Abstract
Grindelia ventanensis (Asteraceae) is an endemic species growing wild in Argentina. In this work, we studied the dichloromethane extract of this plant for their antioxidant activity and cholinesterase inhibition. This extract was fractionated using column chromatography and HPLC. Six diterpenoids were isolated and identified using RMN and MS analyses. The 13-methyl-17-oxo-labda-7,13-diene-15-oic acid elicited the best results in both bioassays, with IC50 = 11.04 µM in the AChE inhibition test and an antioxidant activity comparable to trolox, the reference antioxidant (IC50 = 7.13 µM). Significant bioactivity was also observed for the rest of the isolated compounds.
1. Introduction
Alzheimer’s disease (AD), the most common type of dementia, is a neurodegenerative disorder that progressively impairs the motor and cognitive functions of affected individuals. Cholinergic neurons in the brain deteriorate and the neurotransmitter acetylcholine decreases. The cholinergic hypothesis has led to the discovery of inhibitors of the enzyme acetylcholinesterase (AChE), which prevent the degradation of acetylcholine by this enzyme. The treatment with AChE inhibitors (iAChEs) is the main therapy used for AD cases. This enzyme represents a therapeutic target to improve the cholinergic deficit responsible for the cognitive decline characteristic of AD [1,2].
In addition, the pathogenesis of this disease is associated with oxidative stress. Reactive oxygen species (ROS) lead to neurodegeneration, producing functional alteration, cell loss and death. Therefore, oxidative stress is also considered an effective therapeutic target for the treatment of these neurodegenerative disorders [3,4].
The current therapeutic approach is predominantly directed towards active compounds that could be able to prevent the onset, avoid the progression or alter the outcome of AD. Therefore, the search for new active compounds with antioxidant and anticholinesterase properties, which could be effective in the development of new pharmacological therapies to treat CNS diseases associated with oxidative stress, continues to be a major challenge in pharmacological research [5].
The genus Grindelia (Asteraceae) is represented in South America by 28 species, 25 of which are endemic. Previous phytochemical studies have shown that bicyclic labdane-type diterpenic acids and mannosyl diterpenes are present in plants belonging to this genus. Anti-inflammatory, expectorant, antispasmodic, and antimicrobial activities, as well as antifeedant effects towards insects, have been reported for extracts or secondary metabolites obtained from Grindelia plants [6].
Diterpenoids attract scientific interest due to their chemical diversity and medicinal properties [7]. In the last decade, several natural diterpenoids have been reported as promising cholinesterase inhibitors [8].
Grindelia ventanensis A. Bartoli & Tortosa is an endemic species growing wild in the Buenos Aires province. In a previous study, we reported the cholinesterase inhibition observed for the ethanolic extract of G. ventanensis and the isolation of an active metabolite, 17-hydroxycativic acid, which showed antioxidant activity and moderate acetylcholinesterase (AChE) inhibition, without cytotoxic activity [9]. In this work, we focused our attention on the dichloromethane (DCM) extract of this plant, and its components, looking forward to identifying other bioactive diterpenes. The antioxidant activity was evaluated, using the reduction of the stable free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) [10], for the DCM extract and its components. Also, AChE inhibition was evaluated using the Ellman method [11] for all the samples.
2. Materials and Methods
2.1. General
Gas chromatography-mass spectrometry (GC-MS) analyses were performed with a Hewlett-Packard 6890 chromatograph connected to a Hewlett-Packard 5972A mass spectrometer equipped with a capillary column (HP-5, 25 m × 0.25 mm, 0.25 µm film thickness). The carrier gas was helium with flow 1 mL/min. The GC oven temperature was held at 80 °C for 2 min, programmed at 10 °C/min to 280 °C, then held at this temperature for 10 min. Mass spectra were recorded at 70 eV. Mass range was from m/z 35–700 amu. The temperature of the injection block was 250 °C. HPLC separations were performed in a Waters 600 system equipped with a diode array detector with a Rheodyne manual injector with a 1 mL loading loop and a variable wavelength UV-VIS detector operating at 254 nm and a Phenomenex Gemini 5 µm C18 110Å, 250 × 10 mm column. 1H and 13C NMR spectra were recorded on a Bruker ARX 300 multinuclear spectrometer at 300 MHz and 75 MHz, respectively. UV spectra were recorded on a GBC Spectral UV-VIS spectrophotometer.
