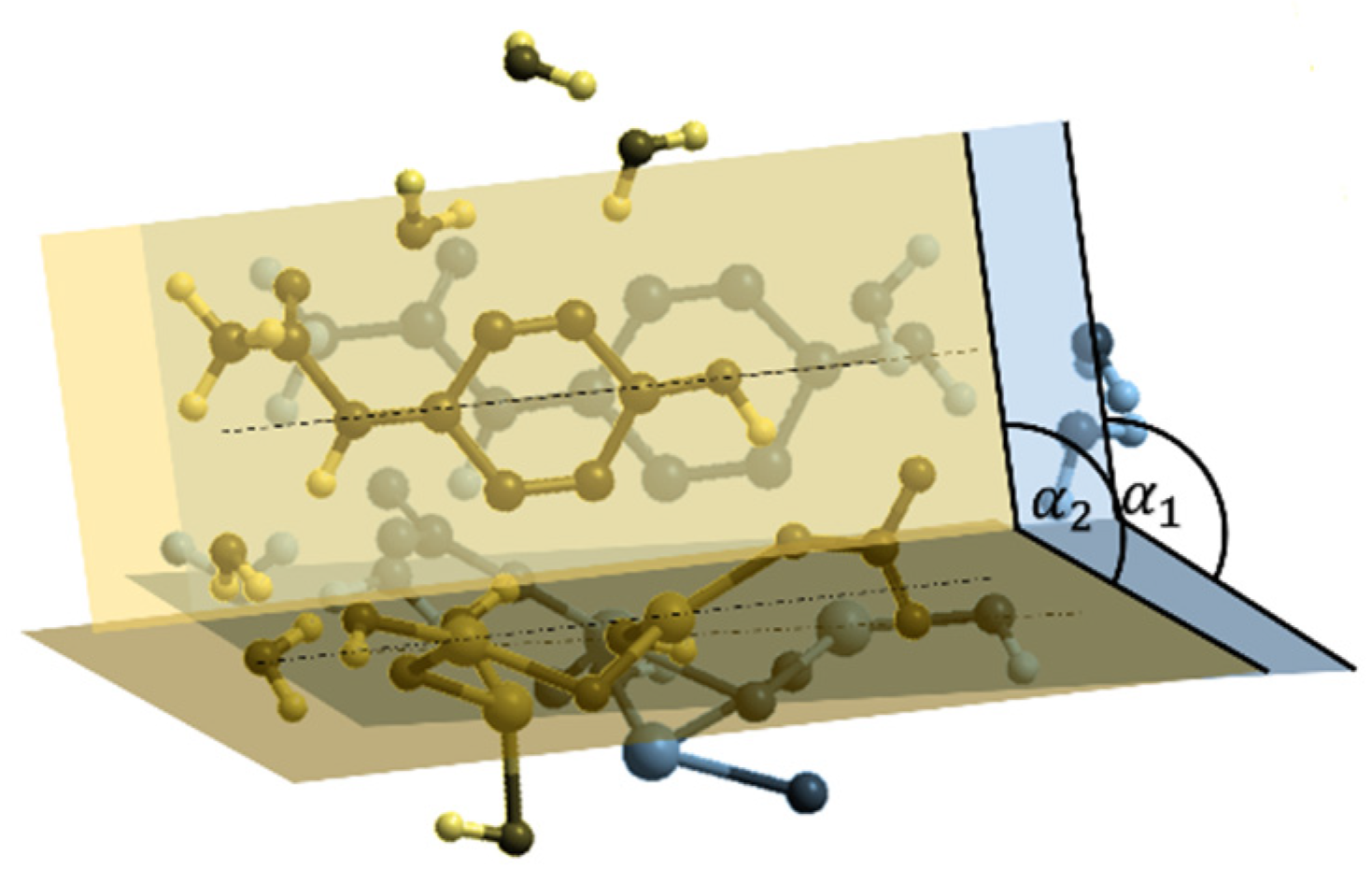
3.2. Computational Evaluation
- (1)
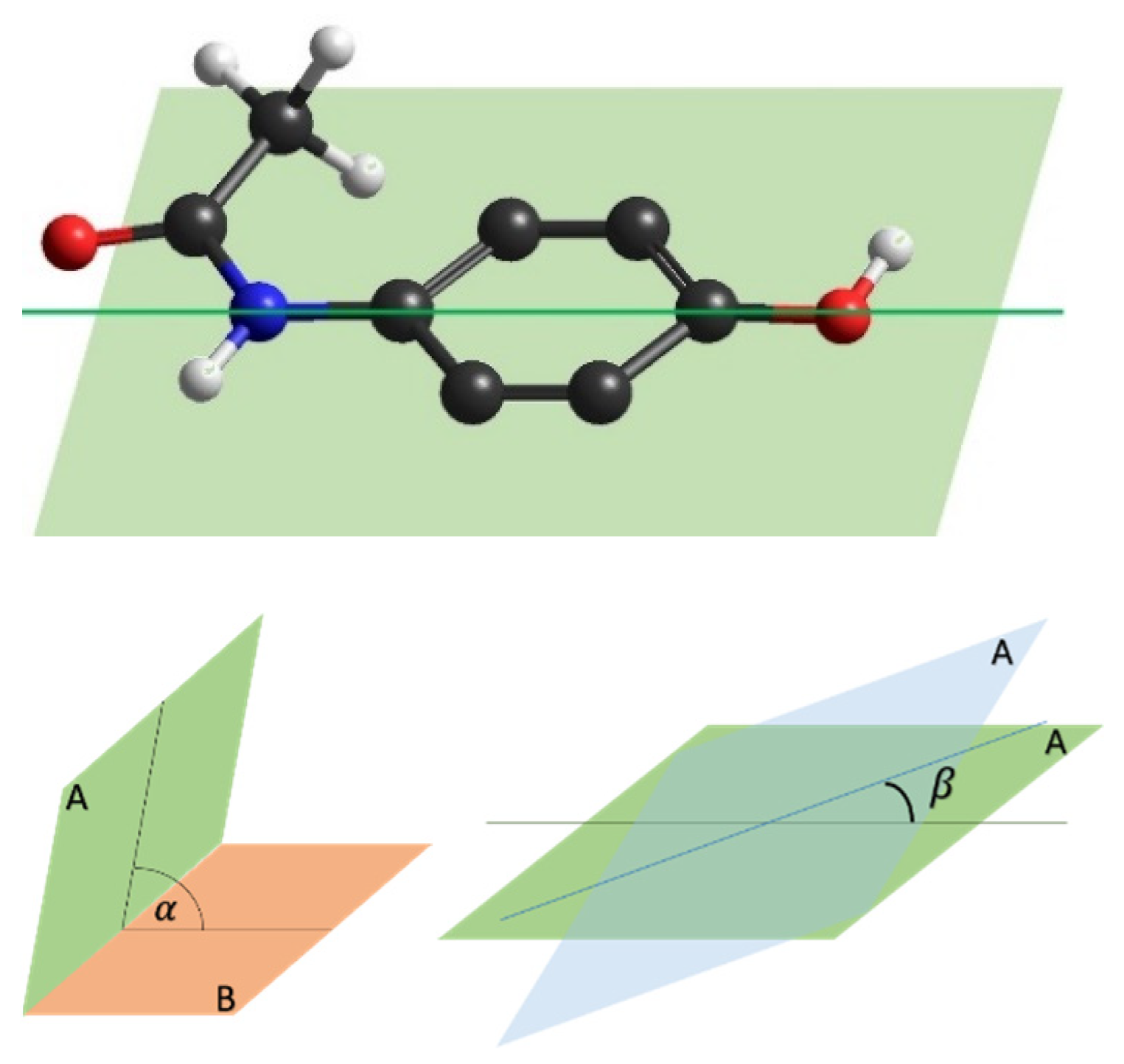
- The symmetric axis passing through the organic compound C atoms that is a C2 axis