1. Introduction
The substantial increase in energy and resource consumption caused by the rapid growth of the worldwide population, fossil fuel reserve depletion, greenhouse gas emissions and global warming are some of the biggest challenges that humanity is facing in the present day. The most promising solution is brought by renewable energies, but it frequently comes at a cost: intermittency. Therefore, energy storage has become an indispensable piece of the puzzle for an effective sustainable transition.
Among the finest energy storage devices are lithium metal batteries (LMB) and lithium-ion batteries (LIB). However, they still face some difficulties, and the ones associated with electrolyte performance are the focus of this work.
Liquid electrolytes (LE) present performance disadvantages due to parasitic reactions between the lithium anode and LE, provoking a decrease in Coulombic efficiency and shortening LMBs’ lifespan. Moreover, leakage risk, insufficient thermal stability and dendrite formation, which can lead to battery short-circuits, pose an important safety hazard [
1]. Among the most promising LEs are ionic liquids (ILs), which are defined as molten salts whose melting point is below 100 °C. These compounds present outstanding properties for the electrochemical industry, such as high ionic conductivity, good thermal stability, low flammability, wide electrochemical windows, etc. However, as liquids, they still face leakage risks [
1].
Aiming to ensure battery safety, solid electrolytes (SE) have been developed. These electrolytes possess the advantage of no leakage risk and prevent dendrite formation. Nevertheless, they lose against LEs in terms of ionic conductivity and ease of manufacture [
1].
These two types of electrolytes find common ground in ionogel electrolytes (IG). Ionogels immobilize IL in a polymer matrix, and therefore are situated midway between liquid and solid electrolytes, combining the advantages of the two worlds. IGs provide a wider temperature range of function compared to LEs, are inert with respect to metallic lithium and can prevent dendrite growth while allowing good contact between electrode and electrolyte and presenting higher ionic conductivity than SE [
2].
The aim of this study is to extend knowledge about ionogels and their opportunities as promising electrolytes for the electrochemical industry. Hence, the conductive behavior of an ionogel based on 1-Ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (EMimTFSI) synthesized by the sol–gel method in comparison with priscine IL is analyzed in this work.
2. Materials and Methods
2.1. Chemicals and Gelation Process
The IL 1-Ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (cas number 174899-82-2), commonly referred to as EMimTFSI, was selected for this study. Even though this IL has been reported to be a hydrophobic compound [
1], it was dried in a vacuum chamber for at least 48h at room temperature to ensure minimal water content and other impurities.
The ionogel based on this IL has been synthesized via the sol–gel method, following a similar approach to Chen et al. [
3]. Tetramethylorthosilicate (TMOS) and Dimethyl Dimethoxysilane (DMDMS) were selected as silica precursors, and formic acid (FA) as the catalyst.
The synthesis procedure was the following: firstly, TMOS was mixed with DMDMS in a molar proportion of 0.7:0.3 and stirred for 10 min. Afterwards, EMimTFSI was added in the relation of 0.5[0.7:0.3] (EMimTFSI[TMOS:DMDMS]) and the mixture was stirred again for another 10 min before the addition of FA, and finally stirred for 2 min more. Lastly, the ionogel was aged for 6 days at room temperature; for the first 2 days it was covered with a lid, followed by another 4 days uncovered.
2.2. Differential Scanning Calorimetry (DSC)
A Differential Scanning Calorimeter (DSC) Q2000 (Waters-TA Instruments, New Castle, DE, USA) was used with hermetically sealed aluminum pans, and sample masses ranged from 5 to 8 mg; experiments were performed under a nitrogen atmosphere. One heating–cooling cycle between −80 °C and 50 °C was performed at a rate of 5 °C/min, with an initial ramp from room temperature to 125 °C at 40 °C/min, and with a 45 min isothermal step at 125 °C to eliminate potential volatile impurities. Transition temperatures of the pure IL were determined at the onset of peaks observed in the DSC curves during the reheating and recooling phases. The transition temperatures were estimated with a 2 °C uncertainty at a 95% confidence level [
4].
