Development of Quantitative Structure–Anti-Inflammatory Relationships of Alkaloids †
Abstract
1. Introduction
2. Materials and Methods
2.1. Alkaloids Database Description
2.2. Molecular Structure of Alkaloids and Feature Representation
2.3. Machine Learning Classifiers
2.4. Supervised Feature Selection
2.5. Validation Performance
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kulinsky, V.I. Biochemical Aspects of Inflammation. Biochemistry 2007, 72, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.A.; Kirkby, N.S. Eicosanoids, prostacyclin and cyclooxygenase in the cardiovascular system. Br. J. Pharmacol. 2019, 176, 1038–1050. [Google Scholar] [CrossRef]
- Souto, A.L.; Tavares, J.F.; Da Silva, M.S.; Diniz, M.d.F.F.M.; de Athayde-Filho, P.F.; Filho, J.M.B. Anti-inflammatory Activity of Alkaloids: An Update from 2000 to 2010. Molecules 2011, 16, 8515–8534. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.F.; Wink, M. Introduction. In Alkaloids: Biochemistry, Ecology, and Medicinal Applications; Roberts, M.F., Wink, M., Eds.; Springer: Boston, MA, USA, 1998; pp. 1–7. [Google Scholar]
- Todeschini, R.; Consonni, V. Molecular Descriptors for Chemoinformatics; WILEY-VCH: Weinheim, Germany, 2009. [Google Scholar]
- OECD. Guidance Document on the Validation of (Quantitative)Structure-Activity Relationships [(Q)SAR] Models; OECD: Paris, France, 2014. [Google Scholar] [CrossRef]
- Barbosa-Filho, J.M.; Piuvezam, M.R.; Moura, M.D.; Silva, M.S.; Lima, K.V.B.; da-Cunha, E.V.L.; Fechine, I.M.; Takemura, O.S. Anti-inflammatory Activity of Alkaloids: A twenty-century Review. Rev. Bras. Farmacogn. 2006, 16, 109–139. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B. PubChem 2023 Update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef] [PubMed]