since the symmetry is maintained by a rotation of 180°
- (2)
- The paracetamol plane A of symmetry on which the C ring lies that is a reflection plane
- (3)
- A second plane B on which the active Bi atoms lie
- (4)
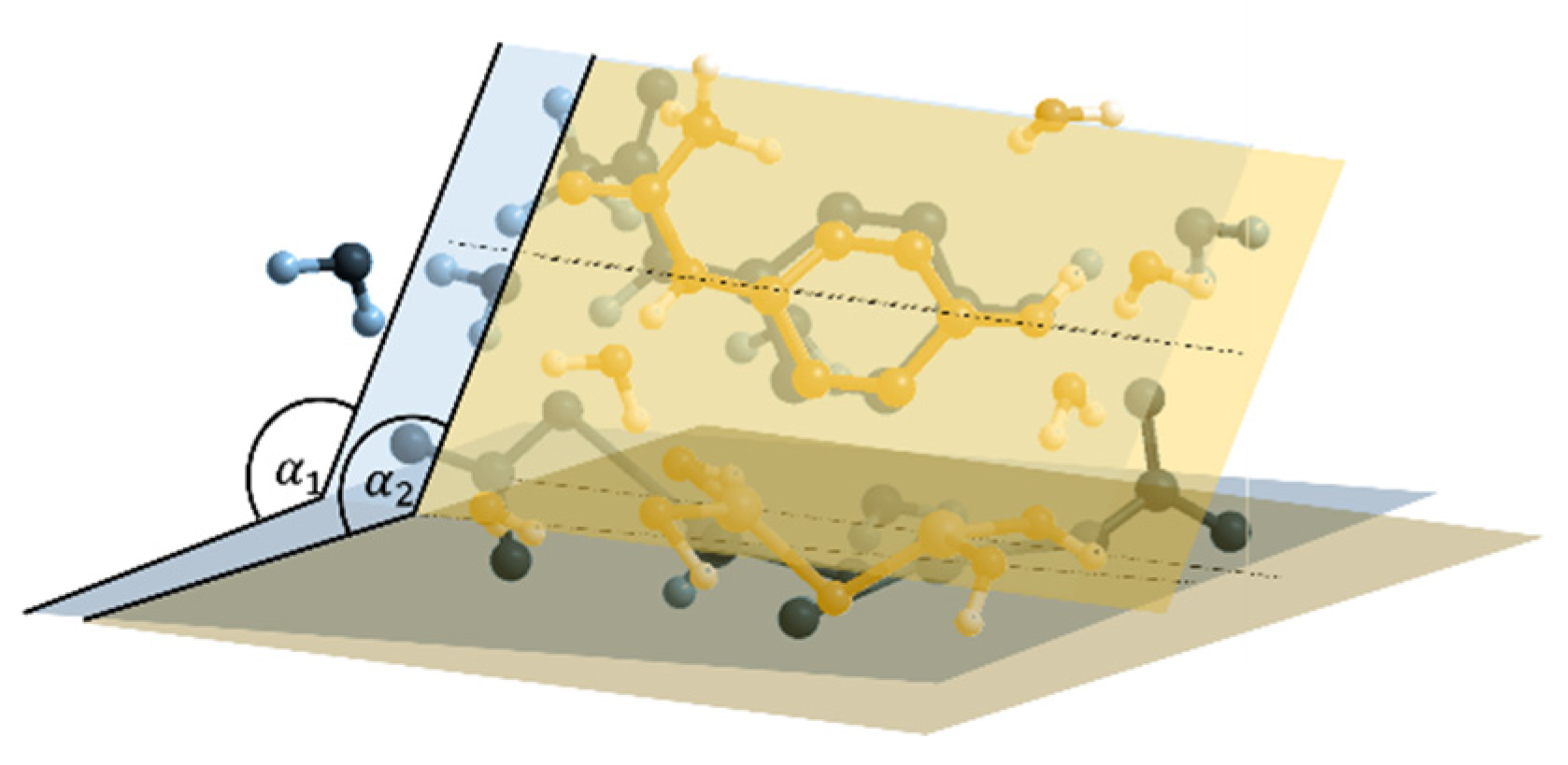
- The dihedral angle between these two planes
- (5)
- The angle β defining the rotation of the paracetamol plane with respect to its symmetric axis.
