Functionalization of Fullerene C60 with Organic Carbonates in the Presence of a Grignard Reagent and Ti(Oi-Pr)4 †
Abstract
1. Introduction
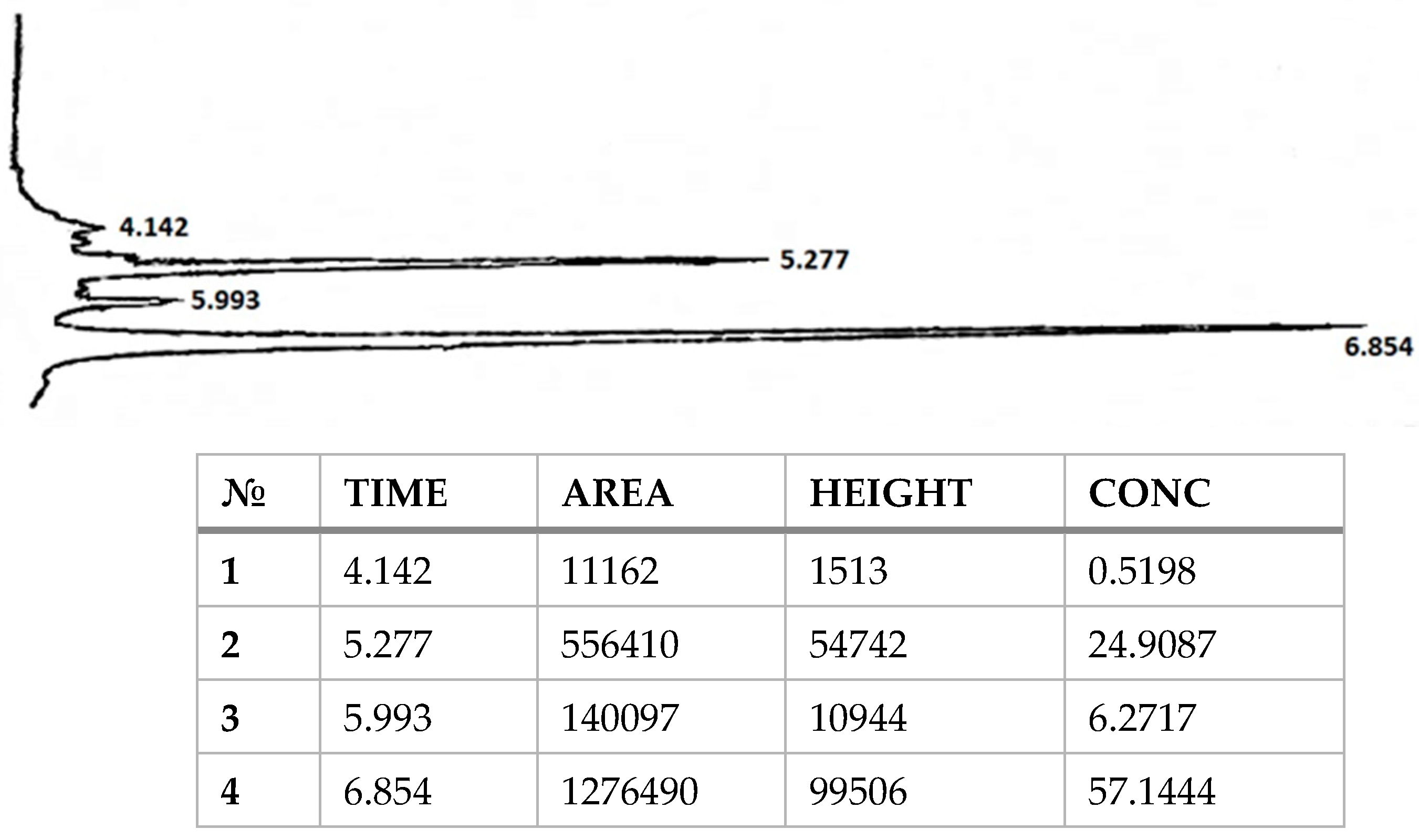
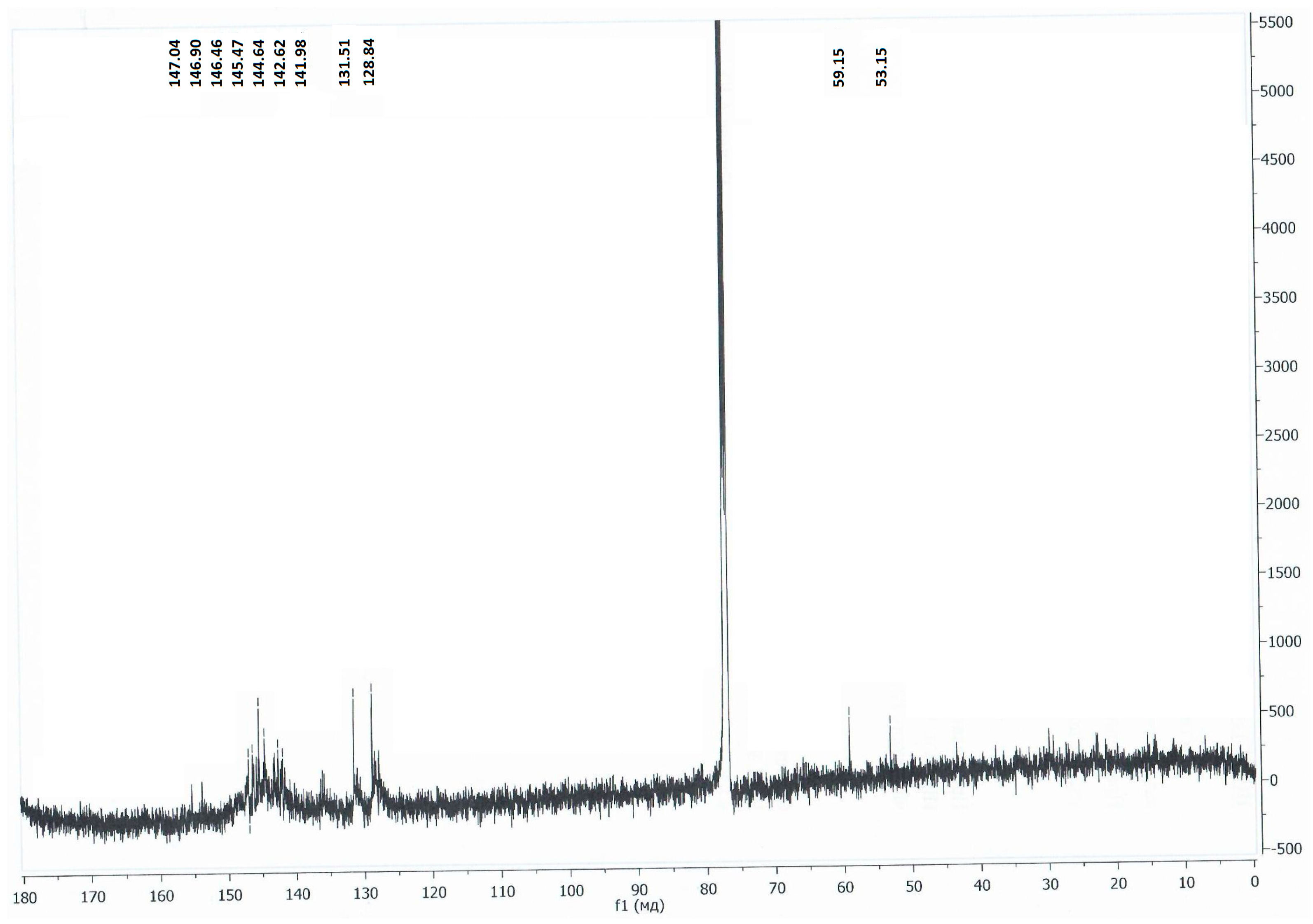
2. Results and Discussion
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parrish, J.P.; Salvatore, R.; Jung, K.W. Perspectives on Alkyl Carbonates in Organic Synthesis. Tetrahedron 2000, 56, 8207–8237. [Google Scholar] [CrossRef]
- Shaikh, A.-A.G.; Sivaram, S. Organic Carbonates. Chem. Rev. 1996, 96, 951–976. [Google Scholar]
- Mohamed, N.; Paull, K.D.; Narayanan, V.L. Computer-Assisted Structure–Anticancer Activity Correlations of Carbamates and Thiocarbamates. J. Pharm. Sci. 1985, 74, 831–836. [Google Scholar]
