Abstract
The focus of this study is the properties of a new oligothiophene grafted with oligo-(D, L-lactide) side chains (OTh-PDLLA) that enable us to establish its prospect as a biomedical material. In this vein, OTh-PDDLA’s self-assembling capability in various solvents was established by measuring its particle size using dynamic light scattering. AFM investigation of the surface topography of the films obtained from several solvents, as well as the results of the interaction with normal human gingival fibroblast (NHGF) cells, showed that OTh-PDLLA offers several ways to topographically modulate the film’s surface, which allows an easy adjustment of interactions with biological entities.
1. Introduction
Conducting π-conjugated polymers (CPs) serendipitously discovered by Shirakawa’s group 50 years ago [1] proved to be complex materials “equipped” with appropriate tools for effectively bridging the interface between electronics and biology [2]. As such, their unique and efficient ion-to-electron conversion combined with their ability to work in physiologically relevant media, alongside their mechanical biomimicry and biocompatibility, already place CPs as the obvious member of synthetic polymers-based biomedical materials to directly interact and/or interface with complex biological systems [3].
Progress in the area of bioelectronics requires sophisticated materials that will ensure not only electrical communication with biological entities but also conformability on non-flat surfaces and/or transience behavior [4]. One of the alternatives to such materials can be graft-CPs (or “hairy-rod” CPs) that have a “rod-graft-coil” architecture. Such topology offers, in addition to the freedom of various combinations of chemistries, the opportunity to program, optimize, and control the molecular design stage, the processing-structure-properties relationship, and the self-assembly pathway [5]. In particular, conjugated polythiophene and its derivatives grafted with flexible, biocompatible, and functional side chains showed their applicability as active surfaces for selective proteins adsorption [6] and as excellent biocompatible and electroactive cellular matrices [7,8,9]. Their applicability in cell imaging [10] or for the sensing of a plethora of bioanalytics [11,12,13] has also been exploited.
Considering the importance of electroactive biomaterials’ biodegradability today, in particular of those based on PTh, we recently, reported on the synthesis and structural characterization of an oligothiophene grafted with oligo-(D, L-lactide) side chains (denoted as OTh-PDLLA) [14]. The environmentally friendly, poly (lactic acid) (PLA) is a biocompatible, biodegradable, sustainable, and non-cytotoxic aliphatic polyester approved by the FDA to be used in medical science and biotechnology [15]. Engineered to function as an efficient bioelectronics interface, being also potentially biocompatible and bioerodible, OTh-PDLLA (Scheme 1) was synthesized by the “grafting through” approach via metal-free photoinduced oxidative homopolymerization. The “grafting through” strategy allows for engineering of both the rigid conjugated backbone and flexible side chains, while the photomediated polymerization of the Th-PDLLA macromonomer was an original, successful approach. Both the structural dissymmetry and particular topology seem to induce unusual features of the new synthesized oligomer [14]. The present report focuses on the study of those properties of OTh-PDLLA that allow the establishing of its suitability as a biomaterial. Thus, its capability for forming thin films, on either rigid or flexible supports used for processing solvents with different polarities and variable concentrations and films surface properties, were explored.
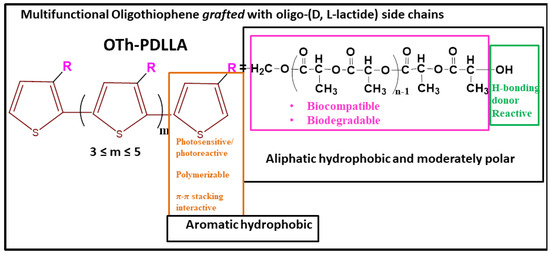
Scheme 1.
Design criteria for the synthesis of the multifunctional OTh-PDLLA.