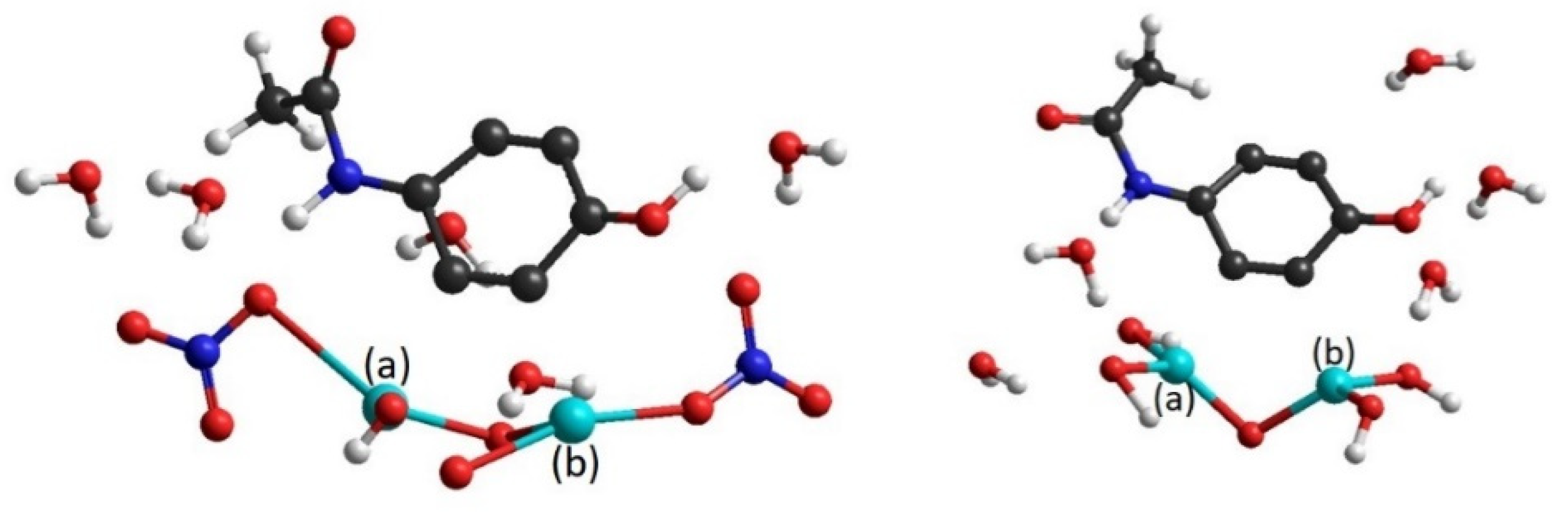
3.2.1. HO–BiONO3 Dimers
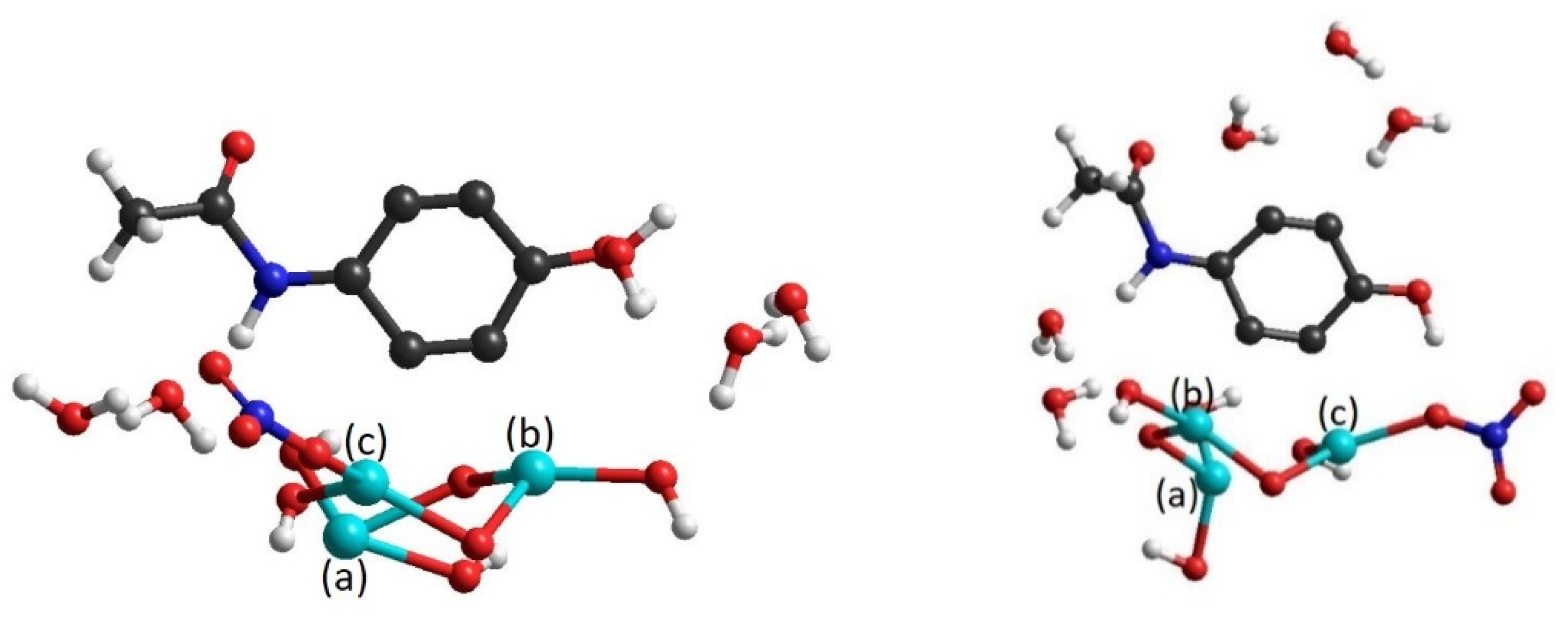
3.2.2. HO–BiONO3 Trimer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kang, X.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. A graphene-based electrochemical sensor for sensitive detection of paracetamol. Talanta 2010, 81, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.; Tan, G.; Jiang, J.; Peng, S.; Deng, M.; Qian, D.; Feng, Y.; Liu, Y. High-sensitivity paracetamol sensor based on Pd/graphene oxide nanocomposite as an enhanced electrochemical sensing platform. Biosens. Bioelectron. 2014, 54, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, W.; Chen, Z.; Song, Z.; Luo, X. Electrochemical determination of paracetamol based on Au@ graphene core-shell nanoparticles doped conducting polymer PEDOT nanocomposite. Sens. Actuators B Chem. 2018, 260, 778–785. [Google Scholar] [CrossRef]
- Madagalam, M.; Bartoli, M.; Tagliaferro, A.; Carrara, S. Bismuth-nanocomposites modified SPCEs for non-enzymatic electrochemical sensors. IEEE Sens. J. 2021, 21, 11155–11162. [Google Scholar] [CrossRef]
- Bartoli, M.; Jagdale, P.; Tagliaferro, A. A Short Review on Biomedical Applications of Nanostructured Bismuth Oxide and Related Nanomaterials. Materials 2020, 13, 5234. [Google Scholar] [CrossRef] [PubMed]
- Aliakbarinodehi, N.; Taurino, I.; Pravin, J.; Tagliaferro, A.; Piccinini, G.; De Micheli, G.; Carrara, S. Electrochemical nanostructured biosensors: Carbon nanotubes versus conductive and semi-conductive nanoparticles. Chem. Pap. 2015, 69, 134–142. [Google Scholar] [CrossRef]