2.3. Thermogravimetry Analysis (TGA)
To study the short-term thermal stability of pure IL, a Mettler Toledo DSC/TGA1 instrument in dynamic mode was used, with a heating rate of 10 °C/min and a purge gas flow of 20 cm
3/min, covering a temperature range from 50 °C to 800 °C under a nitrogen atmosphere. Samples weighing over 40 ± 1 mg were placed in an open platinum pan for analysis [
5,
6].
Since it has been demonstrated multiple times that the onset temperature (t
onset) tends to overestimate the working temperature of the compound [
5,
6], several models have been proposed to obtain a more accurate operating temperature. Wooster et al. [
7] established a method to estimate the maximum operating temperature from dynamic scans using Equation (1):
where
corresponds to the temperature (in Kelvin) at which the first derivative of the weight loss curve with respect to T is not equal to zero.
2.4. Broad-Band Dielectric Spectroscopy (BBDS)
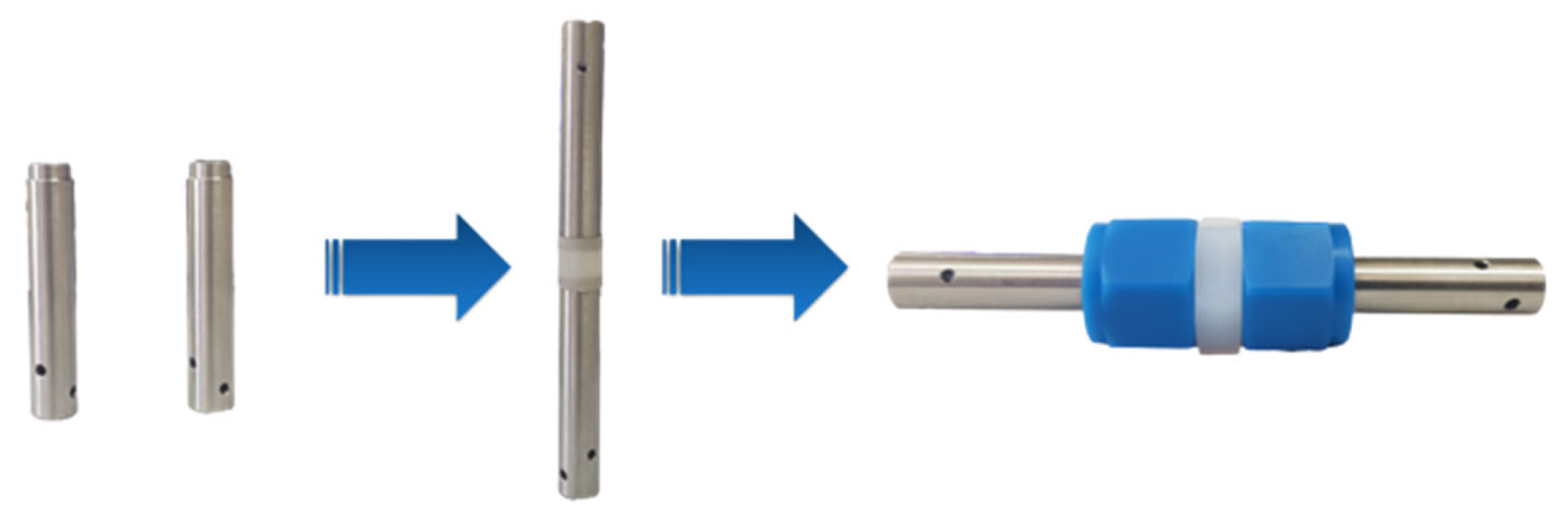
In order to obtain the ionic conductivity of both bulk IL and the ionogel, broad-band dielectric spectroscopy was carried out. For this, an Agilent RLC precision meter HP 4284A (Agilent Technologies, Santa Clara, CA, USA) with a precision of 0.05% was used, coupled with a climate chamber (Memmert ICP400, Schwabahc, Germany) for temperature control. The sample was fixed inside a two-electrode Swagelok cell, ensuring good contact between electrodes and the electrolyte (
Figure 1) [
8].