2. Materials and Methods
Particles size measurement was carried out by dynamic light scattering (DLS) using a Malvern Zetasizer Nano ZS instrument equipped with a 4.0 mW He–Ne laser operating at 633 nm and a detection angle of 173°. The intensity weighted mean hydrodynamic size (Z average) and the polydispersity factor were obtained from analysis of the autocorrelation function. Samples were used as prepared, without filtration at two different concentration, c = 10 mg/mL and c = 1 mg/mL, respectively, in different solvents. The reported values represent the average of three measurements made for each sample, at 25 °C with an equilibration time of 5 min before starting each measurement. The wettability of all the surfaces was investigated by measuring the static contact angle using a DataPhysics OCA 15 (DataPhysics GmbH, Filderstadt, Germany) optical contact angle goniometer. The contact angle was determined under ambient condition within 5 s after placing 1 µL drops of deionized water on the sample’s surface. The images of the water droplets were recorded and analyzed using the software provided by the manufacturer to determine the contact angle values. At least three measurements (five contact angle values) were performed at randomly selected locations across the sample’s surface. AFM micrographs of the polymer’s films were taken in air and on an SPM SOLVER Pro-M instrument. A NSG10/Au Silicon tip with a 35 nm radius of curvature and 255 kHz oscillation mean frequency was used. The apparatus was operated in semi-contact mode, over a 10 × 10 μm2 scan area, with 256 × 256 scan point size images being thus obtained. The polymer’s films were prepared by drop casting on round glass slides and PLA films as substrates. For the biocompatibility evaluation, MTS assay by the direct contact procedure adapted from ISO 10993-5:2009(E) [16] was used. First, each specimen of the test samples was carefully placed into 24-well tissue culture-treated plate and sterilized by UV-radiation for 15 min and then incubated in complete cell culture medium (MEM α medium with 10% fetal bovine serum and 1% Penicillin–Streptomycin–Amphotericin B mixture) in 24-well tissue-culture-treated plate for 1 h. For the in vitro biocompatibility assessment (direct contact method), human gingival fibroblasts (HGF, CLS Cell Lines Service GmbH, Eppelheim, Germany) were seeded into 24-well plates and allowed to adhere, then samples were placed in the wells and incubated for 24 h and 72 h. After incubation, cell viability was determined using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI, USA), according to the manufacturer instructions. Absorbance readings were performed on a FLUOstar® Omega microplate reader (BMG LABTECH, Ortenberg, Germany). Experiments were performed in triplicate and treated cell viability was expressed as percentage of control cells’ viability (means ± standard deviation).
3. Results and Discussion
Designed as a multifunctional material, OTh-PDLLA has encoded in its structure all the “ingredients” that grants it to function as electroactive biointerface or as active layer in flexible and/or implantable transient electronics. As such, its capability for forming thin films, on either rigid or flexible supports compatible with biological milieu and the surface features with direct impact on biological events of the formed films, were investigated by contact-angle measurements, atomic force microscopy (AFM), or dynamic laser-scattering (DLS). Despite the fact that polymeric films obtained by the drop-cast technique could show heterogeneities in the film thickness, we choose this method for the OTh-PDLLA thin films fabrication because it allows slow solvent evaporation into a solvent saturated atmosphere, enabling the polymer’s self-assembling. As such, OTh-PDLLA dispersions (10 mg/mL and 1 mg/mL) in chloroform (Chl) and in dimethyl sulfoxide (DMSO), respectively, were casted on either rigid surface of the round glass slides or flexible, biocompatible and bioerodible PLA sheets. Regarding the solvents used in this study, several aspects, like Hansen solubility parameters, Flory–Huggins solvent-polymer interaction parameters [17,18,19,20], as well as the pharmaceutical nature and therapeutic properties of DMSO [21], were taken into consideration. Thus, while Chl could be considered a good solvent for both conjugated main chain and PDLLA side chains, DMSO is PDLA selective, having the lowest value of the interaction parameter [20] and the best fit of the HS parameters with those of PDLLA [17]. Indeed, all the surface features of OTh-PDLLA with a direct impact on its performance as a biomaterial, like surface chemistry, wettability, or morphology, were found to be solvent and concentration-dependent. Thus, solvent polarity had more influence on the size and morphology of the oligomers ‘self-assembled structures than on its wettability (Table 1, Figure 1 and Figure 2). Unimodal DLS traces (not showed) and almost identical value of the hydrodynamic radii (Dh) of the formed particles were registered by DLS measurement of the OTh-PDLLA dispersions in Chl with different concentrations. As Chl is a mutual solvent for both constitutive parts of OTh-PDLLA, most probable is that their shape dissimilarity is inducing its self-assembling in this solvent. A mixed-population of particles, having 600 nm and 5100 nm, respectively, was formed in the PDLLA selective solvent DMSO. The highest value of 5100 nm could be due (associated with the presence of) either to particles clusters [22], or to the presence of particles with non-spherical geometries [23].