- Đurđić, S.; Vukojević, V.; Vlahović, F.; Ognjanović, M.; Švorc, Ľ.; Kalcher, K.; Mutić, J.; Stanković, D.M. Application of bismuth (III) oxide decorated graphene nanoribbons for enzymatic glucose biosensing. J. Electroanal. Chem. 2019, 850, 113400. [Google Scholar] [CrossRef]
- Liu, G.-Q.; Zhong, H.; Li, X.-R.; Yang, K.; Jia, F.-F.; Cheng, Z.-P.; Zhang, L.-L.; Yin, J.-Z.; Guo, L.-P.; Qian, H.-Y. Research on nonenzymatic electrochemical sensor using HO-BiONO3 nanocomposites for glucose detection. Sens. Actuators B Chem. 2017, 242, 484–491. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods Fundamentals and Applications; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Brunk, E.; Rothlisberger, U. Mixed Quantum Mechanical/Molecular Mechanical Molecular Dynamics Simulations of Biological Systems in Ground and Electronically Excited States. Chem. Rev. 2015, 115, 6217–6263. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods I. Method. J. Comput. Chem. 1989, 10, 209–220. [Google Scholar] [CrossRef] [Green Version]
- Marcus, R.A. Chemical and electrochemical electron-transfer theory. Annu. Rev. Phys. Chem. 1964, 15, 155–196. [Google Scholar] [CrossRef]
- Tachiya, M. Generalization of the Marcus equation for the electron-transfer rate. J. Phys. Chem. 1993, 97, 5911–5916. [Google Scholar] [CrossRef]
- Serpone, N.; Maruthamuthu, P.; Pichat, P.; Pelizzetti, E.; Hidaka, H. Exploiting the interparticle electron transfer process in the photocatalysed oxidation of phenol, 2-chlorophenol and pentachlorophenol: Chemical evidence for electron and hole transfer between coupled semiconductors. J. Photochem. Photobiol. A Chem. 1995, 85, 247–255. [Google Scholar] [CrossRef]


| Working Electrode | Peak Position (mV) | Peak Current (μA) | α | ΔEp (mV) | k (ms−1) | Sensitiviy (μA/mM) | Detection Limit (μM) |