The selected temperature range was 0 °C to 50 °C and the frequency sweep was conducted between 20 Hz and 1 MHz with a logarithmic step to ensure that the measurement included the ohmic region. With the aim of obtaining the ionic conductivity (
), the imaginary part of the dielectric constant (
) was presented against the frequency (
) and fitted to Equation (2) with a tolerance for the slope of −1 ± 0.02 [
2,
8].
3. Results
3.1. Liquid Range of the Pure IL
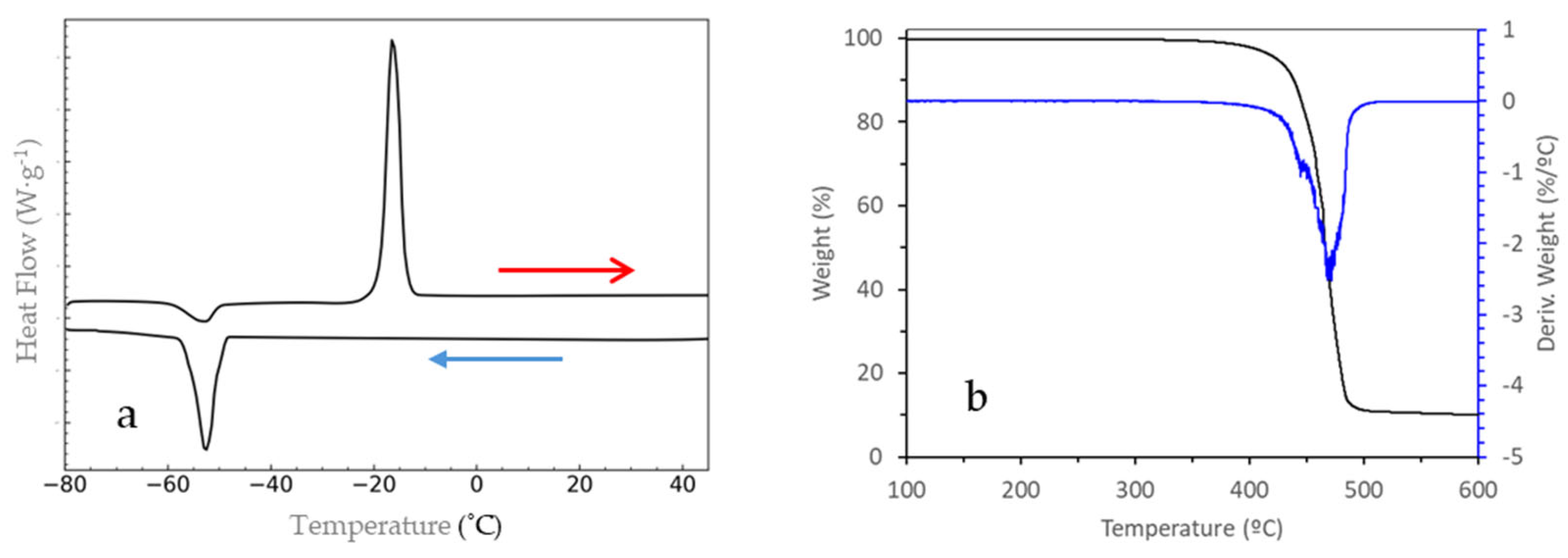
Figure 2a shows the DSC curve of pure IL obtained during the heating and cooling process at 5 °C/min. Upon cooling, an exothermic peak was observed corresponding to the crystallization of the IL with an onset temperature of −50 °C. Additionally, a cold crystallization exothermic process at −53 °C, followed by a characteristic endothermic peak at −19 °C associated with melting, can be observed clearly upon heating.
