1. Introduction
Chile has emerged as a notable producer of olive oil, with an expanding olive cultivation industry driven by favorable climatic conditions, particularly in the central regions. In 2022, Chile produced approximately 19,500 metric tons of olive oil, positioning itself as a competitive player in the global market. The most cultivated varieties, including Arbequina and Arbosana, have gained prominence due to their adaptability to Chile’s agricultural environment and their high oil yield [
1].
In this context, the efficient use of by-products, such as olive pomace (alperujo), plays a critical role in promoting a circular economy. Olive pomace, a residue generated during olive oil extraction, represents a significant environmental challenge due to its volume and organic load. However, its rich composition of bioactive compounds offers an opportunity for valorization, transforming waste into valuable resources. By implementing sustainable extraction methods, it is possible to recover these compounds for potential applications in the food, pharmaceutical, and cosmetic industries, aligning with the goals of waste reduction and resource efficiency [
2].
This study aims to evaluate the efficacy of three extraction techniques—sonication, reflux, and maceration—applied to olive pomace from Arbequina and Arbosana varieties. The extracted compounds were analyzed for their antioxidant capacity using DPPH and ABTS assays, enzymatic inhibition of acetylcholinesterase and butyrylcholinesterase, and FTIR-ATR spectroscopy. The findings of this research are expected to contribute to the sustainable use of olive by-products, enhancing their value within a circular economy framework.
2. Materials and Methods
2.1. Plant Material
Olive pomace (alperujo) from two olive varieties, Arbequina and Arbosana, was provided by local companies from the Maule region, Chile. Samples were collected immediately after the oil extraction process to minimize oxidation of bioactive compounds. The pomace samples were air-dried at room temperature and stored in a dark, cool environment until further processing.
2.2. Extraction Procedures
Three extraction techniques were employed to recover bioactive compounds from the olive pomace:
2.2.1. Sonication
A total of 200 g of dried pomace was suspended in 800 mL of ethanol (100%) for sonication-assisted extraction. The mixture was sonicated using a probe sonicator (output power: 400 W, frequency: 20 kHz) for 30 min at room temperature. After sonication, the mixture was filtered, and the solvent was evaporated under reduced pressure using a rotary evaporator, followed by lyophilization to obtain the dry extract.
2.2.2. Reflux Extraction
For the reflux system extraction, 200 g of pomace was placed in a round-bottom flask with 800 mL of a 7:3 ethanol:water mixture. The reflux process was maintained at 70 °C for 1 h. After the extraction, the liquid phase was filtered, concentrated via rotary evaporation, and lyophilized to recover the extract.
2.2.3. Maceration
In the maceration process, 200 g of pomace was mixed with 800 mL of ethanol (100%). The mixture was stirred at room temperature for 48 h. Following maceration, the extract was filtered, concentrated using a rotary evaporator, and lyophilized for further use.
2.3. Biological Activity
The antioxidant potential of the extracts was assessed using two radical scavenging assays:
2.3.1. DPPH Assay
The free radical scavenging activity was measured by the DPPH (2,2-diphenyl-1-picrylhydrazyl) method [
3]. A solution of 0.1 mM DPPH in methanol was prepared, and 1 mL of each extract was mixed with 2 mL of DPPH solution. After 30 min in the dark, the absorbance was measured at 517 nm. The percentage of inhibition was calculated relative to a control. Ascorbic acid was used as a reference compound with EC
50 = 1.5 mg/mL
2.3.2. ABTS Assay
The ABTS radical cation decolorization assay was performed by mixing ABTS stock solution with potassium persulfate and allowing it to stand overnight to generate ABTS
•+. The working solution was diluted, and the absorbance was set to 0.700 ± 0.020 at 734 nm. Each extract (100 μL) was added to 2.9 mL of ABTS solution, and the absorbance was measured after 30 min [
4]. Ascorbic acid was used as a reference compound with EC
50 = 27.62 mg/mL.
2.4. Enzymatic Inhibition Assays
The enzymatic inhibition of acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) was determined using Ellman’s method [
5]. The extracts were tested at various concentrations, and the reaction was initiated by adding acetylthiocholine or butyrylthiocholine as substrates. The formation of the yellow 5-thio-2-nitrobenzoate anion, resulting from the reaction between thiocholine and Ellman’s reagent (DTNB), was monitored at 405 nm. Galantamine was used as a reference with IC
50 of 0.101 ± 0.01 µg/mL in AChE and 0.58 ± 0.05 µg/mL in BChE.
