Molecular Docking and Dynamics of a Series of Aza-Heterocyclic Compounds Against Penicillin-Binding Protein 2a of Methicillin-Resistant Staphylococcus aureus †
Abstract
1. Introduction
2. Methodology
2.1. Pharmacokinetic Analysis
2.2. Molecular Docking
2.3. Molecular Dynamics Details
3. Results and Discussion
3.1. Pharmacokinetic Results
3.2. Molecular Docking
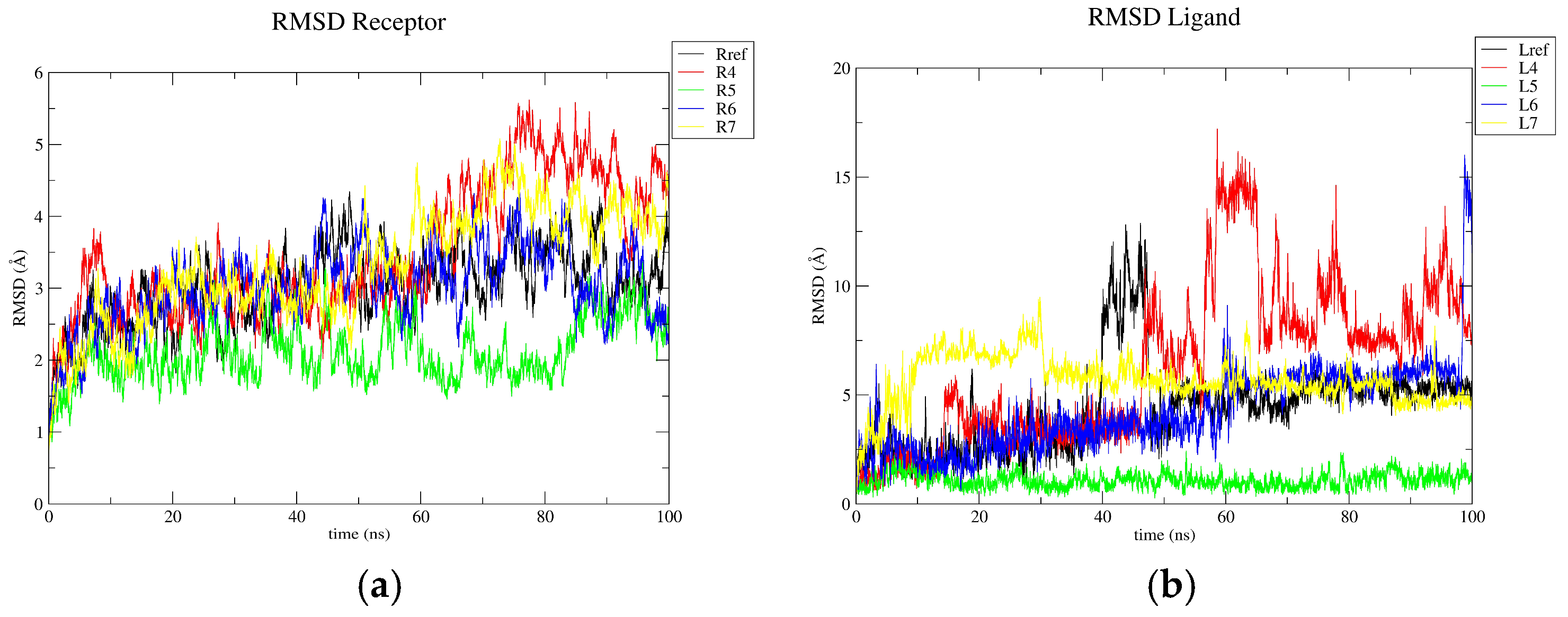
3.3. Molecular Dynamics Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cervantes-García, E.; García-González, R.; Salazar-Schettino, P.M. Características generales del Staphylococcus aureus. Rev. Latinoam. Patol. Clin. Med. Lab. 2014, 61, 28–40. [Google Scholar]
- Aguayo-Reyes, A.; Quezada-Aguiluz, M.; Mella, S.; Riedel, G.; Opazo-Capurro, A.; Bello-Toledo, H.; Domínguez, M.; González-Rocha, G. Bases moleculares de la resistencia a meticilina en Staphylococcus aureus. Rev. Chil. Infectol. 2018, 35, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Chan, A. Among Superbugs, MRSA Is at the Forefront of Antimicrobial Resistance. Available online: https://www.healthdata.org/news-events/insights-blog/acting-data/among-superbugs-mrsa-forefront-antimicrobial-resistance (accessed on 20 July 2024).