Column chromatography (CC) was performed with Silica gel 60 (70–230 mesh, Merck, Darmstadt, Germany) and column flash chromatography with Silica gel 60 (200–425 mesh, Merck, Darmstadt, Germany). Analytical thin-layer chromatography (TLC) was performed on Silica gel 60 F254 sheets (0.2 mm thickness, Merck, Darmstadt, Germany) and the spots were detected with p-anisaldehyde-acetic acid spray reagent
All chemicals and solvents were analytical grade and solvents were purified using general methods before being used. AChE from electric eel (type VI-S), 5,50-dithiobis(2-nitrobenzoic acid) (DTNB), acetylthiocholine iodide (ATCI), tacrine, 2,2-diphenyl-1-picrylhydracil (DPPH) and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), were purchased from Sigma-Aldrich, (Madrid, Spain).
2.2. Plant Material
Aerial parts of G. ventanensis were collected (December 2016) in Sierra de la Ventana, Buenos Aires Province, Argentina, and identified by Dr. Maria Gabriela Murray. A voucher specimen (MGM 546) was deposited with the Herbario del Departamento de Biología, Bioquímica y Farmacia, Universidad Nacional del Sur (BBB).
2.3. Obtention of DCM Extract and Its Active Compounds
Fresh aerial parts from G. ventanensis (356.2 g) were extracted with DCM (2 L) at room temperature for two weeks, filtered and evaporated to dryness to yield 12.9 g of the active extract. This extract was subjected to CC, eluting with mixtures of hexane/EtOAc of increasing polarity, sixteen fractions FA-FO, were obtained. The active fractions (FC, FI and FM) were subsequently purified via reverse-phase semi-preparative HPLC isocratically using MeOH/H2O (90:10). The compound 6, major constituent of the active fraction FC, was isolated and yielded 13.4 mg. From the FI fraction three active compounds were isolated, 3–5, that yielded 29.9, 28.6 and 23.8 mg, respectively. Compounds 1 and 2 were obtained from FM fraction and yielded 27.5 and 26.2 mg, respectively. These compounds were analyzed using GC/MS and they were identified via comparison of their retention indices (Kovats indices) and their mass spectra with those stored in the MS database (NBS75K.L MS DATA). The identity of the compounds was confirmed via its 1H and 13C NMR spectra, recorded in CDCl3.
2.4. Antioxidante Activity
The antioxidant activity was evaluated through its ability as the free radical scavenger of extracts, fractions, and/or pure compounds, as described by Bors et al. [10]. The preliminary test was performed with a rapid TLC screening method using the stable DPPH radical. Analytical TLC were developed with appropriate conditions after application of 5 μL of each test compound solution (1 mg/mL), dried, and then sprayed with a DPPH solution (0.2%, MeOH). After 5 min, active compounds appeared as yellow spots against a purple background. The purple stable free radical 2,2-diphenyl-1-picrylhydrazyl was reduced to the yellow colored diphenylpicryl hydrazine. Trolox was used as positive control.
To determine the quantitative antioxidant activity, each of the samples was tested at different concentrations. Blank solutions were prepared with methanol while the negative control was the DPPH solution (0.002%, MeOH). Test sample solution (1 mL) contained DCM extract or isolated compounds, serially diluted in methanol. Absorbance was measured at 517 nm. Percentage antioxidant activity (AA%) values were calculated using the formula:
(Abs sample is the absorbance of the sample, Abs blank is the absorbance of the blank and Abs control is the absorbance of the control).
AA% = 100 − {[(Abs sample − Abs blank) × 100]/Abs control}.
2.5. Acetylcholinesterase Inhibitory Activity
Electric eel (Torpedo californica) AChE was used as source of cholinesterase. AChE inhibitory activity was measured in vitro using the spectrophotometric method developed by Ellman [11], with slight modifications, as previously reported [9]. Enzyme activity was calculated by comparing reaction rates for the samples to the blank. All reactions were performed in triplicate. IC50 values were determined with GraphPad Prism 5. Tacrine (99%) was used as the reference AChE inhibitor.