2.5. FTIR-ATR Analysis
The chemical composition of the extracts was characterized using Fourier-transform infrared (FTIR) spectroscopy with an attenuated total reflectance (ATR) accessory. Spectra were recorded over a range of 4000 to 400 cm−1 at a resolution of 2 cm−1 with 32 scans per sample. Key functional groups were identified, and the results were compared with the literature for validation.
3. Results and Discussion
3.1. Extraction Yields
The extraction yields obtained from the three methods (sonication, reflux, and maceration) show significant differences across the two olive pomace varieties, Arbequina and Arbosana. Reflux extraction yielded the highest amounts for both varieties, likely due to the use of heat, which facilitated the breakdown of cell walls and enhanced the release of bioactive compounds. Sonication provided moderate yields, while maceration, being the least aggressive method, resulted in the lowest yields. These results are consistent with previous findings that suggest heat-assisted extraction methods tend to be more efficient in recovering bioactive compounds from plant materials [
6,
7].
3.2. Antioxidant Activity
3.2.1. DPPH Radical Scavenging Activity
The antioxidant capacity of the extracts was evaluated using the DPPH assay, where the reflux method consistently demonstrated the highest radical scavenging activity for both olive varieties. Arbequina showed slightly better results than Arbosana, which may be attributed to differences in their polyphenolic composition. The EC
50 values of the reflux extracts were the lowest, indicating a higher efficiency in neutralizing free radicals compared to sonication and maceration extracts. These results align with previous literature findings, where heat treatments, such as reflux, have been shown to increase the solubility of phenolic compounds, thereby enhancing antioxidant activity [
8,
9].
Table 1 shows the results of the antioxidant activity.
3.2.2. ABTS Radical Cation Scavenging Activity
The ABTS assay confirmed the antioxidant trend observed in the DPPH assay. Reflux extracts displayed the highest ABTS radical cation scavenging capacity, followed by sonication and maceration. This further highlights the superiority of the reflux method in extracting compounds with high antioxidant potential. The results obtained from both DPPH and ABTS assays suggest that the higher temperature employed during reflux extraction enhances the release of phenolics and flavonoids, which are key contributors to antioxidant activity.
Table 1 shows the results of the percentage of discoloration of the radical solution at three concentrations of each extract analyzed.
3.3. Enzymatic Inhibition Assays
Cholinesterase Inhibition
The AChE inhibitory activity was highest in the extracts obtained from the reflux method as shown on
Table 2, particularly in Arbosana samples, with inhibition reaching 83.21% at 500 µg/mL and 49.19% at 125 µg/mL. Reflux extracts from Arbequina also demonstrated strong inhibition, with 80.75% at 500 µg/mL. Sonication extracts exhibited moderate inhibitory activity, with inhibition values ranging from 47.85% to 80.98% for Arbequina and 47.85% to 80.81% for Arbosana. The maceration extracts displayed more variable results, with Arbequina showing inhibition levels between 50.83% and 74.29%, while Arbosana exhibited a higher inhibition at 82.41% at 500 µg/mL but lower activity at 43.02% at 125 µg/mL.
These results suggest that the reflux method is highly efficient for extracting compounds with AChE inhibitory activity. The differences between Arbequina and Arbosana varieties were less pronounced in the sonication method but more variable in maceration. These findings are consistent with previous studies that highlight the role of phenolic compounds, including flavonoids and secoiridoids, in inhibiting cholinesterase enzymes. The high inhibitory activity of these extracts makes them promising candidates for the development of neuroprotective agents, especially in neurodegenerative disorders like Alzheimer’s disease [
1,
2].
The maceration method exhibited the highest BChE inhibition, with Arbequina reaching 56.74% and Arbosana at 52.95% at 500 µg/mL, making it the most effective extraction technique for BChE across all concentrations. The sonication method showed moderate inhibition, with Arbequina ranging from 44.61% to 21.23% and Arbosana from 44.01% to 25.10%. In contrast, the reflux method, which was highly effective for acetylcholinesterase inhibition, displayed lower BChE inhibition, with Arbequina at 40.63% and Arbosana at 43.37% at 500 µg/mL, dropping significantly at lower concentrations. These findings suggest that maceration is better suited for extracting compounds that inhibit BChE, highlighting how different extraction methods favor distinct bioactive compounds for enzyme inhibition. In general, the extracts showed activity and selectivity for AChE.