Table 1.
Hydrodynamic radii, water contact angle, surface roughness of OTh-PDLLA films, and particles size in dry state measured by AFM.
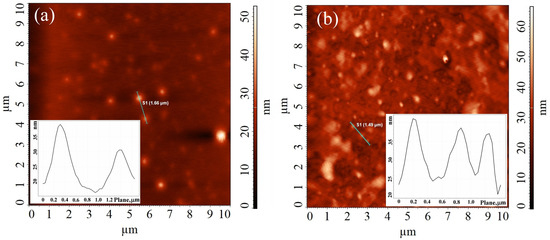
Figure 1.
AFM images for OTh-PDLLA films obtained from its dispersion in Chl at c = 10 mg/mL (a) and c = 1 mg/mL (b).
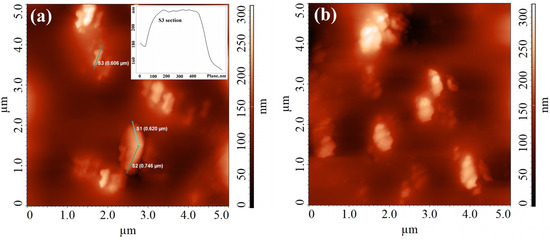
Figure 2.
AFM images for OTh-PDLLA films obtained from its dispersion in DMSO at c = 10 mg/mL deposited on glass (a) and PLA film (b).
Following the dictum “seeing is believing”, the AFM images confirmed that the self-assembled structures of OTh-PDLLA in Chl (Figure 1), “sensed” by DLS, are round in shape with average size of around 600 nm (for Chl solution concentration of 10 mg/mL) and 450 nm (for concentration of 1 mg/mL), arranged as a uniformly scattered network. A similar morphology was observed for PEDOT-co-PDLLA films obtained by the slow cast of chloroform PEDOT-co-PDLLA (1:05, 1:25, and 1: 50) solutions on the gold mylar substrates [24].
A completely different, sophisticated morphology was noticed for the films of OTh-PDLA from DMSO on glass slides or on PLA sheets (Figure 2). Thus, depending on the nature of the used support, nanorods of 600 nm in width with bending propensity (Figure 2a) or twist supermolecular nanostructures (Figure 2b) were observed. Apart from the inter-particles’ interaction on the one hand and the particles–support interactions on the other hand, there are other important aspects like solvophobic/solvophilic interactions or the already-mentioned shape dissimilarity of the oligomer constitutive parts, as well as the thiophene rings’ tendency for π-π stacking during aggregation, that could contribute to formation of the observed morphologies [5,19,25].
Due to its important role in cellular response, the film’s wettability was evaluated by measuring the water contact angle (WCA) outlined in Table 1. The WCA of bare substrates were also registered, the found values being consistent with those already reported in the literature [26]. The investigated OTh-PDLLA films are quite hydrophobic, as the average values of WCA are between 74 and 85°. The films obtained from DMSO and from Chl showed almost the same value of WCA (83° and 85°, respectively), which means that neither the polarity of the solvent nor the roughness of films had no influence on surfaces wettability. On the other hand, a decrease in the films’ hydrophobicity with the concentration decreasing was registered (Table 1). This concentration-dependent wettability is a very important feature from the prospect of the synthesized oligomer’s applicability as a biomaterial. In this scenario, the effects of the OTh-PDLLA films’ surface features on the normal human gingival fibroblasts (NHGF) viability were further evaluated. It has been reported that the attachment and proliferation of these cells are sensitive to the surface topography, as well to the surface hydrophobicity [27], having a maximum adhesion on surfaces with WCA values between 60 and 80° [28].