3. Results and Discussion
3.1. Extraction and Isolation
The DCM extract of the aerial parts of G. ventanensis was subjected to a bioassay-guided fractionation. The active extract was subjected to silica gel chromatographic column and reverse phase HPLC purification, in order to obtain pure bioactive compounds. Six bioactive diterpenoids were isolated and identified as 13-methyl-17-oxo-labda-7,13-diene-15-oic acid (1), 17-hydroxy-13-methyl-labda-7,13-diene-15-oic acid (2), 18-acetoxy-17- hydroxy cativic acid (3), 17-acetoxycativic acid (4), 17-hydroxy cativic acid (5) and grindelic acid (6) (Figure 1). The compounds were identified using 1D and 2D-NMR and mass spectrometry and by comparison with literature data [12].
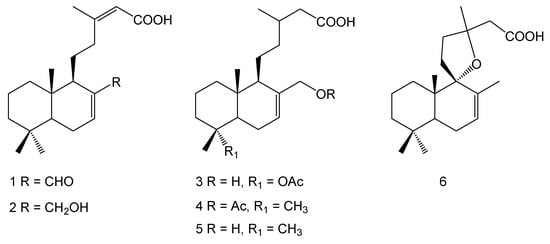
Figure 1.
Chemical structures.
3.2. Antioxidante Activity
The antioxidant activity was evaluated using the reduction of the stable radical 2,2 diphenyl-1-picrylhydrazyl (DPPH). The DCM extract obtained from aerial parts of G. ventanensis showed a good antioxidant activity (IC50 = 27.03 ± 0,28 μg/mL). Compounds 1 and 2 were found to be 4.29 and 3.54 times more active, respectively, than Trolox, the antioxidant reference compound (Table 1). The presence of these metabolites, together with others of an activity similar to trolox (3–6), could explain the free radical-scavenging activity observed for this extract.

Table 1.
IC50 values for DPPH and AChE of extracts and isolated compounds obtained from G. ventanensis.
3.3. Acetylcholinesterase Inhibitory Activity
The AChE inhibition was determined spectrophotometrically using Ellman’s method, with tacrine as the reference inhibitor [11]. The DCM extract showed good AChE inhibition (IC50 = 121.9 µg/mL). The AChE inhibitory activity of compounds 1–4 and 6 was evaluated and compared to that of the natural diterpenoid 17-hydroxycativic acid (5), as previously reported [9]. The results show that most of these compounds are better AChE inhibitors than diterpenoid 5 (Table 1). Compounds 1 and 2, showed the most effective AChE inhibition with IC50 values of 11.04 and 18.12 µM, respectively. Compound 6 showed AChE inhibition comparable to that of compound 5, while compounds 3 and 4 presented higher IC50 values, eliciting weaker inhibition (Table 1).
The presence of these active metabolites could explain the AChE inhibition observed for the DCM extract of G. ventanensis.
4. Conclusions
A previous study by our group had shown that Grindelia ventanensis is an interesting source of natural bioactive compounds and can lead to the development of new derivatives with AChE inhibition and other valuable properties that could be relevant in the treatment of various neurodegenerative conditions of the CNS, including Parkinson’s and AD [9,13]. In the current study, the DCM extract obtained from the aerial part of G. ventanensis was selected, due to its cholinesterase inhibition and antioxidant activity, to be submitted to a bioassay-guided fractionation. This strategy led to the isolation and identification of its active components, that were identified as promising candidates to develop potent new AChE inhibitors as well as antioxidants agents.
Multiple factors are involved in the development of neurodegenerative disease. Phytochemicals that have antioxidant as well as AChE inhibitory activity have been considered to be safer therapeutic candidates for treating AD. Antioxidant therapy has proven successful for improving cognitive function and behavioral deficits in patients with mild-to-moderate AD [14].
Our results suggest that this plant and/or its diperpenoids could lead to the development of new anti-Alzheimer agents, although further evaluation to assess safety and bioavailability using in vivo animal models is required.