Figure 2b shows the thermogravimetric curves corresponding to the dynamic study of EMim TFSI, showing the mass loss curve and its corresponding derivative. It can be observed that this compound undergoes the mass loss process in a single step (one peak in the DTG), as previously observed by other authors [
9,
10]. Furthermore, we can determine the characteristic thermogravimetric parameters of the IL,
tonset (which represents the beginning of the mass loss process),
tendset (which represents the ending of the mass loss process),
Wonset (mass loss percentage at onset temperature),
t1st (maximum at DTG curve) and
twooster (1% degradation in 10 h), presented in
Table 1, which are similar to those reported by other authors for the same IL [
9,
10].
Additionally, a discrepancy of 185 °C can be observed between the tonset and the tWooster, highlighting the need for isothermal studies. These studies involve maintaining a constant temperature for a specified period to analyze the percentage of mass loss at a given temperature, which, as demonstrated by other authors, will be significantly lower than the tonset.
Then, it can be concluded that this IL has a large liquid range between its melting temperature and its thermal stability temperature, between −19 °C and 280 °C, enough to be used as electrolyte in high-temperature solid-state batteries.
3.2. Ionic Conductivity
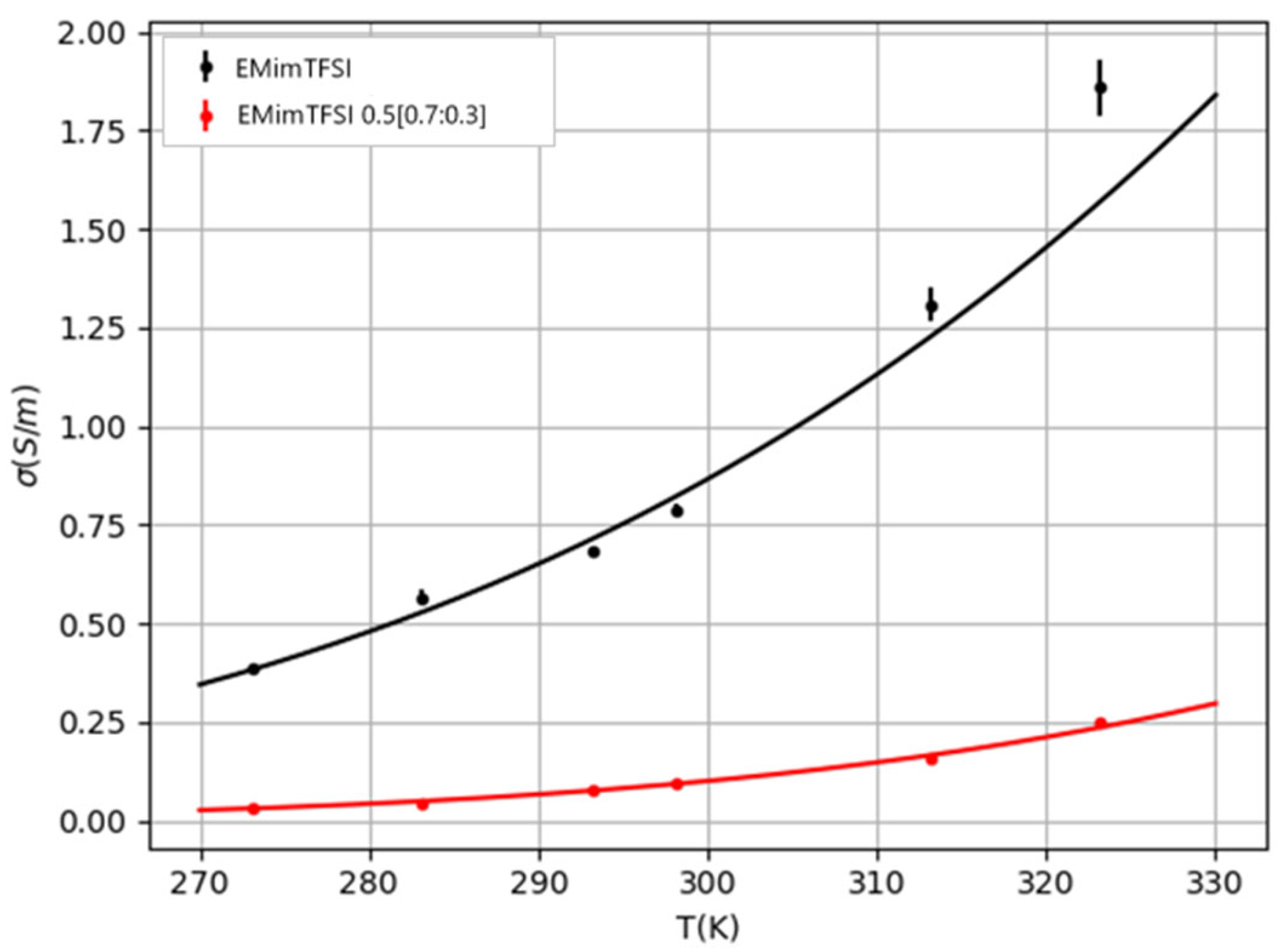
The comparison between the ionic conductivity of bulk EMimTFSI and the ionogel EMimTFSI 0.5[0.7:0.3] is shown in
Figure 3 and
Table 2. Results of bulk IL are in good agreement with those previously published in the literature [
3], presenting conductivities of several mS/cm. In the case of the ionogel, it drops approximately one order of magnitude. However, as it is still close to 1 mS/cm at ambient temperature, it is considered adequate for electrochemistry applications.
Both the liquid and gel samples exhibit a similar trend in ionic conductivity with increasing temperature. As expected, conductivity rises with temperature, which is related to the decrease in viscosity, but the increase is more pronounced in the liquid sample [
11,
12].
This behavior of ionic conductivity with temperature is explained by the Arrhenius Equation (3), with
