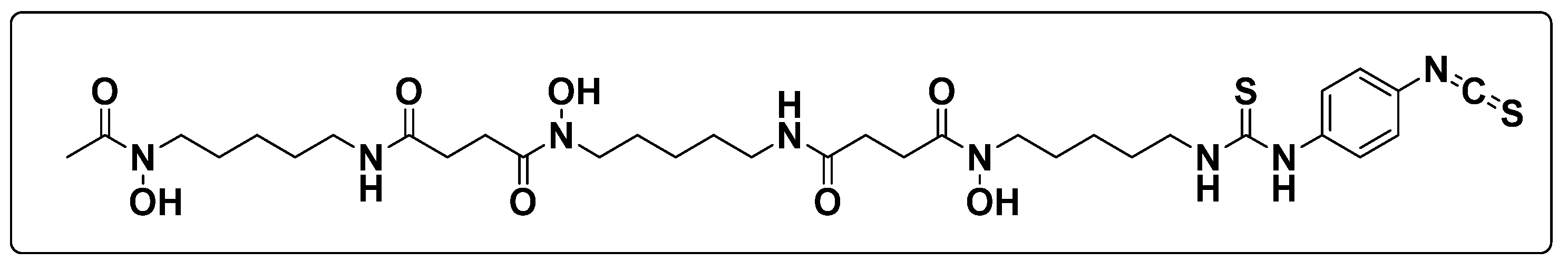
Development and Validation of the Stability of p-SCN-Bn-Df via the Reversed-Phase Chromatography Method: Practical Experiences †
Abstract
1. Introduction
2. Materials and Methods
Chemicals and Instruments
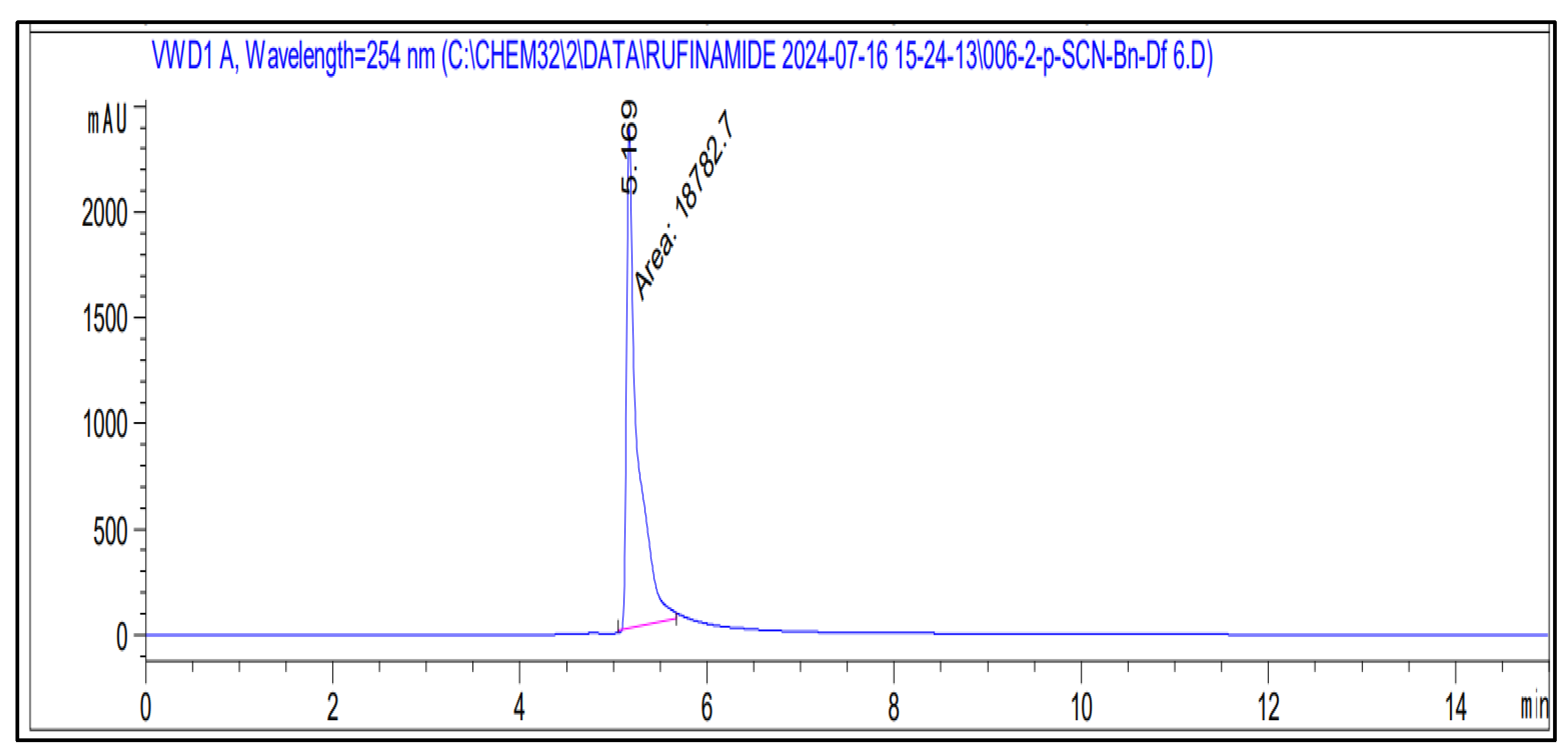
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, D.; Severin, G.W.; Dougherty, C.A.; Lombardi, R.; Chen, D.; Van Dort, M.E.; Barnhart, T.E.; Ross, B.D.; Mazar, A.P.; Hong, H. Antibody-based PET of uPA/uPAR signaling with broad applicability for cancer imaging. Oncotarget 2016, 7, 73912. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.; Wang, X.; Lemma, K.; Ashby, M.T. Reactive sulfur species: Hydrolysis of hypothiocyanite to give thiocarbamate-S-oxide. J. Am. Chem. Soc. 2007, 129, 15756–15757. [Google Scholar] [CrossRef]
- Davey, P.R.; Paterson, B.M. Modern developments in bifunctional chelator design for gallium radiopharmaceuticals. Molecules 2022, 28, 203. [Google Scholar] [CrossRef]
- Christenson Emily, A. Effects of Complexation with the Siderophore Desferrioxamine b on Transition Metal Removal from Seawater. Master’s Thesis, University of Maryland, College Park, MD, USA, 2013. [Google Scholar]
- Jones, K.E.; Batchler, K.L.; Zalouk, C.; Valentine, A.M. Ti (IV) and the siderophore desferrioxamine B: A tight complex has biological and environmental implications. Inorg. Chem. 2017, 56, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Mular, A.; Shanzer, A.; Kozłowski, H.; Hubmann, I.; Misslinger, M.; Krzywik, J.; Decristoforo, C.; Gumienna-Kontecka, E. Cyclic analogs of desferrioxamine e siderophore for 68Ga nuclear imaging: Coordination chemistry and biological activity in staphylococcus aureus. Inorg. Chem. 2021, 60, 17846–17857. [Google Scholar] [CrossRef] [PubMed]