Author Contributions
Conceptualization, S.A.R. and A.P.M.; methodology, S.A.R., M.V. and E.C.C.; software, S.A.R.; validation, S.A.R., M.V., E.C.C. and A.P.M.; formal analysis, S.A.R.; investigation, S.A.R.; re-sources, S.A.R.; data curation, S.A.R.; writing—original draft preparation, S.A.R.; writing—review and editing, S.A.R.; visualization, S.A.R.; supervision, S.A.R. and A.P.M.; project administration, S.A.R. and A.P.M.; funding acquisition, A.P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Research Council of Argentina (CONICET), Universidad Nacional del Sur (Argentina), PIP CODE 11220200100834CO.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Selkoe, D. Preventing Alzheimer’s disease. Science 2012, 337, 1488–1492. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Shen, X.; Yu, H.; Sun, L.; Lin, W.; Zhang, C. Water-soluble ginseng oligosaccharides protect against scopolamine-induced cognitive impairment by functioning as an antineuroinflammatory agent. J. Ginseng. Res. 2016, 40, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cai, P.; Liu, Q.; Xu, D.; Yang, X.; Wu, J.; Kong, L.; Wang, X. Rational modification of donepezil as multifunctional acetylcholinesterase inhibitors for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2016, 123, 282–297. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Dou, J.; Wu, T.; Aisa, H. Investigating the antioxidant and acetylcholinesterase inhibition activities of Gossypium herbaceam. Molecules 2013, 18, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Tong, T.; Wan, S.; Yan, T.; Ren, F.; Bi, K.; Jia, Y. Protective effects of puerarin against Ab 1-42-Induced Learning and Memory Impairments in Mice. Planta Med. 2017, 83, 224–231. [Google Scholar] [PubMed]
- Zuloaga, F.; Morrone, O.; Belgrano, M.; Marticorena, C.; Marchesi, E. (Eds.) Catálogo de las Plantas Vasculares del Cono Sur (Argentina, Sur de Brasil, Chile, Paraguay y Uruguay). In Monographs in Systematic Botany from the Missouri Botanical Garden; Missouri Botanical Garden Press: St. Louis, MO, USA, 1323; Volume 107, pp. 1323–1326. [Google Scholar]
- Hung, T.; Luan, T.; Vinh, B.; Cuong, T.; Min, B. Labdane-type diterpenoids from Leonurus heterophyllus and their cholinesterase inhibitory activity. Phytother. Res. 2011, 25, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Selenge, E.; Oikawa, S.; Ageishi, K.; Batkhuu, J.; Sasaki, K.; Yoshizaki, F. Cholinesterase-inhibitory diterpenoids and chemical constituents from aerial parts of Caryopteris mongolica. J. Nat. Med. 2015, 69, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Alza, N.; Richmond, V.; Baier, C.; Freire, E.; Baggio, R.; Murray, A.P. Synthesis and cholinesterase inhibition of cativic acid derivatives. Bioorg. Med. Chem. 2014, 22, 3838–3849. [Google Scholar] [CrossRef] [PubMed]
- Bors, W.; Saran, M.; Eltsner, E. Modern Methods Plant Analysis. New Ser. 1992, 13, 277–295. [Google Scholar]
- Ellman, G.; Courtney, K.; Andres, V.; Featherstone, R. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, A.G.; de Oliveira, P.M.; Piló-Veloso, D.; de Carvalho Alcântara, A.F. 13C-NMR Data of Diterpenes Isolated from Aristolochia Species. Molecules 2009, 14, 1245–1262. [Google Scholar] [CrossRef] [PubMed]
- Alza, N.; Murray, A.; Salvador, G. Cativic acid-caffeic acid hybrid exerts cytotoxic effects and induces apoptotic death in human neuroblastoma cells. Naunyn Schmiedebergs Arch. Pharmacol. 2017, 390, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, D.; Baek, J.; Jung, E.; Baek, Y.; Lee, I.; Jang, T.; Kang, K.; Kim, K. In Vitro assessment of selected Korean plants for antioxidant and antiacetylcholinesterase activities. Pharm. Biol. 2017, 55, 2205–2210. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).