- Hypercube Inc. HyperChemTM Professional Version 8.0. Available online: http://www.hypercubeusa.com/ (accessed on 1 July 2024).
- Alvascience Software Solutions for Cheminformatics and QSAR Research. 2023. Available online: https://www.alvascience.com (accessed on 1 July 2024).
- Mauri, A.; Bertola, M. Alvascience: A New Software Suite for the QSAR Workflow Applied to the Blood–Brain Barrier Permeability. Int. J. Mol. Sci. 2022, 23, 12882. [Google Scholar] [CrossRef] [PubMed]
- Ballabio, D.; Consonni, V.; Mauri, A.; Claeys-Bruno, M.; Sergent, M.; Todeschini, R. A Novel Variable Reduction Method Adapted from Space-filling Designs. Chemom. Intell. Lab. Syst. 2014, 136, 147–154. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A Basic Tool of Chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Kowalski, B.; Bender, C. k-Nearest Neighbor Classification Rule (Pattern Recognition) Applied to Nuclear Magnetic Resonance Spectral Interpretation. Anal. Chem. 1972, 44, 1405–1411. [Google Scholar] [CrossRef]
- Todeschini, R.; Ballabio, D.; Cassotti, M.; Consonni, V. N3 and BNN: Two New Similarity Based Classification Methods in Comparison with Other Classifiers. J. Chem. Inf. Model. 2015, 55, 2365–2374. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Freund, Y.; Schapire, R.E. A Desicion-Theoretic Generalization of On-line Learning and an Application to Boosting. In Proceedings of the Computational Learning Theory, Barcelona, Spain, 13–15 March 1995; pp. 23–37. [Google Scholar]
- Leardi, R. Genetic Algorithms in Chemistry. In Comprehensive Chemometrics: Chemical and Biochemical Data Analysis, 2nd ed.; Brown, S., Tauler, R., Walczak, B., Eds.; Elsevier: Oxford, UK, 2020; Volume 1, pp. 617–634. [Google Scholar]
