Straightforward Synthesis of BHQ-3 Amine: An Azo Dark-Quencher for FRET-Based Protease Activity Assays †
Abstract
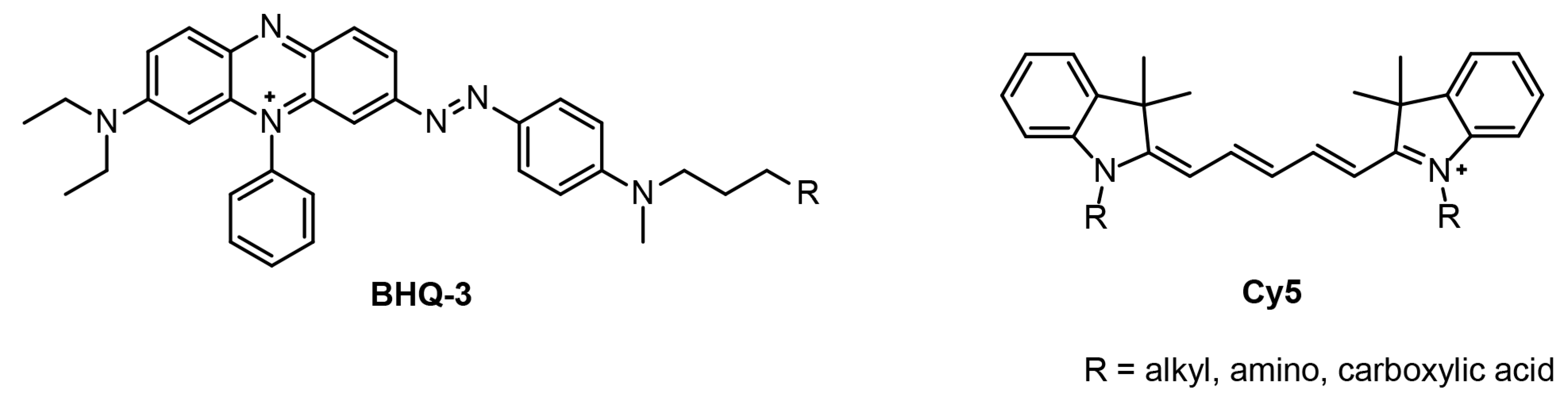
1. Introduction
2. Experimental Section
2.1. General Synthesis
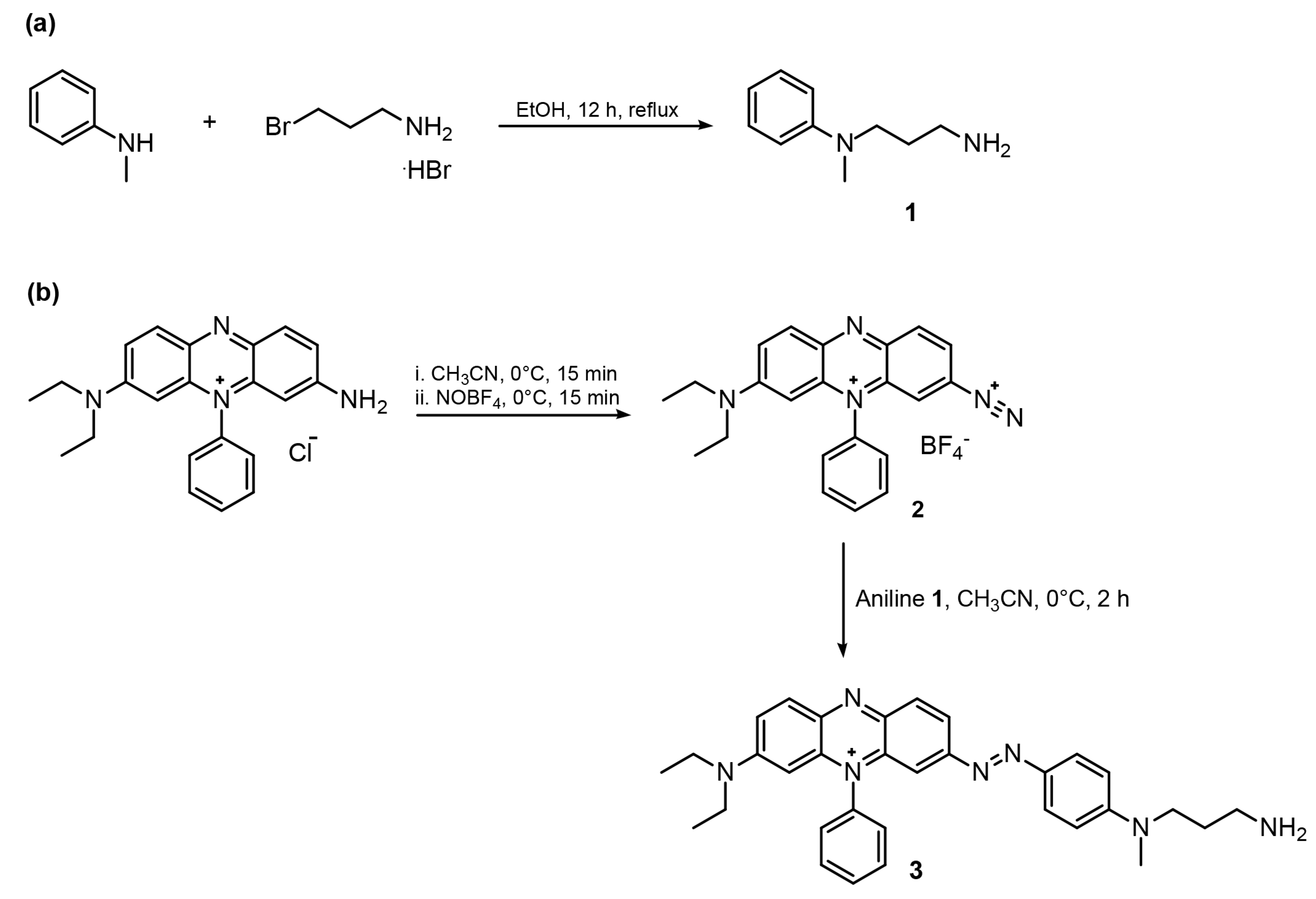
2.2. Synthesis
2.2.1. Synthesis of N′-Methyl-N′-phenylpropane-1,3-diamine (1)
2.2.2. Synthesis of Black Hole Quencher Amine (BHQ-3, 3)
2.3. General UV/Vis and Fluorescence Studies
3. Results and Discussion
3.1. Synthesis of the Functionalized BHQ-3 Amine Dye
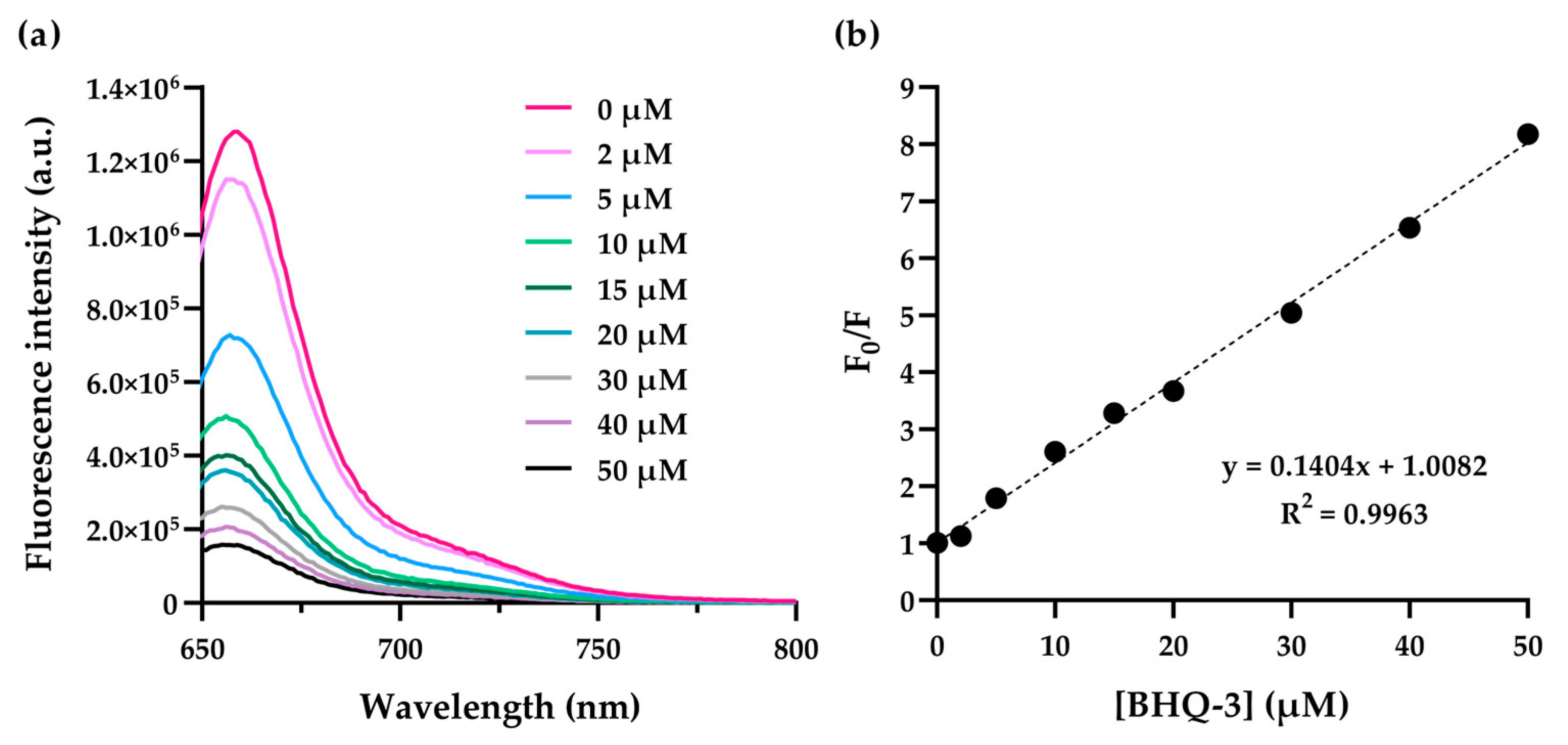
3.2. UV–Vis and Fluorescence Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodriguez-Rios, M.; Megia-Fernandez, A.; Norman, D.J.; Bradley, M. Peptide Probes for Proteases—Innovations and Applications for Monitoring Proteolytic Activity. Chem. Soc. Rev. 2022, 51, 2081–2120. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Shen, Y.; Peng, B.; Bai, H.; Wang, L.; Zhang, J.; Hu, W.; Fu, L.; Zhang, W.; Li, L.; et al. Small-Molecule Quenchers for Förster Resonance Energy Transfer: Structure, Mechanism, and Applications. Angew. Chem. Int. Ed. Engl. 2022, 61, e202207188. [Google Scholar] [CrossRef]
- Chevalier, A.; Renard, P.-Y.; Romieu, A. Azo-Based Fluorogenic Probes for Biosensing and Bioimaging: Recent Advances and Upcoming Challenges. Chem. Asian J. 2017, 12, 2008–2028. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, A.; Massif, C.; Renard, P.-Y.; Romieu, A. Bioconjugatable Azo-Based Dark-Quencher Dyes: Synthesis and Application to Protease-Activatable Far-Red Fluorescent Probes. Chem. Eur. J. 2013, 19, 1686–1699. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, A.; Renard, P.-Y.; Romieu, A. Straightforward Synthesis of Bioconjugatable Azo Dyes. Part 1: Black Hole Quencher-1 (BHQ-1) Scaffold. Tetrahedron Lett. 2014, 55, 6759–6763. [Google Scholar] [CrossRef]
- Simard, B.; Tomanek, B.; van Veggel, F.C.J.M.; Abulrob, A. Optimal Dye-Quencher Pairs for the Design of an “Activatable” Nanoprobe for Optical Imaging. Photochem. Photobiol. Sci. 2013, 12, 1824–1829. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; He, B.; Tao, J.; He, Y.; Deng, H.; Wang, X.; Zheng, Y. Application of Förster Resonance Energy Transfer (FRET) Technique to Elucidate Intracellular and In Vivo Biofate of Nanomedicines. Adv. Drug Deliv. Rev. 2019, 143, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.I.; Deng, Q.; Vendrell, M. Near-Infrared Fluorescent Probes for the Detection of Cancer-Associated Proteases. ACS Chem. Biol. 2021, 16, 1304–1317. [Google Scholar] [CrossRef]
- Yi, X.; Wang, F.; Qin, W.; Yang, X.; Yuan, J. Near-Infrared Fluorescent Probes in Cancer Imaging and Therapy: An Emerging Field. Int. J. Nanomed. 2014, 9, 1347–1365. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhang, E.; Su, Y.; Cheng, T.; Shi, C. A Review of NIR Dyes in Cancer Targeting and Imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.D.F.; Raposo, M.M.M.; Costa, S.P.G. A New Fluorogenic Substrate for Granzyme B Based on Fluorescence Resonance Energy Transfer. Chem. Proc. 2021, 3, 6. [Google Scholar]