- Mahasenan, K.V.; Molina, R.; Bouley, R.; Batuecas, M.T.; Fisher, J.F.; Hermoso, J.A.; Chang, M.; Mobashery, S. Conformational Dynamics in Penicillin-Binding Protein 2a of Methicillin-Resistant Staphylococcus aureus, Allosteric Communication Network and Enablement of Catalysis. J. Am. Chem. Soc. 2017, 139, 2102–2110. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.; Strynadka, N.C.J. Structural basis for the β lactam resistance of PBP2a from methicillin-resistant Staphylococcus aureus. Nat. Struc. Biol. 2002, 9, 870–876. [Google Scholar] [CrossRef]
- Alhadrami, H.A.; Hamed, A.A.; Hassan, H.M.; Belbahri, L.; Rateb, M.E.; Sayed, A.M. Flavonoids as Potential anti-MRSA Agents through Modulation of PBP2a: A Computational and Experimental Study. Antibiotics 2020, 9, 562. [Google Scholar] [CrossRef]
- Masumi, M.; Noormohammadi, F.; Kianisaba, F.; Nouri, F.; Taheri, M.; Taherkhani, A. Methicillin-Resistant Staphylococcus aureus: Docking-Based Virtual Screening and Molecular Dynamics Simulations to Identify Potential Penicillin-Binding Protein 2a Inhibitors from Natural Flavonoids. Int. J. Microbiol. 2022, 2022, 9130700. [Google Scholar] [CrossRef]
- Gutiérrez, R.U.; Correa, H.C.; Bautista, R.; Vargas, J.L.; Jerezano, A.V.; Delgado, F.; Tamariz, J. Regioselective Synthesis of 1,2-Dihydroquinolines by a Solvent-Free MgBr2-Catalyzed Multicomponent Reaction. J. Org. Chem. 2013, 78, 9614–9626. [Google Scholar] [CrossRef]
- Gutiérrez, R.U.; Rebollar, A.; Bautista, R.; Pelayo, V.; Várgas, J.L.; Montenegro, M.M.; Espinoza-Hicks, C.; Ayala, F.; Bernal, P.M.; Carrasco, C.; et al. Functionalized α-oximinoketones as building blocks for the construction of imidazoline-based potential chiral auxiliaries. Tetrahedron Asymmetry 2015, 26, 230–246. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.; Thiessen, P.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Wavefunction_Inc. Spartan’20 (Version 20.1.3); Q-CHEM: Pleasanton, CA, USA, 2020. [Google Scholar]
- BIOVIA Dassault Systèmes BIOVIA. Discovery Studio Modeling Environment; Dassault Systèmes: San Diego, CA, USA, 2019. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexiblity. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, P.A.; Forli, S.; Goodsell, D.S.; Olson, A.J.; Sanner, M.F. AutoDockFR: Advances in Protein-Ligand Docking with Explicitly Specified Binding Site Flexibility. PLoS Comput. Biol. 2015, 11, e1004586. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Jo, S.; Brooks, C.L., III; Lee, H.S.; Im, W. CHARMM-GUI ligand reader and modeler for CHARMM force field generation of small molecules. J. Comput. Chem. 2017, 38, 1879–1886. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Huang, J.; Rauscher, S.; Nawrocki, G.; Ran, T.; Feig, M.; de Groot, B.L.; Grubmüller, H.; MacKerell, A.D. CHARMM36m: An improved force field for folded and intrinsically disordered proteins. Nat. Methods 2017, 14, 71–73. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Phillips, J.C.; Hardy, D.J.; Maia, J.D.C.; Stone, J.E.; Ribeiro, J.V.; Bernardi, R.C.; Buch, R.; Fiorin, G.; Hénin, J.; Jiang, W.; et al. Scalable molecular dynamics on CPU and GPU architectures with NAMD. J. Chem. Phys. 2020, 153, 044130. [Google Scholar] [CrossRef]
- Fletcher, R.; Reeves, C.M. Function minimization by conjugate gradients. Comput. J. 1964, 7, 149–154. [Google Scholar] [CrossRef]
- Martyna, G.J.; Tobias, D.J.; Klein, M.L. Constant pressure molecular dynamics algorithms. J. Chem. Phys. 1994, 101, 4177–4189. [Google Scholar] [CrossRef]
- Feller, S.E.; Zhang, Y.; Pastor, R.W.; Brooks, B.R. Constant pressure molecular dynamics simulation: The Langevin piston method. J. Chem. Phys. 1995, 103, 4613–4621. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. gmx_MMPBSA: A New Tool to Perform End-State Free Energy Calculations with GROMACS. J. Chem. Theory Comput. 2021, 17, 6281–6291. [Google Scholar] [CrossRef]

