Ionogels: Polimeric and Sol-Gel Silica Nanoscaffolds of Ionic Liquids as Smart Materials †
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Gel Preparation
- Firstly, TMOS and DMDMS with a molar rate 0.65 to 0.35 are mixed and stirred for 10 min.
- Secondly, [BMPyrr][TFSI] at a 0.8 molar rate is added to the previous mixture and stirred for an additional 10 min.
- Lastly, formic acid in a molar proportion of 3.3 is added, left in agitation for 2 min and finally, introduced in a Teflon mold.
- Mix 8% PVDF in mass with 92% DMF and stir for 3 h.
- Next, considering that PVDF is the hosting matrix (and DMF is only a solute to trigger the gelation process), the mass of PVDF vs IL is adjusted again, in mass proportions of 13% of PVDF and 87% of IL, and then mixed for 3 min and kept in a Teflon mold.
2.3. Experimental Procedure
3. Results
4. Conclusions
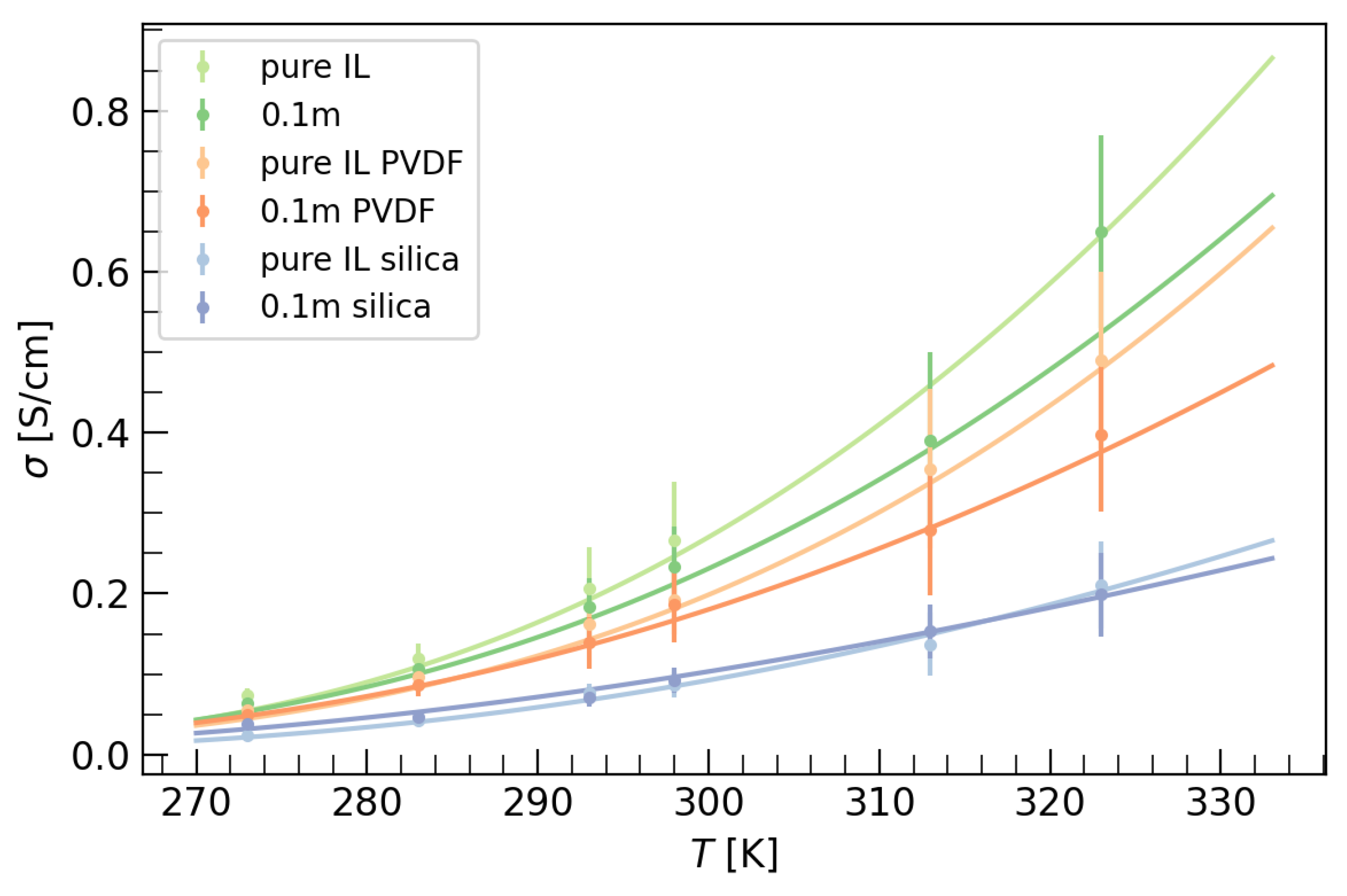
- The mobility of ions becomes greater when the temperature increases.