Table 2 shows the percentage values of enzyme inhibition at the three highest concentrations of each extract analyzed.
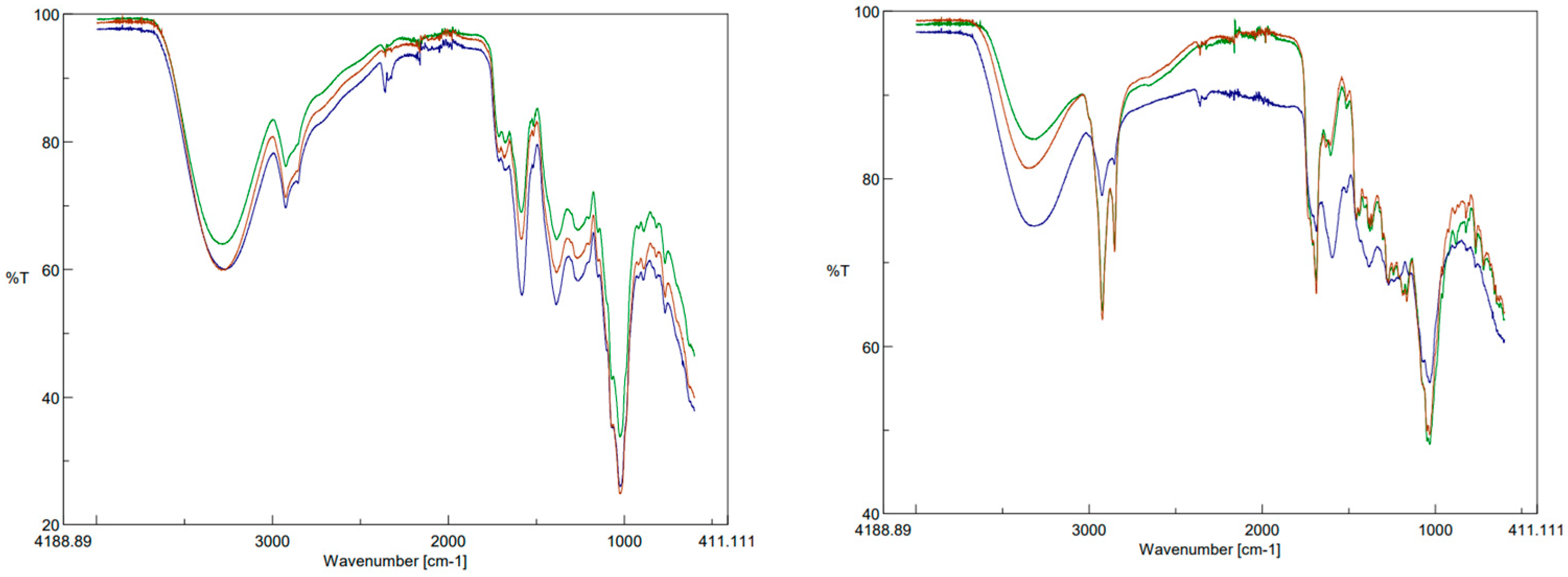
3.4. FTIR-ATR Spectral Analysis
The IR spectra of the olive residue with the three methods of extracts are given in
Figure 1. It can be seen that the position of the characteristic peaks did not change with the extraction methods used, but only the peak intensity changed. The FTIR-ATR spectra of the extracts revealed the presence of key functional groups commonly associated with phenolic compounds, such as hydroxyl (-OH) and carbonyl (C=O) groups. All extracts showed a broad O-H stretching band around 3300 cm
−1, indicative of alcohols and phenols. Strong peaks observed at 1735 cm
−1 correspond to C=O stretching in ester and carboxylic acid groups, which are characteristic of phenolic acids and flavonoids [
10].
Comparing these spectra with previously reported spectra in the literature, the presence of polyphenolic compounds such as oleuropein and hydroxytyrosol in the olive pomace extracts was confirmed. These compounds are known for their strong antioxidant and anti-inflammatory properties. The spectral regions between 1600–1500 cm
−1, attributed to aromatic C=C stretching, further support the identification of flavonoid structures, aligning with earlier studies on olive leaves and pomace [
10].
