Abstract
1-Hydroxy-N-phenylnaphthalene-2-carboxamide and a series of seventeen other carboxanilides in the anilide part of dichlorinated, trichlorinated, dibrominated, tribrominated, and chlorinated/brominated variants have recently been reported as biologically active compounds mainly with antibacterial, antimycobacterial, and anticancer effects. Since lipophilicity is one of the factors influencing the bioavailability (absorption, distribution, metabolism, and elimination), activity, and even toxicity of bioactive compounds, all the derivatives were investigated for their lipophilic and hydrophilic properties. All eighteen compounds were analyzed by reversed-phase high-performance liquid chromatography (RP-HPLC). The procedure was performed under isocratic conditions with methanol as the organic modifier in the mobile phase using an end-capped non-polar C18 stationary reversed-phase column. The lipophilicity values are expressed as the logarithm of the capacity factor k (for the mobile phase water/methanol) and the distribution coefficients D at pH values of 6.5 and 7.4 (for the mobile phase buffer/methanol), as well as the calculated values of log P/Clog P by various methods. 1-Hydroxy-N-(3,4,5-trichlorophenyl)naphtha- lene-2-carboxamide and N-(4-bromo-3-chlorophenyl)-1-hydroxynaphthalene-2-carboxamide are the most lipophilic compounds of the whole series; on the other hand, surprisingly, unsubstituted 1-hydroxy-N-phenylnaphthalene-2-carboxamide is not the least lipophilic derivative. The mutual correlations between the experimental and predicted lipophilicity values are low; in addition, there are large deviations in the cross-correlations between log k and log D, which are due to the presence of a free ionizable phenolic group in the molecule.
1. Introduction
Designing and optimizing the structure/properties of new drugs are two of the priorities of modern science. The relationships between the structure and effects of biologically active compounds have been analyzed in previous research [1,2]. Lipophilicity is an important property of all bioactive molecules. The determination of lipophilicity is a central step in studies devoted to the design of new agents with potential biological effects and is also useful in optimizing the effects of existing agents. Lipophilicity, a key property of compounds, is responsible for their solubility, transport across membranes, and binding to plasma proteins, as well as the interactions of molecules with receptors, which give rise to the pharmacological action of drugs. It is one of the key properties for modeling biological response, correlating with absorption, distribution, metabolism, excretion, and toxicity (ADMET) processes [3,4,5].
A common quantitative descriptor of lipophilicity Is the log P partition coefficient. Studies show that the optimal range of lipophilicity, expressed as the logarithm of the n-octanol/water partition coefficient (log P), for optimal gastrointestinal absorption by passive diffusion after oral administration is from 0 to 3 [3,4,6]. However, log P values include only the neutral form of the compound and are independent of ionization under physiological conditions. If the molecule contains basic or acidic groups, it can be ionized and its distribution in the n-octanol/water system depends on the pH. It is estimated that 95% of drugs are ionizable. Therefore, another descriptor of lipophilicity for ionizable molecules is expressed as the distribution coefficient D or its logarithm log D. Log D is dependent on the pH of the environment, and its value includes the contribution of all ionized forms of the substance present at a given pH [3,6].
Lipophilicity can be determined experimentally using several methods. They are usually divided into direct and indirect methods [7,8,9]. In this work, aimed at determining the lipophilicity of a series of anilides of 1-hydroxynaphthalene-2-carboxylic acid, an indirect chromatographic method using reversed-phase high-performance liquid chromatography (RP-HPLC) was used. Hydroxynaphthoic acid derivatives have a wide spectrum of biological effects, as recently described [10,11,12], and thus offer a source of promising molecules for drug development.
2. Results and Discussion
The discussed anilides were synthesized using microwave-assisted synthesis as illustrated in Scheme 1 and described by Gonec et al. [10,11]. The reaction of 1-hydroxynaphthalene-2-carboxylic acid with ring-substituted aniline using phosphorus trichloride in chlorobenzene provided a series of eighteen 1-hydroxynaphthalene- 2-carboxanilides 1–18. All the target compounds are listed in Table 1.
