Activity of Satureja montana Allelochemical Volatiles Against the Pinewood Nematode †
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. In Vitro Cultures of the Pinewood Nematode
2.3. Nematicidal Activity of Volatiles
2.4. Data Treatment and Statistical Analysis
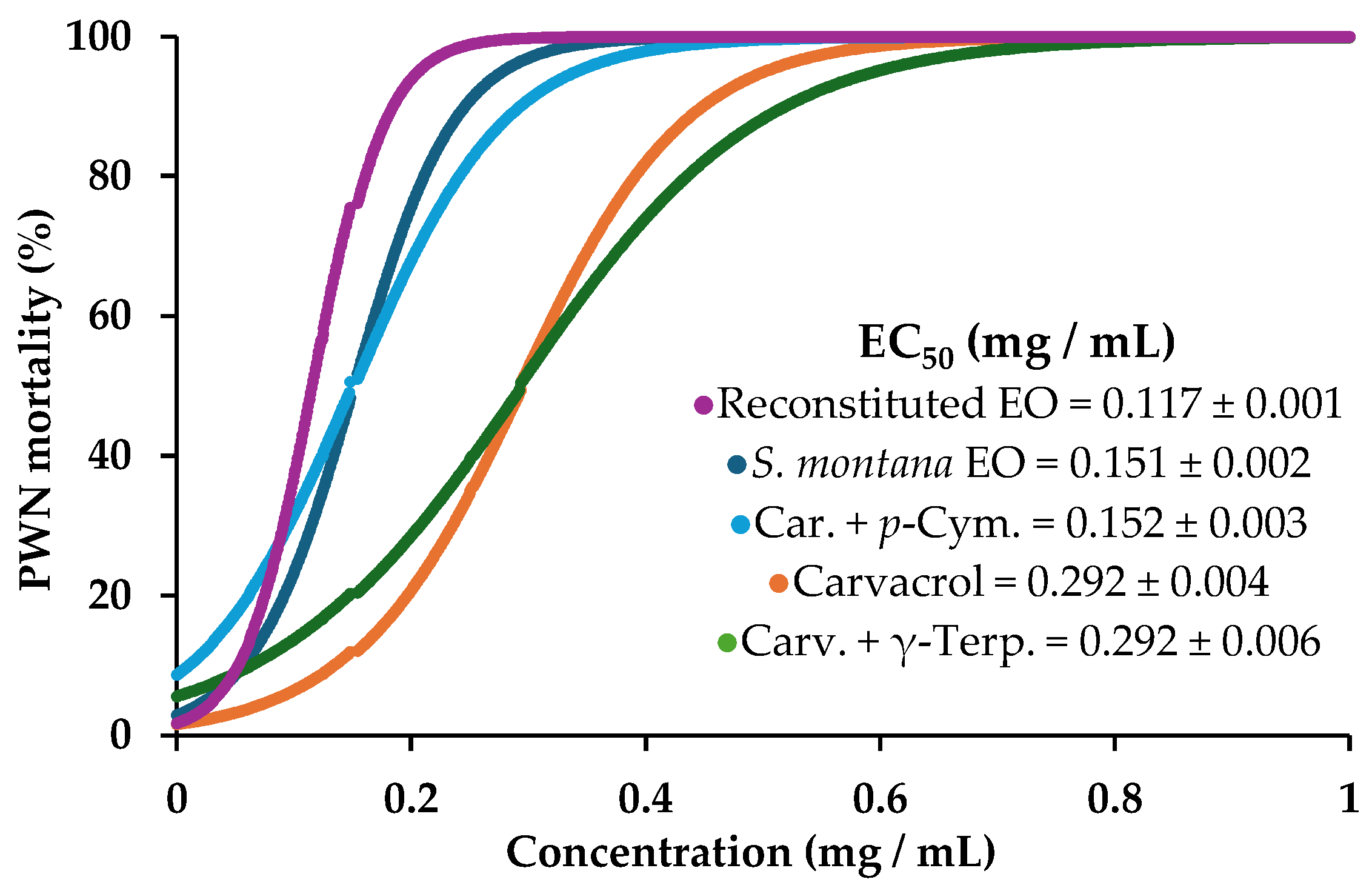
3. Results and Discussion
Nematicidal Activity of Essential Oils and Mixtures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological Control of Plant-Parasitic Nematodes by Filamentous Fungi Inducers of Resistance: Trichoderma, Mycorrhizal and Endophytic Fungi. Front. Microbiol. 2020, 11, 992. [Google Scholar] [CrossRef] [PubMed]
- Back, M.A.; Bonifácio, L.; Inácio, M.L.; Mota, M.; Boa, E. Pine Wilt Disease: A Global Threat to Forestry. Plant Pathol. 2024, 73, 1026–1041. [Google Scholar] [CrossRef]
- Vicente, C.; Espada, M.; Vieira, P.; Mota, M. Pine Wilt Disease: A Threat to European Forestry. Eur. J. Plant Pathol. 2012, 133, 89–99. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Barbosa, P.; Vieira, P.; Vicente, C.S.L.; Figueiredo, A.C.; Mota, M. Phytochemicals as Biopesticides against the Pinewood Nematode Bursaphelenchus Xylophilus: A Review on Essential Oils and Their Volatiles. Plants 2021, 10, 2614. [Google Scholar] [CrossRef] [PubMed]
- Takai, K.; Soejima, T.; Suzuki, T.; Kawazu, K. Development of a Water-Soluble Preparation of Emamectin Benzoate and Its Preventative Effect against the Wilting of Pot-Grown Pine Trees Inoculated with the Pine Wood Nematode,Bursaphelenchus Xylophilus. Pest Manag. Sci. 2001, 57, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Cavaco, T.; Faria, J.M.S. Phytochemical Volatiles as Potential Bionematicides with Safer Ecotoxicological Properties. Toxics 2024, 12, 406. [Google Scholar] [CrossRef] [PubMed]
- Eurostat Agri-Environmental Indicator—Consumption of Pesticides—Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Agri-environmental_indicator_-_consumption_of_pesticides#cite_note-1 (accessed on 28 February 2024).