In the present report, the NHGF viability, at 24 and 72 h (Figure 3), was evaluated by MTS assay based on the mitochondrial activity of cells in direct contact with the material surfaces.
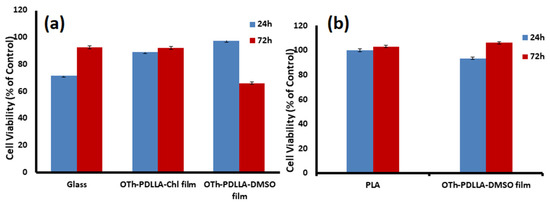
Figure 3.
In vitro cell viability of NHGF seeded on OTh-PDLLA films obtained from its dispersion at c = 10 mg/mL deposited on glass (a) and PLA film (b).
The obtained data showed the good biocompatibility and non-cytotoxic effect of the OTh-PDLLA, highlighting the impact of the films’ topographic properties on cellular behavior. As such, when keeping the concentration of OTh-PDLLA in the used solvents as well as the rigidity of the films’ support constant, the viability of NHGF after 24 h was higher for the OTh-PDLLA-coated glass slides compared to uncovered glass (Figure 3a). A slight increase in the cell viability value for the DMSO-casted films compared with those obtained from the Chl solution was noticed as well. However, after 3 days of direct cells–polymers contact, the viability of the cells seeded onto the DMSO-casted films decreased considerably, while a very good viability (around 90%), was registered for the cells seeded onto Chl-films. As the tested films have similar WCA values, the smoother Chl-films’ surface, measured directly from AFM topographic scans (Table 1), seems to support better the NHGF cells growth. Regarding the influence of the surface roughness of the films, it seems that this parameter is neutral if we take into account the cells’ viability seeded on either uncovered or OTh-PDLLA-covered PLA sheets (Figure 3b). Analyzing the NHGF viability for the DMSO-casted films, it can be assumed that the particles’ shape modulates the cellular behavior.
4. Conclusions
Understanding the structure–property relationship is the “key” to control morphology, which is the functions origin of any (bio) material. In this context, the present study showed that OTh-PDLLA offers several ways to modulate a film’s surface topography properties in order to adjust its interactions with biological entities such as proteins or cells. Employing techniques like dynamic laser-scattering (DLS), contact–angle measurement, and atomic force microscopy (AFM), changes in the particle’s size, wettability, and the film’s surface topography were investigated. Notably, by keeping constant the nature of the solvent but varying the solution’s concentration, the films roughness can be varied, while the solvent polarity has more influence on the film’s morphology than on their wettability. Using MTS assay, the oligomer’s biocompatibility was confirmed, this being the first study to advocate for OTh-PDLLA’s potential as an electroactive biointerface or as active layer in flexible and/or implantable transient electronics.