- An enhancement of ionic conductivity is observed with an increase of temperature, which could be justified by a reduction of viscosity.
- Conductivity decreases with the addition of salt due to an increase of viscosity, and also decreases with the nanoconfinement of the IL in the matrix scaffolds, probably due to electrostatic interactions between IL and the nano-scaffold.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arbabzadeh, M.; Sioshansi, R.; Johnson, J.X.; Keoleian, G.A. The Role of Energy Storage in Deep Decarbonization of Electricity Production. Nat. Commun. 2019, 10, 3413. [Google Scholar] [CrossRef] [PubMed]
- Menne, S.; Pires, J.; Anouti, M.; Balducci, A. Protic Ionic Liquids as Electrolytes for Lithium-Ion Batteries. Electrochem. Commun. 2013, 31, 39–41. [Google Scholar] [CrossRef]
- Liew, C.W.; Ramesh, S.; Ramesh, K.; Arof, A.K. Preparation and Characterization of Lithium Ion Conducting Ionic Liquid-Based Biodegradable Corn Starch Polymer Electrolytes. J. Solid State Electrochem. 2012, 16, 1869–1875. [Google Scholar] [CrossRef]
- Russina, O.; Caminiti, R.; Méndez-Morales, T.; Carrete, J.; Cabeza, O.; Gallego, L.J.; Varela, L.M.; Triolo, A. How Does Lithium Nitrate Dissolve in a Protic Ionic Liquid? J. Mol. Liq. 2015, 205, 16–21. [Google Scholar] [CrossRef]
- Brachet, M.; Gaboriau, D.; Gentile, P.; Fantini, S.; Bidan, G.; Sadki, S.; Brousse, T.; le Bideau, J. Solder-Reflow Resistant Solid-State Micro-Supercapacitors Based on Ionogels. J. Mater. Chem. A Mater. 2016, 4, 11835–11843. [Google Scholar] [CrossRef]
- le Bideau, J.; Viau, L.; Vioux, A. Ionogels, Ionic Liquid Based Hybrid Materials. Chem. Soc. Rev. 2011, 40, 907–925. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, S.; Kumbharkhane, A.; Chaudhari, A. Theoretical and Experimental Aspects of Time Domain Permittivity Spectroscopy. In Binary Polar Liquids; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–43. [Google Scholar]
- Vadhva, P.; Hu, J.; Johnson, M.J.; Stocker, R.; Braglia, M.; Brett, D.J.L.; Rettie, A.J.E. Electrochemical Impedance Spectroscopy for All-Solid-State Batteries: Theory, Methods and Future Outlook. ChemElectroChem 2021, 8, 1930–1947. [Google Scholar]
- Rana, V.A.; Barot, D.K.; Vankar, H.P.; Pandit, T.R.; Karakthala, J.B. AC/DC Conductivity and Dielectric Relaxation Behavior of Ionic Solutions of 1-Butyl-3-Methylimidazolium Chloride in Methanol. J. Mol. Liq. 2019, 296, 111804. [Google Scholar] [CrossRef]
- Kremer, F.; Schönhals, A. (Eds.) Broadband Dielectric Spectroscopy; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2003; ISBN 978-3-642-62809-2. [Google Scholar]
- Vallet, P.; Bouzón-Capelo, S.; Méndez-Morales, T.; Gómez-González, V.; Arosa, Y.; de la Fuente, R.; López-Lago, E.; Rodríguez, J.R.; Gallego, L.J.; Parajó, J.J.; et al. On the Physical Properties of Mixtures of Nitrate Salts and Protic Ionic Liquids. J. Mol. Liq. 2022, 350, 118483. [Google Scholar] [CrossRef]