being the limit of the ionic conductivity when the temperature (T) tends to infinity,
the ideal gas constant and
the activation energy. The fitting of the obtained results to the Arrhenius equation is shown in
Figure 3, and its parameters are presented in
Table 3. The increment of the activation energy with the gelation of the compound is consistent with the decrease in conductivity.
4. Conclusions
This work aimed to analyze the changes in the conductive behavior of an ionogel synthesized by the sol–gel method in comparison with the bulk ionic liquid. Additionally, the transition temperatures and thermal stability of pure IL EMimTFSI were also analyzed to ensure the liquid state in all the temperature ranges studied. The main conclusions are summarized as follows:
The selected compound exhibits excellent thermal properties, with a melting point at −19 °C and thermal stability higher than 250 °C.
As expected, the ionic conductivity of the liquid sample increases with temperature, displaying values well-suited for the intended application.
Gelation reduces the ionic conductivity, but it remains adequate for energy storage applications.
Author Contributions
Conceptualization, J.J.P. and J.S.; methodology, R.S.E., A.S.-A. and P.V.; validation, M.V., J.J.P. and J.S.; formal analysis, R.S.E., A.S.-A., P.V., M.V., J.J.P. and J.S.; data curation, R.S.E., A.S.-A. and P.V.; writing—original draft preparation, R.S.E. and A.S.-A. writing—review and editing, M.V., J.J.P. and J.S.; supervision, J.J.P. and J.S.; project administration, J.J.P. and J.S.; funding acquisition, M.V., J.J.P. and J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Xunta de Galicia ED431C 2024/06. A. Santiago-Alonso, P. Vallet and J. J. Parajó thank funding support of the Doutoramento industrial program from GAIN-Xunta de Galicia (14_IN606D_2022_2702732), the FPI Program from the Spanish Ministry of Science, Education and Universities and the I2C postdoctoral Program of Xunta de Galicia (ED481D 2023/014), respectively.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data utilized in this paper are included in tables and figures or can be accessed through the respective citations provided.