- Barbosa, P.; Faria, J.M.S.; Cavaco, T.; Figueiredo, A.C.; Mota, M.; Vicente, C.S.L. Nematicidal Activity of Phytochemicals against the Root-Lesion Nematode Pratylenchus Penetrans. Plants 2024, 13, 726. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors Affecting Secondary Metabolite Production in Plants: Volatile Components and Essential Oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Barbosa, P. Cymbopogon Citratus Allelochemical Volatiles as Potential Biopesticides against the Pinewood Nematode. Plants 2024, 13, 2233. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.M.S.; Barbosa, P.; Bennett, R.N.; Mota, M.; Figueiredo, A.C. Bioactivity against Bursaphelenchus Xylophilus: Nematotoxics from Essential Oils, Essential Oils Fractions and Decoction Waters. Phytochemistry 2013, 94, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.M.S.; Cavaco, T.; Gonçalves, D.; Barbosa, P.; Teixeira, D.M.; Moiteiro, C.; Inácio, M.L. First Report on the Synergistic Interaction between Essential Oils against the Pinewood Nematode Bursaphelenchus Xylophilus. Plants 2023, 12, 2438. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, A.G.; Hemming, J.R. A Comparison of Some Quantitative Methods of Extracting Small Vermiform Nematodes from Soil. Ann. Appl. Biol. 1965, 55, 25–38. [Google Scholar] [CrossRef]
| Compound/Combination | Concentration in 1 mg/mL of EO (mg/mL) 1 | ||
|---|---|---|---|
| Carvacrol (64 2) | γ-Terpinene (18 2) | p-Cymene (8 2) | |
| carvacrol | 0.64 | ||
| γ-terpinene | 0.18 | ||
| p-cymene | 0.08 | ||
| carvacrol + γ-terpinene | 0.64 | 0.18 | |
| carvacrol + p-cymene | 0.64 | 0.08 | |
| γ-terpinene + p-cymene | 0.18 | 0.08 | |
| carvacrol + γ-terpinene + p-cymene 3 | 0.64 | 0.18 | 0.08 |
| EO/Compounds | Mortality % at 1 mg/mL | EC50 24h (mg/mL) | EC100 24h (mg/mL) | p | R2 |
|---|---|---|---|---|---|
| Satureja montana EO | 100 ± 0.0 | 0.151 ± 0.002 | 0.59–0.78 | 10.1 ± 0.5 | 0.98 |
| carvacrol | 100 ± 0.0 | 0.292 ± 0.004 | 0.84–0.99 | 6.1 ± 0.3 | 0.99 |
| γ-terpinene | 23.5 ± 0.3 | ||||
| p-cymene | 16.4 ± 0.4 | ||||
| carvacrol + γ-terpinene | 100 ± 0.0 | 0.292 ± 0.006 | 0.71–0.81 | 4.2 ± 0.2 | 0.97 |
| carvacrol + p-cymene | 100 ± 0.0 | 0.152 ± 0.003 | 0.66–0.79 | 6.7 ± 0.3 | 0.97 |
| γ-terpinene + p-cymene | 63.7 ± 1.2 | ||||
| γ-terpinene + p-cymene + carvacrol 1 | 100 ± 0.0 | 0.117 ± 0.001 | 0.38–0.62 | 14.9 ± 0.8 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, G.; Faria, J.M.S. Activity of Satureja montana Allelochemical Volatiles Against the Pinewood Nematode. Chem. Proc. 2024, 16, 8. https://doi.org/10.3390/ecsoc-28-20154
Pereira G, Faria JMS. Activity of Satureja montana Allelochemical Volatiles Against the Pinewood Nematode. Chemistry Proceedings. 2024; 16(1):8. https://doi.org/10.3390/ecsoc-28-20154
Chicago/Turabian StylePereira, Gonçalo, and Jorge M. S. Faria. 2024. "Activity of Satureja montana Allelochemical Volatiles Against the Pinewood Nematode" Chemistry Proceedings 16, no. 1: 8. https://doi.org/10.3390/ecsoc-28-20154
APA StylePereira, G., & Faria, J. M. S. (2024). Activity of Satureja montana Allelochemical Volatiles Against the Pinewood Nematode. Chemistry Proceedings, 16(1), 8. https://doi.org/10.3390/ecsoc-28-20154