- Ballabio, D.; Grisoni, F.; Todeschini, R. Multivariate Comparison of Classification Performance Measures. Chemom. Intell. Lab. Syst. 2018, 174, 33–44. [Google Scholar] [CrossRef]
- Randić, M. On Characterization of Chemical Structure. J. Chem. Inf. Comput. Sci. 1997, 37, 672–687. [Google Scholar] [CrossRef]
- Labute, P. A Widely Applicable Set of Descriptors. J. Mol. Graph. Model. 2000, 18, 464–477. [Google Scholar] [CrossRef] [PubMed]
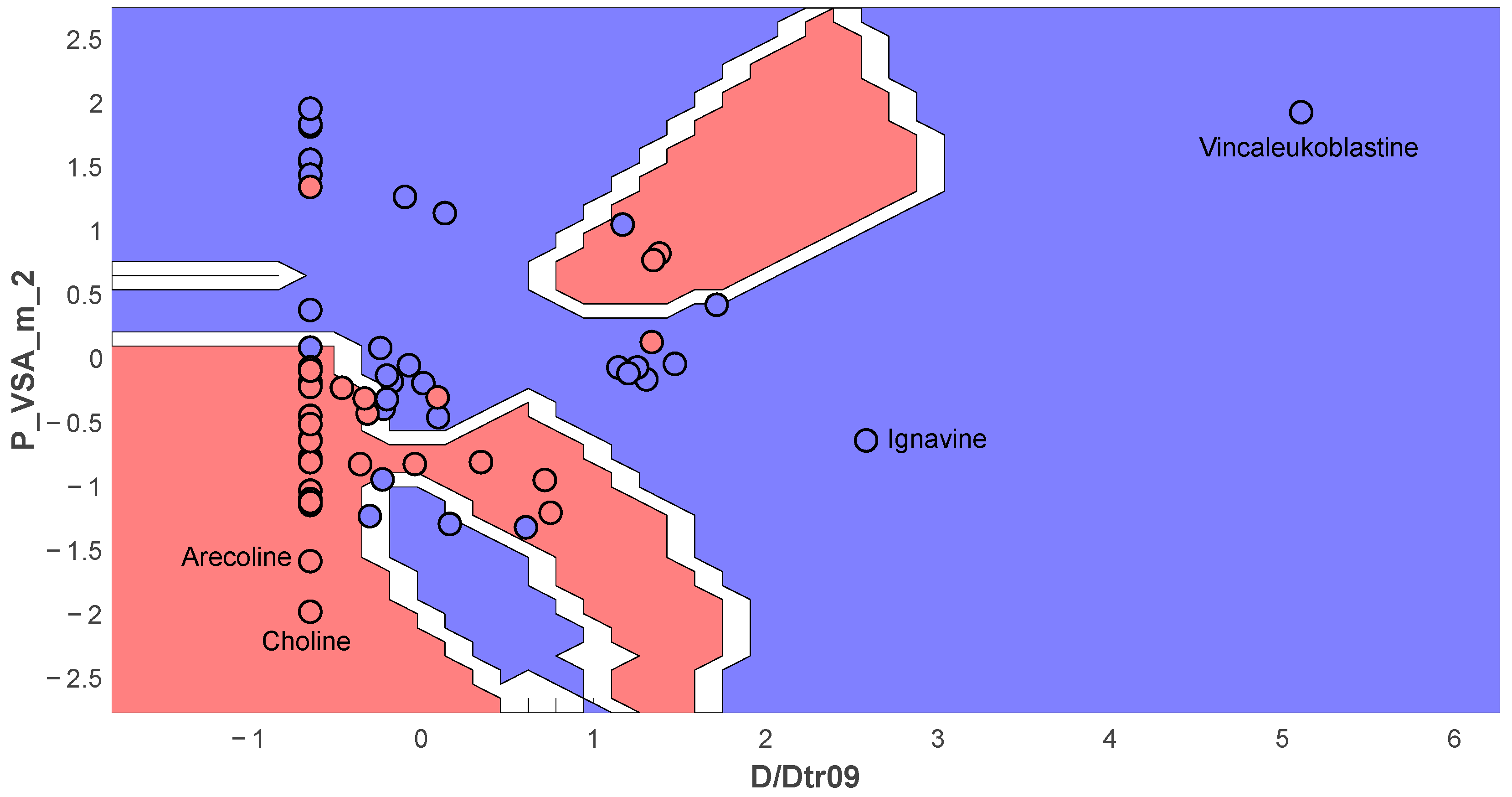
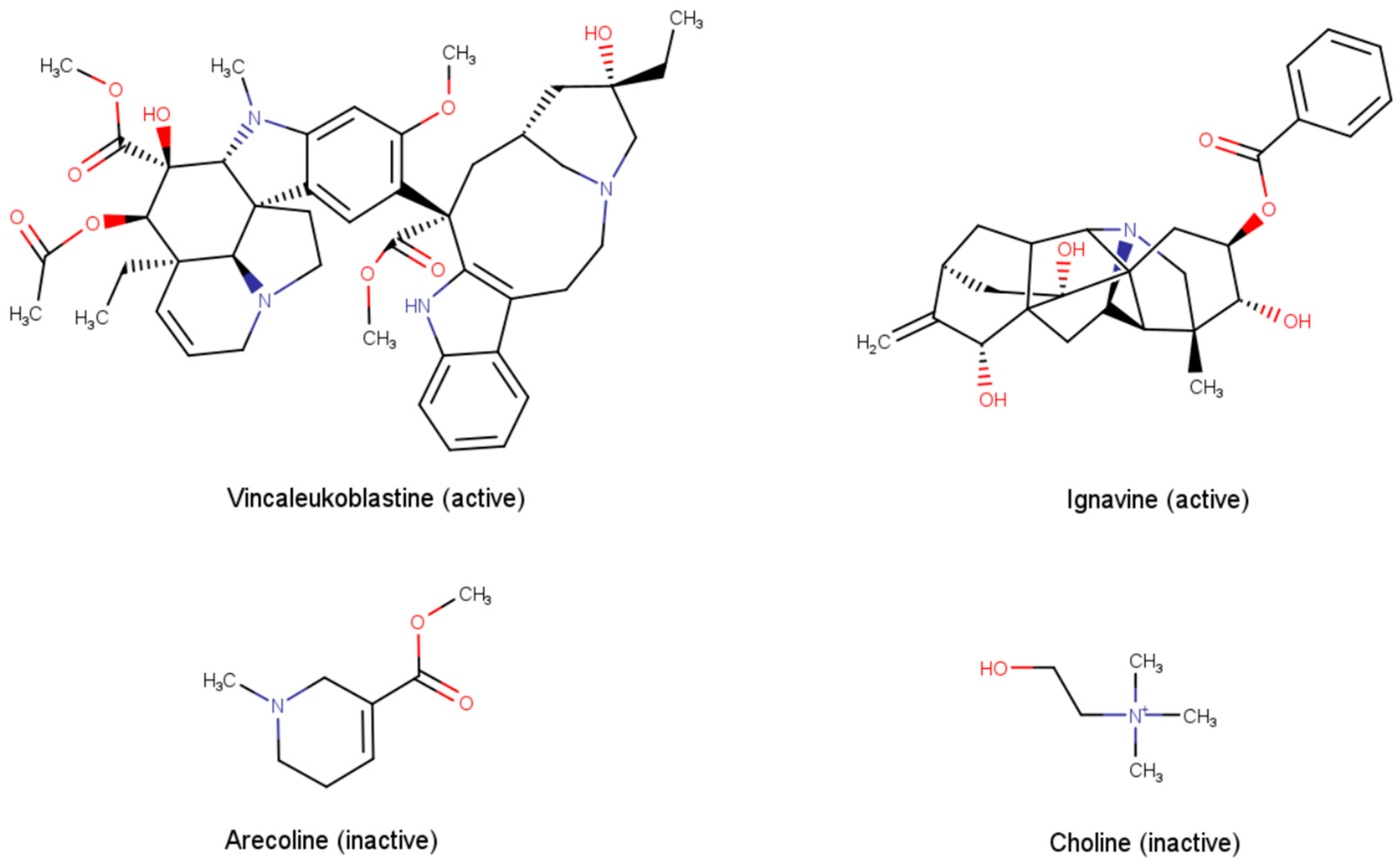
| Molecular Feature Type | Classifier | Optimal Parameters | Training Set | Cross-Validation | Test Set |
|---|---|---|---|---|---|
| Molecular descriptors | kNN | k = 6; MDs = 2 | 0.71 | 0.76 | 0.77 |
| Extended-connectivity fingerprints | BNN | α = 0.9 | 0.75 | 0.75 | 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas, C.; Muñoz, D.; Cordero, I.; Tenesaca, B.; Ballabio, D. Development of Quantitative Structure–Anti-Inflammatory Relationships of Alkaloids. Chem. Proc. 2024, 16, 77. https://doi.org/10.3390/ecsoc-28-20159
Rojas C, Muñoz D, Cordero I, Tenesaca B, Ballabio D. Development of Quantitative Structure–Anti-Inflammatory Relationships of Alkaloids. Chemistry Proceedings. 2024; 16(1):77. https://doi.org/10.3390/ecsoc-28-20159
Chicago/Turabian StyleRojas, Cristian, Doménica Muñoz, Ivanna Cordero, Belén Tenesaca, and Davide Ballabio. 2024. "Development of Quantitative Structure–Anti-Inflammatory Relationships of Alkaloids" Chemistry Proceedings 16, no. 1: 77. https://doi.org/10.3390/ecsoc-28-20159
APA StyleRojas, C., Muñoz, D., Cordero, I., Tenesaca, B., & Ballabio, D. (2024). Development of Quantitative Structure–Anti-Inflammatory Relationships of Alkaloids. Chemistry Proceedings, 16(1), 77. https://doi.org/10.3390/ecsoc-28-20159