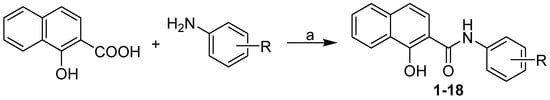
Scheme 1.
Synthesis of ring-substituted 1-hydroxynaphthalene-2-carboxanilides 1–18. Reagents and conditions: (a) PCl3, chlorobenzene, microwave synthesis (500 W, 130 °C, 15 min) [10,11].

Table 1.
Structure of ring-substituted 1-hydroxynaphthalene-2-carboxanilides 1–18; experimentally determined log k, log D6.5, and log D7.4 values; and predicted lipophilicity (log P/Clog P) values of investigated compounds.
The lipophilicity of all eighteen compounds was determined using RP-HPLC as capacity factors k (with a subsequent calculation of log k) and as the distribution coefficients D at pH values of 6.5 and 7.4 (with a subsequent calculation of log D6.5 and log D7.4). The retention times of individual compounds were determined under isocratic conditions with methanol as an organic modifier in the mobile phase using end-capped non-polar C18 stationary RP columns. In addition, the lipophilicities (log P/Clog P data) of all target anilides were calculated using two commercially available programs: ACD/Percepta ver. 2012 and ChemBioDraw Ultra 13.0. All results are shown in Table 1.
Log P and Clog P calculations in ChemBioDraw software are based on the fragment method, whereby the log P calculation algorithm in this software neglects the position of the substituents and therefore calculates the same log P values for individual positional isomers. According to the Clog P algorithm, which also includes possible chemical interactions of the molecule, the lipophilicity values were identical for the 2,4- and 2,5-disubstituted isomers (i.e., for derivatives 3/5, 11/12, 15/16). For this reason, the lipophilicity values predicted by the ChemBioDraw software are only listed in Table 1 without further discussion. Thus, only the log P values calculated by ACD/Percepta are unique for each individual isomer.
Very poor agreement is evident from the graphs in Figure 1, where experimentally determined lipophilicity values (log k, log D6.5, log D7.4) are plotted against log P values; the correlation coefficients r (n = 18) are 0.5225, 0.4774, and 0.4084, respectively. These low correlations appear to be due to the phenolic moiety at the 1-position of naphthalene. It follows that predicted log P values cannot be used to search for structure–activity relationships.
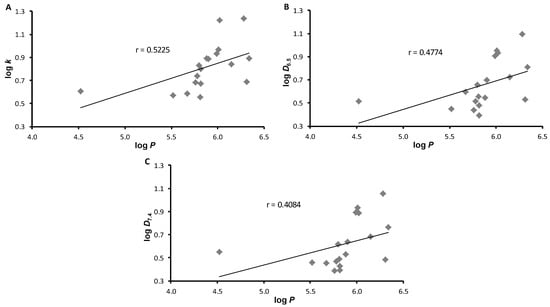
Figure 1.
Comparison of experimentally determined values of log k (A), log D6.5 (B), and log D7.4 (C) with calculated log P (ACD/Percepta) of ring-substituted 1-hydroxynaphthalene-2-carboxanilides 1–18.
Figure 2 shows the mutual correlations of all three measured experimental values. The best correlation was obtained when comparing the distribution parameters, i.e., logD6.5 versus logD7.4 (r = 0.9842, n = 18). The correlations of log k versus logD6.5 or logD7.4 were lower (r = 0.8506 and 0.8526, respectively). This fact again confirms the significant influence of the free phenolic group, which manifests itself in media with a different pH.
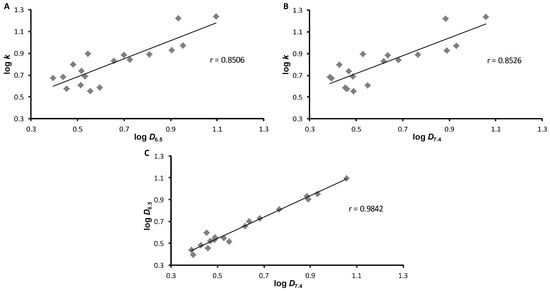
Figure 2.