- Costa da Silva, M.; Vieira Rocha, C.; Bañobre-López, M.; Gallo, J. Stimulation and Suppression of the Innate Immune System through Nanotechnology. ACS Appl. Nano Mater. 2021, 4, 2303–2316. [Google Scholar] [CrossRef]
- Martins, C.D.F.; Raposo, M.M.M.; Costa, S.P.G. Synthesis and Characterization of a Water-Soluble Pentamethine Indocyanine Dye for Peptide Labeling. Chem. Proc. 2022, 8, 91. [Google Scholar] [CrossRef]
- Martins, C.D.F.; Raposo, M.M.M.; Costa, S.P.G. Dabcyl as a Naked Eye Colorimetric Chemosensor for Palladium Detection in Aqueous Medium. Molecules 2023, 28, 6111. [Google Scholar] [CrossRef] [PubMed]
- Kubba, R.; Kumar Singh, M.; Jyoti; Yadav, O.; Kumar, A. Fӧrster Resonance Energy Transfer (FRET) between CdSe Quantum Dots and ABA Phosphorus(V) Corroles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 291, 122345. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J. Introduction to Fluorescence. In Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 1, pp. 1–26. [Google Scholar]
- Kotresh, M.G.; Adarsh, K.S.; Shivkumar, M.A.; Mulimani, B.G.; Savadatti, M.I.; Inamdar, S.R. Spectroscopic Investigation of Alloyed Quantum Dot-Based FRET to Cresyl Violet Dye. Luminescence 2016, 31, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Texier, I.; Goutayer, M.; Da Silva, A.; Guyon, L.; Djaker, N.; Josserand, V.; Neumann, E.; Bibette, J.; Vinet, F. Cyanine-Loaded Lipid Nanoparticles for Improved In Vivo Fluorescence Imaging. J. Biomed. Opt. 2009, 14, 054005. [Google Scholar] [CrossRef]