| Ligand | Physicochemical Properties | Lipophilicity | Water Solubility | Drug-Likeness | Medicinal Chemistry | ||||
|---|---|---|---|---|---|---|---|---|---|
| MW | NRB | HBA | HBD | Log Po/w | Log S | Class | Lipinski | PAINS | |
| Lig_ref | 382.43 | 8 | 7 | 3 | −2.38 | −3.25 | Soluble | Yes; 0 violation | 0 alert |
| 1 | 319.33 | 3 | 5 | 2 | 1.04 | −3.70 | Soluble | Yes; 0 violation | 0 alert |
| 2 | 238.24 | 2 | 2 | 1 | 1.88 | −5.19 | Moderately S. | Yes; 0 violation | 0 alert |
| 3 | 236.27 | 2 | 2 | 2 | 2.24 | −5.33 | Moderately S. | Yes; 0 violation | 0 alert |
| 4 | 327.42 | 3 | 1 | 1 | 4.35 | −8.54 | Poorly S. | Yes; 1 violation | 0 alert |
| 5 | 291.30 | 5 | 5 | 1 | 1.06 | −3.68 | Soluble | Yes; 0 violation | 0 alert |
| 6 | 356.42 | 6 | 3 | 1 | 3.29 | −8.64 | Poorly S. | Yes; 0 violation | 0 alert |
| 7 | 458.55 | 6 | 2 | 2 | 4.34 | −12.25 | Insoluble | Yes; 1 violation | 0 alert |
| 8 | 358.88 | 5 | 1 | 0 | 4.41 | −5.96 | Moderately S. | Yes; 1 violation | 0 alert |
| Ligand | Affinity Energies |
|---|---|
| Lig_ref | −7.6 |
| 1 | −7.5 |
| 2 | −8.5 |
| 3 | −8.4 |
| 4 | −7.4 |
| 5 | −8.4 |
| 6 | −9.5 |
| 7 | −7.3 |
| 8 | −8.9 |
| Ligand | H-bonds Involving the Amino Acids of the Catalytic Site | Other H-Bonds | ||||
|---|---|---|---|---|---|---|
| Donator | Acceptor | % of Occupancy | Donator | Acceptor | % of Occupancy | |
| Lig_ref | LIG | GLU602 | 42.88% | GLN613 | LIG | 17.56% |
| THR600 | LIG | 9.48% | ||||
| THR600 | LIG | 5.58% | ||||
| GLU602 | LIG | 6.18% | ||||
| 4 | TYR446 | LIG | 27.58% | GLU447 | LIG | 5.76% |
| 5 | ASN464 | LIG | 65.86% | ALA642 | LIG | 24.24% |
| LIG | THR600 | 78.04% | ||||
| TYR446 | LIG | 37.36% | ||||
| ASN464 | LIG | 5.16% | ||||
| 6 | TYR446 | LIG | 50.78% | ARG445 | LIG | 10.70% |
| ASN464 | LIG | 14.44% | THR444 | LIG | 5.72% | |
| 7 | GLN613 | LIG | 5.58% | |||
| Ligand | Binding Free Energies |
|---|---|
| Lig_ref | −26.07 |
| 4 | −10.92 |
| 5 | −30.05 |
| 6 | −17.13 |
| 7 | −18.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Vargas, K.A.; Gutierrez-Aguilar, R.U.; Avina-Verduzco, J.A.; Garcia-Gutierrez, H.A.; Ontiveros-Rodriguez, J.C.; Herrera-Bucio, R.; Navarro-Santos, P. Molecular Docking and Dynamics of a Series of Aza-Heterocyclic Compounds Against Penicillin-Binding Protein 2a of Methicillin-Resistant Staphylococcus aureus . Chem. Proc. 2024, 16, 4. https://doi.org/10.3390/ecsoc-28-20221
Ortiz-Vargas KA, Gutierrez-Aguilar RU, Avina-Verduzco JA, Garcia-Gutierrez HA, Ontiveros-Rodriguez JC, Herrera-Bucio R, Navarro-Santos P. Molecular Docking and Dynamics of a Series of Aza-Heterocyclic Compounds Against Penicillin-Binding Protein 2a of Methicillin-Resistant Staphylococcus aureus . Chemistry Proceedings. 2024; 16(1):4. https://doi.org/10.3390/ecsoc-28-20221
Chicago/Turabian StyleOrtiz-Vargas, Karen Astrid, Rsuini Uri Gutierrez-Aguilar, Judit Araceli Avina-Verduzco, Hugo A. Garcia-Gutierrez, Julio Cesar Ontiveros-Rodriguez, Rafael Herrera-Bucio, and Pedro Navarro-Santos. 2024. "Molecular Docking and Dynamics of a Series of Aza-Heterocyclic Compounds Against Penicillin-Binding Protein 2a of Methicillin-Resistant Staphylococcus aureus " Chemistry Proceedings 16, no. 1: 4. https://doi.org/10.3390/ecsoc-28-20221
APA StyleOrtiz-Vargas, K. A., Gutierrez-Aguilar, R. U., Avina-Verduzco, J. A., Garcia-Gutierrez, H. A., Ontiveros-Rodriguez, J. C., Herrera-Bucio, R., & Navarro-Santos, P. (2024). Molecular Docking and Dynamics of a Series of Aza-Heterocyclic Compounds Against Penicillin-Binding Protein 2a of Methicillin-Resistant Staphylococcus aureus . Chemistry Proceedings, 16(1), 4. https://doi.org/10.3390/ecsoc-28-20221