Author Contributions
The manuscript was written through contributions of all authors. Conceptualization, A.-D.B., L.C., I.C. and M.P.; methodology, A.-D.B. and L.C.; validation, A.-D.B., I.C. and L.C.; investigation A.-D.B., N.S., L.C. and E.-G.H.; data curation, N.S., E.-G.H., A.-D.B. and L.C.; writing—original draft preparation, A.-D.B. writing—review and editing, A.-D.B., L.C., I.C. and M.P.; visualization, L.C.; supervision, I.C. and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the articles.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Müllen, K.; Scherf, U. Conjugated Polymers: Where We Come from, Where We Stand, and Where We Might Go. Macromol. Chem. Phys. 2023, 224, 2200337. [Google Scholar] [CrossRef]
- Zeglio, E.; Rutz, A.L.; Winkler, T.E.; Malliaras, G.G.; Herland, A. Conjugated Polymers for Assessing and Controlling Biological Functions. Adv. Mater. 2019, 31, 1806712. [Google Scholar] [CrossRef]
- Inal, S.; Rivnay, J.; Suiu, A.-O.; Malliaras, G.G.; McCulloch, I. Conjugated polymers in bioelectronics. Acc. Chem. Res. 2018, 51, 1368. [Google Scholar] [CrossRef] [PubMed]
- Morsada, Z.; Hossain, M.M.; Islam, M.T.; Mobin, M.A.; Saha, S. Recent progress in biodegradable and bioresorbable materials: From passive implants to active electronics. App. Mat. Today 2021, 25, 101257. [Google Scholar] [CrossRef]
- Hicks, G.E.J.; Li, S.; Obhi, N.K.; Jarrett-Wilkins, C.N.; Seferos, D.S. Programmable Assembly of π-Conjugated Polymers. Adv. Mater. 2021, 33, 2006287. [Google Scholar] [CrossRef] [PubMed]
- Bendrea, A.-D.; Fabregat, G.; Cianga, L.; Estrany, F.; del Valle, L.J.; Cianga, I.; Aleman, C. Hybrid materials consisting of an all-conjugated polythiophene backbone and grafted hydrophilic poly(ethylene glycol) chains. Polym. Chem. 2013, 4, 2709–2723. [Google Scholar] [CrossRef]
- Molina, B.G.; Bendrea, A.-D.; Lanzalaco, S.; Franco, L.; Cianga, L.; del Valle, L.J.; Puiggali, J.; Turon, P.; Armelin, E.; Cianga, I.; et al. Smart design for a flexible, functionalized an electroresponsive hybrid platform based on poly(3,4 ethylenedioxythiophene) derivatives to improve cell viability. J. Mater. Chem. B 2020, 8, 8864–8877. [Google Scholar] [CrossRef]
- Bendrea, A.-D.; Fabregat, G.; Torras, J.; Maione, S.; Cianga, L.; del Valle, L.J.; Cianga, I.; Aleman, C. Polythiophene-g-poly(ethylene glycol) graft copolymers for electroactive scaffolds. J. Mater. Chem. B 2013, 1, 4135–4145. [Google Scholar] [CrossRef] [PubMed]
- Maione, S.; Fabregat, G.; del Valle, L.J.; Bendrea, A.-D.; Cianga, L.; Cianga, I.; Estrany, F.; Aleman, C. Effect of the Graft Ratio on the Properties of Polythiophene-g-poly(ethylene glycol). J. Polym. Sci. Part B Polym. Phys. 2015, 53, 239–252. [Google Scholar] [CrossRef]
- Cianga, L.; Bendrea, A.-D.; Fifere, N.; Nita, L.E.; Doroftei, F.; Ag, D.; Seleci, M.; Timur, S.; Cianga, I. Fluorescent micellar nanoparticles by self assembly of amphiphilic, nonionic and water self-dispersible polythiophenes with “hairy rod” architecture. RSC Adv. 2014, 4, 56385–56405. [Google Scholar] [CrossRef]
- Molina, B.G.; Bendrea, A.D.; Cianga, L.; Armelin, E.; del Valle, L.J.; Cianga, I.; Alemán, C. The biocompatible polythiophene-g-polycaprolactone copolymer as an efficient dopamine sensor platform. Polym. Chem. 2017, 8, 6112–6122. [Google Scholar] [CrossRef]
- Molina, B.G.; Cianga, L.; Bendrea, A.-D.; Cianga, I.; Alemán, C.; Armelin, E. An amphiphilic, heterografted polythiophene copolymer containing biocompatible/biodegradable side chains for use as an (electro)active surface in biomedical applications. Polym. Chem. 2019, 10, 5010–5022. [Google Scholar] [CrossRef]
- Demir, B.; Yilmaz, T.; Guler, E.; Pinar Gumus, Z.; Akbulut, H.; Aldemir, E.; Coskunol, H.; Colak, D.G.; Cianga, I.; Yamada, S.; et al. Polypeptide with electroactive end groups as sensing platform for the abused drug ‘methamphetamine’ by bioelectrochemical method. Talanta 2016, 161, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Bendrea, A.D.; Cianga, L.; Göen Colak, D.; Constantinescu, D.; Cianga, I. Thiophene End-Functionalized Oligo-(D,L)-Lactide as a New Electroactive Macromonomer for the “Hairy-Rod” Type Conjugated Polymers Synthesis. Polymers 2023, 15, 1094. [Google Scholar] [CrossRef]
- Davachi, S.M.; Kaffashi, B. Polylactic Acid in Medicine. Polym.-Plast. Technol. 2015, 54, 944–967. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices. Part 5: Tests for in Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009.