- Vosjan, M.J.; Perk, L.R.; Visser, G.W.; Budde, M.; Jurek, P.; Kiefer, G.E.; Van Dongen, G.A. Conjugation and radiolabeling of monoclonal antibodies with zirconium-89 for PET imaging using the bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine. Nat. Protoc. 2010, 5, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Deri, M.A.; Ponnala, S.; Kozlowski, P.; Burton-Pye, B.P.; Cicek, H.T.; Hu, C.; Lewis, J.S.; Francesconi, L.C. p-SCN-Bn-HOPO: A superior bifunctional chelator for 89Zr immunoPET. Bioconjugate Chem. 2015, 26, 2579–2591. [Google Scholar] [CrossRef] [PubMed]
- Gohar, M.S.; Rahman, T.U.; Bahadur, A.; Ali, A.; Alharthi, S.; Al-Shaalan, N.H. Development and Validation of Novel HPLC Methods for Quantitative Determination of Vitamin D3 in Tablet Dosage Form. Pharmaceuticals 2024, 17, 505. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, N.V.S.D.K.; Younes, A.; Cao, M.; Ali, J.; Cicek, H.T.; Tully, K.M.; Ponnala, S.; Babich, J.W.; Deri, M.A.; Lewis, J.S.; et al. Improved synthesis of the bifunctional chelator p-SCN-Bn-HOPO. Org. Biomol. Chem. 2019, 17, 6866–6871. [Google Scholar] [CrossRef] [PubMed]
- Nikolin, B.; Imamović, B.; Medanhodžić-Vuk, S.; Sober, M. High perfomance liquid chromatography in pharmaceutical analyses. Bosn. J. Basic Med. Sci. 2004, 4, 5. [Google Scholar] [CrossRef]
- Ali, A.H. High-performance liquid chromatography (HPLC): A review. Ann. Adv. Chem. 2022, 6, 010–020. [Google Scholar]
- Liu, L.X.; Zhang, Y.; Zhou, Y.; Li, G.H.; Yang, G.J.; Feng, X.S. The application of supercritical fluid chromatography in food quality and food safety: An overview. Crit. Rev. Anal. Chem. 2020, 50, 136–160. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, V.; Avellone, G.; Bongiorno, D.; Cunsolo, V.; Muccilli, V.; Sforza, S.; Dossena, A.; Drahos, L.; Vékey, K. Applications of liquid chromatography–mass spectrometry for food analysis. J. Chromatogr. A 2012, 1259, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tian, Y.; Jiang, P.; Lin, Y.; Liu, X.; Yang, H. Recent advances in the application of metabolomics for food safety control and food quality analyses. Crit. Rev. Food Sci. Nutr. 2021, 61, 1448–1469. [Google Scholar] [CrossRef] [PubMed]