Cross-correlations of experimentally determined values of log k versus log D6.5 (A), log k versus log D7.4 (B), and log D6.5 versus log D7.4 (C) of ring-substituted 1-hydroxynaphthalene- 2-carboxanilides 1–18.
This influence is also evidenced by the fact that the least lipophilic compound is not the unsubstituted 1-hydroxy-N-phenylnaphthalene-2-carboxamide (1), as expected (and as predicted by all the used programs), but N-(2,5-dibromophenyl)-1-hydroxynaphthalene- 2-carboxamide (12, log k = 0.5551). The above-mentioned differences between log k and log D lead to the fact that, according to log D6.5, N-(2,5-dichlorophenyl)-1-hydroxynaph- thalene-2-carboxamide (4) is the least lipophilic (log D6.5 = 0.3949), while according to logD7.4, N-(2,3-dichlorophenyl)-1-hydroxynaphthalene-2-carboxamide (2) is the least lipophilic (log D7.4 = 0.3870). 1-Hydroxy-N-(3,4,5-trichlorophenyl)naphthalene-2-carboxamide (10) is the most lipophilic derivative, both based on the measurements performed under all three conditions and the fact that this compound also has the highest lipophilicity according to the predicted log P/Clog P values.
In general, the lipophilicities represented by log k values are nominally the highest by a great distance, followed by logD6.5 values and then logD7.4 values, which are the lowest. The order of compounds arranged according to increasing log k values and their overall comparison is shown in Figure 3. The greatest mutual differences can be observed for compounds 2 (R = 2,3-Cl), 15 (R = 2-Cl-4-Br), 16 (R = 2-Cl-5-Br), and 4 (R = 2,5-Cl).
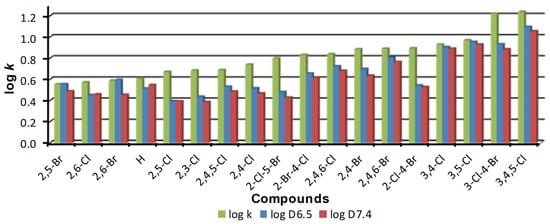
Figure 3.
Order of individual derivatives arranged according to increasing log k values.
Regarding all these observations, it should be summarized that for these 1-hydroxynaphthalene-2-carboxamides on the ring-multisubstituted anilide, standard commercially available lipophilicity prediction programs are unable to provide relevant data due to the high incidence of intra- and intermolecular interactions. Due to the presence of an ionizable acidic phenolic group in the vicinity of the amide bond, there are also differences in the experimental values obtained for different mobile phase properties/compositions.
3. Experimental Section
3.1. Synthesis
Detailed synthesis and characterization of 1-hydroxy-N-phenylnaphthalene- 2-carboxamide (1) was reported by Gonec et al. [10], while a characterization of ring-substituted chlorinated and brominated carboxanilides 2–17 is provided in [11,12].
3.2. Lipophilicity Determination by HPLC
An HPLC system Agilent 1200 equipped with a DAD detector (Agilent, Santa Clara, CA, USA) was used. A chromatographic column Symmetry® C18 5 μm, 4.6 mm × 250 mm, part No. WAT054275 (Waters Corp., Milford, MA, USA) was used. The HPLC separation process was monitored and evaluated with EZChrom Elite software ver. 3.3.2 (Agilent). Isocratic elution by a mixture of MeOH p.a. (72%) and H2O-HPLC Mili-Q grade (28%) as a mobile phase was used for the determination of capacity factor k. Isocratic elution by a mixture of MeOH p.a. (72%) and acetate-buffered saline (pH 7.4 and pH 6.5) (28%) as a mobile phase was used for the determination of distribution coefficients expressed as D7.4 and D6.5. The total flow of the column was 1.0 mL/min, injection volume was 20 μL, column temperature was 40 °C, and sample temperature was 10 °C. The detection wavelength of 210 nm was chosen. A KI methanolic solution was used for the determination of the dead times (td). Retention times (tr) were measured in minutes. The capacity factors k were calculated according to the formula k = (tr − td)/td, where tR is the retention time of the solute, and td is the dead time obtained using an unretained analyte. The distribution coefficients DpH were calculated according to the formula DpH = (tr − td)/td. Each experiment was repeated three times. The experimental values of lipophilicity of individual compounds are shown in Table 1.