- Tu, W.; Richert, R.; Adrjanowicz, K. Dynamics of Pyrrolidinium-Based Ionic Liquids under Confinement. I. Analysis of Dielectric Permittivity. J. Phys. Chem. C 2020, 124, 5389–5394. [Google Scholar] [CrossRef]
- Kim, J.-K.; Lim, D.-H.; Scheers, J.; Pitawala, J.; Wilken, S.; Johansson, P.; Ahn, J.-H.; Matic, A.; Jacobsson, P. Properties of N-Butyl-N-Methyl-Pyrrolidinium Bis(Trifluoromethanesulfonyl) Imide Based Electrolytes as a Function of Lithium Bis(Trifluoromethanesulfonyl) Imide Doping. J. Korean Electrochem. Soc. 2011, 14, 92–97. [Google Scholar] [CrossRef]
- Noor, S.A.M.; Bayley, P.M.; Forsyth, M.; MacFarlane, D.R. Ionogels Based on Ionic Liquids as Potential Highly Conductive Solid State Electrolytes. Electrochim. Acta 2013, 91, 219–226. [Google Scholar] [CrossRef]

| Name | Molecular Mass (g∙mol−1) | Short Name | CAS Number | Purity Supplier |
|---|---|---|---|---|
| Ionic liquid: 1-butyl-1methyl-pyrrolidinium bis(trifluoromethanesulfonyl)imide | 422.41 | [BMPyrr][TFSI] | 223437-11-4 | >99% IoLiTec |
| Salt: Lithium bis(trifluoromethanesulfonyl)imide | 287.09 | [Li][TFSI] | 90076-65-6 | >99% Sigma Aldrich |
| Gel Precursor: Poly(vinylidene fluoride) | Mw ∼ 534,000 | PVDF | 24937-79-9 | Alfa Aesar |
| Gel Precursor: Tetramethyl orthosilicate | 152.22 | TMOS | 681-84-5 | 98% Sigma Aldrich |
| Gel Precursor: Dimethoxydimethylsilane | 120.22 | DMDMS | 1112-39-6 | 98% Sigma Aldrich |
| T [°C] | Pure IL | 0.1 m | Pure PVDF | 0.1 m PVDF | Pure Silica | 0.1 m Silica |
|---|---|---|---|---|---|---|
| 0 | 0.0735(90) | 0.0642(63) | 0.0546(51) | 0.0495(59) | 0.0240(22) | 0.0388(37) |
| 10 | 0.0119(90) | 0.106(13) | 0.096(13) | 0.086(13) | 0.0425(51) | 0.0473(53) |
| 20 | 0.207(51) | 0.183(36) | 0.162(26) | 0.139(32) | 0.076(12) | 0.071(11) |
| 25 | 0.266(73) | 0.233(51) | 0.192(43) | 0.186(47) | 0.087(16) | 0.092(16) |
| 40 | 0.39(11) | 0.355(99) | 0.279(81) | 0.137(39) | 0.153(34) | |
| 50 | 0.65(12) | 0.49(11) | 0.397(95) | 0.211(54) | 0.199(52) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago, A.; Vallet, P.; Parajó, J.J.; Villanueva, M.; Cabeza, Ó.; Varela, L.M.; Salgado, J. Ionogels: Polimeric and Sol-Gel Silica Nanoscaffolds of Ionic Liquids as Smart Materials. Chem. Proc. 2022, 12, 70. https://doi.org/10.3390/ecsoc-26-13686
Santiago A, Vallet P, Parajó JJ, Villanueva M, Cabeza Ó, Varela LM, Salgado J. Ionogels: Polimeric and Sol-Gel Silica Nanoscaffolds of Ionic Liquids as Smart Materials. Chemistry Proceedings. 2022; 12(1):70. https://doi.org/10.3390/ecsoc-26-13686
Chicago/Turabian StyleSantiago, Antía, Pablo Vallet, Juan José Parajó, María Villanueva, Óscar Cabeza, Luis Miguel Varela, and Josefa Salgado. 2022. "Ionogels: Polimeric and Sol-Gel Silica Nanoscaffolds of Ionic Liquids as Smart Materials" Chemistry Proceedings 12, no. 1: 70. https://doi.org/10.3390/ecsoc-26-13686
APA StyleSantiago, A., Vallet, P., Parajó, J. J., Villanueva, M., Cabeza, Ó., Varela, L. M., & Salgado, J. (2022). Ionogels: Polimeric and Sol-Gel Silica Nanoscaffolds of Ionic Liquids as Smart Materials. Chemistry Proceedings, 12(1), 70. https://doi.org/10.3390/ecsoc-26-13686