| Equipment | Agilent, California, USAhigh-performance liquid chromatography equipped with Auto Sampler and VWD detector. |
| Column | Eclipse Plus C18 (4.6 × 250 mm, 5 μm) |
| Flow rate | 0.5 mL/min |
| Wavelength | 254 nm |
| Injection volume | 100 μL |
| Column oven | Ambient |
| Run time | 15.0 min |
| Sample No. | Conc. (μg/µL) | Mean Peak Area ± SD | RSD % |
|---|---|---|---|
| 1 | 10 | 5276.3 ± 58.12 | 1.102 |
| 2 | 20 | 11,998.3 ± 108.82 | 0.907 |
| 3 | 30 | 18,070.45 ± 91.56 | 0.507 |
| 4 | 40 | 25,065.3 ± 110.24 | 0.440 |
| Parameter | UV Method |
|---|---|
| λmax (nm) | 254 |
| Beer’s law limits (mcg/mL) | 20–40 |
| Molar extinction coefficient (L mol−1 cm−1) | 25 |
| Sandell’s sensitivity (mcg/cm2−0.001 absorbance units) | 0.0004 |
| Regression equation (Y) | y = 25,417x − 50 |
| Slope (b) | 25 |
| Correlation coefficient (r2) | 0.9995 |
| Precision (% RSD) | 0.739 |
| Limit of detection (mcg/mL) | 12.16 |
| Limit of quantitation (mcg/mL) | 36.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrivastav, A.; Faheem, M.; Pandey, V.; Dixit, M. Development and Validation of the Stability of p-SCN-Bn-Df via the Reversed-Phase Chromatography Method: Practical Experiences. Chem. Proc. 2024, 16, 39. https://doi.org/10.3390/ecsoc-28-20175
Shrivastav A, Faheem M, Pandey V, Dixit M. Development and Validation of the Stability of p-SCN-Bn-Df via the Reversed-Phase Chromatography Method: Practical Experiences. Chemistry Proceedings. 2024; 16(1):39. https://doi.org/10.3390/ecsoc-28-20175
Chicago/Turabian StyleShrivastav, Anjli, Mohd. Faheem, Vaibhav Pandey, and Manish Dixit. 2024. "Development and Validation of the Stability of p-SCN-Bn-Df via the Reversed-Phase Chromatography Method: Practical Experiences" Chemistry Proceedings 16, no. 1: 39. https://doi.org/10.3390/ecsoc-28-20175
APA StyleShrivastav, A., Faheem, M., Pandey, V., & Dixit, M. (2024). Development and Validation of the Stability of p-SCN-Bn-Df via the Reversed-Phase Chromatography Method: Practical Experiences. Chemistry Proceedings, 16(1), 39. https://doi.org/10.3390/ecsoc-28-20175