|---|---|---|---|---|---|---|---|
| SPCE | 459.9 ± 1.0 | 33.1 ± 1.5 | 0.44 ± 0.10 | 477 ± 45 | 0.60 ± 0.33 | 20.02 ± 0.23 | 2.34± 0.03 |
| HO–BiONO3 | 433.6 ± 6.1 | 42.0 ± 1.5 | 0.45 ± 0.04 | 392 ± 19 | 2.20 ± 0.82 | 34.88 ± 0.15 | 2.79 ± 0.01 |
| PEG HO–BiONO3 | 385 ± 30 | 52.1 ± 3.0 | 0.40 ± 0.04 | 243 ± 10 | 42.0 ± 9.8 | 43.50 ± 0.54 | 2.24 ± 0.03 |
| Dimer | ||
|---|---|---|
| NO3-NO3 | ||
| OH-OH | ||
| OH-NO3 | ||
| NO3-OH |
| Trimer | ||
|---|---|---|
| NO3-NO3-NO3 | ||
| NO3-OH-NO3 | ||
| NO3-OH-OH | ||
| OH-OH-NO3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madagalam, M.; Catania, F.; Bartoli, M.; Tagliaferro, A.; Carrara, S. Nanostructured Bismuth Electrodes for Non-Enzymatic Paracetamol Sensing: Development, Testing, and Computational Approach. Chem. Proc. 2021, 5, 33. https://doi.org/10.3390/CSAC2021-10427
Madagalam M, Catania F, Bartoli M, Tagliaferro A, Carrara S. Nanostructured Bismuth Electrodes for Non-Enzymatic Paracetamol Sensing: Development, Testing, and Computational Approach. Chemistry Proceedings. 2021; 5(1):33. https://doi.org/10.3390/CSAC2021-10427
Chicago/Turabian StyleMadagalam, Mallikarjun, Federica Catania, Mattia Bartoli, Alberto Tagliaferro, and Sandro Carrara. 2021. "Nanostructured Bismuth Electrodes for Non-Enzymatic Paracetamol Sensing: Development, Testing, and Computational Approach" Chemistry Proceedings 5, no. 1: 33. https://doi.org/10.3390/CSAC2021-10427
APA StyleMadagalam, M., Catania, F., Bartoli, M., Tagliaferro, A., & Carrara, S. (2021). Nanostructured Bismuth Electrodes for Non-Enzymatic Paracetamol Sensing: Development, Testing, and Computational Approach. Chemistry Proceedings, 5(1), 33. https://doi.org/10.3390/CSAC2021-10427