Conflicts of Interest
Author Antía Santiago-Alonso was employed by the company ABCR LABORATORIOS, Lg. Vilapouca (PG Industrial). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Han, C.; Shui, X.; Chen, G.; Xu, G.; Ma, J.; Dong, S.; Wang, S.; Zhou, X.; Cui, Z.; Qiao, L.; et al. Recent Progress in Gel Polymer Electrolyte for Lithium Metal Batteries. Giant 2024, 20, 100337. [Google Scholar] [CrossRef]
- Tripathi, A.K. Ionic Liquid–Based Solid Electrolytes (Ionogels) for Application in Rechargeable Lithium Battery. Mater. Today Energy 2021, 20, 100643. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, H.; Li, L.; Chen, R.; Guo, S. Ionogel Electrolytes for High-Performance Lithium Batteries: A Review. Adv. Energy Mater. 2018, 8, 1702675. [Google Scholar] [CrossRef]
- Vallet, P.; Parajó, J.J.; Santiago-Alonso, A.; Villanueva, M.; Varela, L.M.; Salgado, J. Thermal Characterization of [C2Im][NO3] and Multivalent Nitrate Salts Mixtures. Crystals 2024, 14, 502. [Google Scholar] [CrossRef]
- Parajó, J.J.; Teijeira, T.; Fernández, J.; Salgado, J.; Villanueva, M. Thermal Stability of Some Imidazolium [NTf2] Ionic Liquids: Isothermal and Dynamic Kinetic Study through Thermogravimetric Procedures. J. Chem. Thermodyn. 2017, 112, 105–113. [Google Scholar] [CrossRef]
- Salgado, J.; Villanueva, M.; Parajó, J.J.; Fernández, J. Long-Term Thermal Stability of Five Imidazolium Ionic Liquids. J. Chem. Thermodyn. 2013, 65, 184–190. [Google Scholar] [CrossRef]
- Wooster, T.J.; Johanson, K.M.; Fraser, K.J.; MacFarlane, D.R.; Scott, J.L. Thermal Degradation of Cyano Containing Ionic Liquids. Green Chem. 2006, 8, 691–696. [Google Scholar] [CrossRef]
- Santiago-Alonso, A.; Sánchez-Pico, J.M.; Emeterio, R.S.; Villanueva, M.; Salgado, J.; Parajó, J.J. Pyrrolidinium-Based Ionic Liquids as Advanced Non-Aqueous Electrolytes for Safer Next Generation Lithium Batteries. Batteries 2024, 10, 319. [Google Scholar] [CrossRef]
- Zaitsau, D.H.; Abdelaziz, A. The Study of Decomposition of 1-Ethyl-3-Methyl-Imidazolium Bis(Trifluoromethylsulfonyl)Imide by Using Termogravimetry: Dissecting Vaporization and Decomposition of ILs. J. Mol. Liq. 2020, 313, 113507. [Google Scholar] [CrossRef]
- Liu, S.H.; Chen, C.C.; Zhang, B.; Wu, J.H. Fire and Explosion Hazards of 1-Ethyl-3-Methylimidazolium Bis(Trifluoromethylsulfonyl)Imide. RSC Adv. 2020, 10, 22468–22479. [Google Scholar] [CrossRef] [PubMed]
- Parajó, J.J.; Vallet, P.; Villanueva, M.; Cabeza, O.; Fernández-Carretero, F.; García Luis, A.; Di Pietro, M.E.; Mele, A.; Castiglione, F.; Salgado, J.; et al. Ionogels Based on Protic Ionic Liquid-Lithium Salt Mixtures. J. Mol. Liq. 2024, 397, 124093. [Google Scholar] [CrossRef]
- Vallet, P.; Parajó, J.J.; Santiago-Alonso, A.; Villanueva, M.; Cabeza, Ó.; Varela, L.M.; Salgado, J. Anomalous Behaviour of the Ionic Conductivity of Nanoconfined IL-Lithium Salt Mixtures. J. Mol. Liq. 2024, 401, 124630. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).