- Sandberg, E.; Piguet, J.; Liu, H.; Widengren, J. Combined Fluorescence Fluctuation and Spectrofluorometric Measurements Reveal a Red-Shifted, Near-IR Emissive Photo-Isomerized Form of Cyanine 5. Int. J. Mol. Sci. 2023, 24, 1990. [Google Scholar] [CrossRef] [PubMed]


| Compound | UV-Vis | Fluorescence | ||
|---|---|---|---|---|
| λmax (nm) | log ε | λemi (nm) | Stokes’ Shift (nm) | |
| Cy5-NHS | 640 | 4.82 | 659 | 19 |
| BHQ-3 amine (3) | 625 | 4.33 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, C.D.F.; Raposo, M.M.M.; Costa, S.P.G. Straightforward Synthesis of BHQ-3 Amine: An Azo Dark-Quencher for FRET-Based Protease Activity Assays. Chem. Proc. 2024, 16, 48. https://doi.org/10.3390/ecsoc-28-20170
Martins CDF, Raposo MMM, Costa SPG. Straightforward Synthesis of BHQ-3 Amine: An Azo Dark-Quencher for FRET-Based Protease Activity Assays. Chemistry Proceedings. 2024; 16(1):48. https://doi.org/10.3390/ecsoc-28-20170
Chicago/Turabian StyleMartins, Cátia D. F., Maria Manuela M. Raposo, and Susana P. G. Costa. 2024. "Straightforward Synthesis of BHQ-3 Amine: An Azo Dark-Quencher for FRET-Based Protease Activity Assays" Chemistry Proceedings 16, no. 1: 48. https://doi.org/10.3390/ecsoc-28-20170
APA StyleMartins, C. D. F., Raposo, M. M. M., & Costa, S. P. G. (2024). Straightforward Synthesis of BHQ-3 Amine: An Azo Dark-Quencher for FRET-Based Protease Activity Assays. Chemistry Proceedings, 16(1), 48. https://doi.org/10.3390/ecsoc-28-20170