The presence of oleanolic acid and maslinic acid could be expected based on the characteristic absorption bands around 2925 cm
−1 and 2854 cm
−1, which correspond to the methylene and methyl groups of these triterpenoids. These results suggest the possibility that these compounds are present, aligning with previous studies where these triterpenoids have been identified as key components in olive by-products with significant bioactivity, although their presence has not been confirmed in this specific case [
11].
4. Conclusions
This study demonstrated the potential of olive pomace extracts from Arbequina and Arbosana as valuable sources of bioactive compounds with significant antioxidant and enzyme inhibitory properties. Among the extraction methods tested, the reflux system showed the highest efficiency for AChE inhibition, while maceration proved the most effective for BChE inhibition. The FTIR-ATR analysis confirmed the presence of key phenolic compounds, including oleuropein and hydroxytyrosol, along with triterpenoids such as oleanolic acid and maslinic acid, further supporting the bioactive potential of these extracts. These findings highlight the importance of optimizing extraction techniques to maximize the recovery of functional compounds from olive by-products, contributing to sustainable applications in the food, pharmaceutical, and cosmetic industries.
Author Contributions
Conceptualization, C.A. and M.G.; methodology, C.A. and M.G.; investigation, C.A. resources, M.G. writing—original draft preparation, C.A. writing—review and editing, C.A. and M.G.; supervision, M.G. project administration, M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Chilean National Agency of Research and Development (ANID) through the project ANILLO ACT210025.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Required data or information can be requested from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chileoliva. Estadísticas de Producción de Aceite de Oliva en Chile. Available online: www.chileoliva.cl (accessed on 5 September 2024).
- Hermund, D.B.; Plaza, M.; Turner, C.; Jónsdóttir, R.; Kristinsson, H.G.; Jacobsen, C.; Nielsen, K.F. Structure dependent antioxidant capacity of phlorotannins from Icelandic Fucus vesiculosus by UHPLC-DAD-ECD-QTOFMS. Food Chem. 2018, 240, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Maestre, A.B.; Hernández-Ruiz, J.; Arnao, M.B. ABTS/TAC Methodology: Main Milestones and Recent Applications. Processes 2023, 11, 185. [Google Scholar] [CrossRef]
- Pohanka, M.; Hrabinova, M.; Kuca, K.; Simonato, J.P. Assessment of Acetylcholinesterase Activity Using Indoxylacetate and Comparison with the Standard Ellman’s Method. Int. J. Mol. Sci. 2011, 12, 2631–2640. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Irfan, S.; Lorenzo, J.M.; Shafique, B.; Kanwal, R.; Pateiro, M.; Arshad, R.N.; Wang, L.; Nayik, G.A.; Roobab, U.; et al. Sonication, a Potential Technique for Extraction of Phytoconstituents: A Systematic Review. Processes 2021, 9, 1406. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Extraction of Bioactive Compounds from Plant Materials Using Combination of Various Novel Methods: A Review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Yamauchi, M.; Kitamura, Y.; Nagano, H.; Kawatsu, J.; Gotoh, H. DPPH Measurements and Structure—Activity Relationship Studies on the Antioxidant Capacity of Phenols. Antioxidants 2024, 13, 309. [Google Scholar] [CrossRef] [PubMed]
- Abualzulof, G.W.A.; Scandar, S.; Varfaj, I.; Costa, V.D.; Sardella, R.; Filippini, R.; Piovan, A.; Marcotullio, M.C. The Effect of Maturity Stage on Polyphenolic Composition, Antioxidant and Anti-Tyrosinase Activities of Ficus rubiginosa Extracts. Antioxidants 2024, 13, 1129. [Google Scholar] [CrossRef]
- Hssaini, L.; Razouk, R.; Bouslihim, Y. Rapid Prediction of Fig Phenolic Acids and Flavonoids Using Mid-Infrared Spectroscopy Combined with Partial Least Square Regression. Front. Plant Sci. 2022, 13, 782159. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.A.; Pimentel, F.B.; Costa, A.S.; Alves, R.C.; Oliveira, M.B.P. Olive By-Products for Functional and Food Applications: Challenging Opportunities to Face Environmental Constraints. Innov. Food Sci. Emerg. Technol. 2020, 35, 139–148. [Google Scholar] [CrossRef]
- Medina, E.; Romero, C.; Brenes, M. Residual Olive Paste as a Source of Phenolic Compounds and Triterpenic Acids. Eur. J. Lipid Sci. Technol. 2018, 120, 1700368. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).