Author Contributions
Conceptualization, T.G. and J.J.; methodology, T.G. and L.V.; investigation, L.V.; writing—original draft preparation, T.G., L.V. and J.J.; supervision, J.J.; funding acquisition, J.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Slovak Research and Development Agency (project APVV-22-0133) and by grant GUK/1143/2024.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wu, K.; Karapetyan, E.; Schloss, J.; Vadgama, J.; Wu, Y. Advancements in small molecule drug design: A structural perspective. Drug Discov. Today 2023, 28, 103730. [Google Scholar] [CrossRef] [PubMed]
- Pennington, L.D.; Hesse, M.J.; Koester, D.C.; McAtee, R.C.; Qunies, A.M.; Hu, D.X. Property-based drug design merits a Nobel prize. J. Med. Chem. 2024, 67, 11452–11458. [Google Scholar] [PubMed]
- Kerns, E.H.; Di, L. Drug-Like Properties: Concepts. Structure Design and Methods: From ADME to Toxicity Optimization; Academic Press: San Diego, CA, USA, 2008. [Google Scholar]
- Pliska, V.; Testa, B.; van der Waterbeemd, H. Lipophilicity in Drug Action and Toxicology; Wiley-VCH: Weinheim, Germany, 1996. [Google Scholar]
- Giaginis, C.; Tsopelas, F.; Tsantili-Kakoulidou, A. The impact of lipophilicity in drug discovery: Rapid measurements by means of reversed-phase HPLC. Methods Mol. Biol. 2018, 1824, 217–228. [Google Scholar] [PubMed]
- Rutkowska, E.; Pajak, K.; Jozwiak, K. Lipophilicity – methods of determination and its role in medicinal chemistry. Acta Pol. Pharm. 2013, 70, 3–18. [Google Scholar] [PubMed]
- Tsopelas, F.; Giaginis, C.; Tsantili-Kakoulidou, A. Lipophilicity and biomimetic properties to support drug discovery. Expert Opin. Drug Discov. 2017, 12, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Kempinska, D.; Chmiel, T.; Kot-Wasik, A.; Mroz, A.; Mazerska, Z.; Namiesnik, J. State of the art and prospects of methods for determination of lipophilicity of chemical compounds. Trends Anal. Chem. 2019, 113, 54–73. [Google Scholar] [CrossRef]
- Soares, J.X.; Santos, A.; Fernandes, C.; Pinto, M.M.M. Liquid chromatography on the different methods for the determination of lipophilicity: An essential analytical tool in medicinal chemistry. Chemosensors 2022, 10, 340. [Google Scholar] [CrossRef]
- Gonec, T.; Kos, J.; Zadrazilova, I.; Pesko, M.; Keltosova, S.; Tengler, J.; Bobal, P.; Kollar, P.; Cizek, A.; Kralova, K.; et al. Antimycobacterial and herbicidal activity of ring-substituted 1-hydroxynaphthalene-2-carboxanilides. Bioorg. Med. Chem. 2013, 21, 6531–6541. [Google Scholar] [PubMed]
- Gonec, T.; Kos, J.; Pesko, M.; Dohanosova, J.; Oravec, M.; Liptaj, T.; Kralova, K.; Jampilek, J. Halogenated 1-hydroxynaphthalene-2-carboxanilides affecting photosynthetic electron transport in photosystem II. Molecules 2017, 22, 1709. [Google Scholar] [CrossRef] [PubMed]
- Spaczynska, E.; Mrozek-Wilczkiewicz, A.; Malarz, K.; Kos, J.; Gonec, T.; Oravec, M.; Gawecki, R.; Bak, A.; Dohanosova, J.; Kapustikova, I.; et al. Design and synthesis of anticancer 1-hydroxynaphthalene-2-carboxanilides with a p53 independent mechanism of action. Sci. Rep. 2019, 9, 6387. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).