- Sato, S.; Gondo, D.; Wada, T.; Kanehashi, S.; Nagai, K. Effects of various liquid organic solvents on solvent-induced crystallization of amorphous poly(lactic acid) film. J. Appl. Polym. Sci. 2013, 129, 3. [Google Scholar] [CrossRef]
- Hansen, C.M.; Smith, A.L. Using Hansen solubility parameters to correlate solubility of C60 fullerene in organic solvents and in polymers. Carbon 2004, 42, 1591–1597. [Google Scholar] [CrossRef]
- Kim Tremel, K.; Ludwigs, S. Morphology of P3HT in Thin Films in Relation to Optical and Electrical Properties. Adv. Polym. Sci. 2014, 265, 39–82. [Google Scholar]
- Orwoll, R.A.; Arnold, P.A. Polymer–Solvent Interaction Parameter χ. In Physical Properties of Polymers Handbook; Mark, J.E., Ed.; Springer: New York, NY, USA, 2007. [Google Scholar] [CrossRef]
- Hoang, B.X.; Han, B.; Fang, W.H.; Tran, H.D.; Hoang, C.; Shaw, D.G.; Nguyen, T.Q. The Rationality of Implementation of Dimethyl Sulfoxide as Differentiation-inducing Agent in Cancer Therapy. Cancer Diagn. Progn. 2023, 3, 1–8. [Google Scholar] [CrossRef]
- Bendrea, A.-D.; Cianga, L.; Ailiesei, G.-L.; Ursu, E.-L.; Colak, D.G.; Cianga, I. 3,4-Ethylenedioxythiophene (EDOT) End-Group Functionalized Poly-ε-caprolactone (PCL): Self-Assembly in Organic Solvents and Its Coincidentally Observed Peculiar Behavior in Thin Film and Protonated Media. Polymers 2021, 13, 2720. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Da Silva, A.C.; Higgins, M.J.; Cordoba de Torresi, S.I. The effect of nanoscale surface electrical properties of partially biodegradable PEDOT-co-PDLLA conducting polymers on protein adhesion investigated by atomic force microscopy. Mater. Sci. Eng. C 2019, 99, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Ma, C.Q.; Bauerle, P. Functional oligothiophenes: Molecular design for multidimensional nanoarchitectures and their applications. Chem. Rev. 2009, 109, 1141–1276. [Google Scholar] [CrossRef] [PubMed]
- Khakalo, A.; Filpponen, I.; Rojas, O.J. Protein adsorption tailors the surface energies and compatibility between polylactide and cellulose nanofibrils. Biomacromolecules 2017, 18, 1426–1433. [Google Scholar] [CrossRef]
- Aktas, O.C.; Metzger, W.; Haidar, A.; Açil, Y.; Gülses, A.; Wiltfang, J.; Marques Sacramento, C.; Nothdurft, F.P. Enhancing adhesion and alignment of human gingival fibroblasts on dental implants. J. Cranio-Maxxillo-Facial Surg. 2019, 47, 661–667. [Google Scholar] [CrossRef]
- Tamada, Y.; Ikada, Y. Effect of Preadsorbed proteins on cell adhesion to polymer surfaces. J. Coll. Interf. Sci. 1993, 155, 334–339. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).