- Kole, H.K.; Akamatsu, M.; Ye, B.; Yan, X.J.; Barford, D.; Roller, P.P.; Burke, T.R. Protein-Tyrosine Phosphatase Inhibition by a Peptide Containing the Phosphotyrosyl Mimetic, L-O-Malonyltyrosine (L-OMT). Biochem. Biophys. Res. Commun. 1995, 209, 817–822. [Google Scholar] [CrossRef]
- Kulinkovich, O.G.; Meijere, A. n-Dicarbanionic Titanium Intermediates from Monocarbanionic Organometallics and Their Application in Organic Synthesis. Chem. Rev. 2000, 100, 2789–2834. [Google Scholar] [CrossRef]
- Holden, D.A. Synthesis and spreading behavior of some reactive derivatives of long-chain alcohols and carboxylic acids. Can. J. Chem. 1983, 62, 574–579. [Google Scholar] [CrossRef]
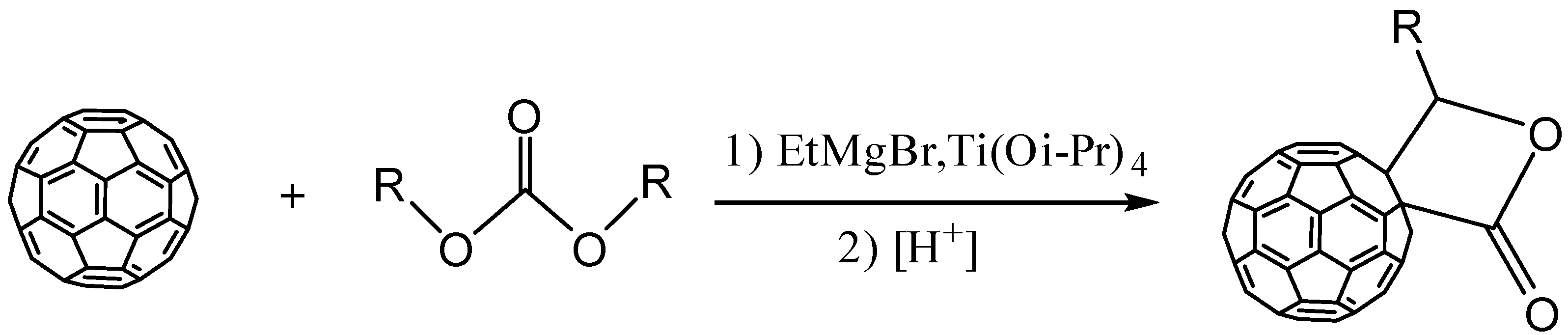

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khuzina, L.; Khuzin, A. Functionalization of Fullerene C60 with Organic Carbonates in the Presence of a Grignard Reagent and Ti(Oi-Pr)4 . Chem. Proc. 2024, 16, 66. https://doi.org/10.3390/ecsoc-28-20108
Khuzina L, Khuzin A. Functionalization of Fullerene C60 with Organic Carbonates in the Presence of a Grignard Reagent and Ti(Oi-Pr)4 . Chemistry Proceedings. 2024; 16(1):66. https://doi.org/10.3390/ecsoc-28-20108
Chicago/Turabian StyleKhuzina, Liliya, and Artur Khuzin. 2024. "Functionalization of Fullerene C60 with Organic Carbonates in the Presence of a Grignard Reagent and Ti(Oi-Pr)4 " Chemistry Proceedings 16, no. 1: 66. https://doi.org/10.3390/ecsoc-28-20108
APA StyleKhuzina, L., & Khuzin, A. (2024). Functionalization of Fullerene C60 with Organic Carbonates in the Presence of a Grignard Reagent and Ti(Oi-Pr)4 . Chemistry Proceedings, 16(1), 66. https://doi.org/10.3390/ecsoc-28-20108





