The Antiviral Activity of Trifluoromethylthiolane Derivatives †
Abstract
1. Introduction
2. Materials and Methods
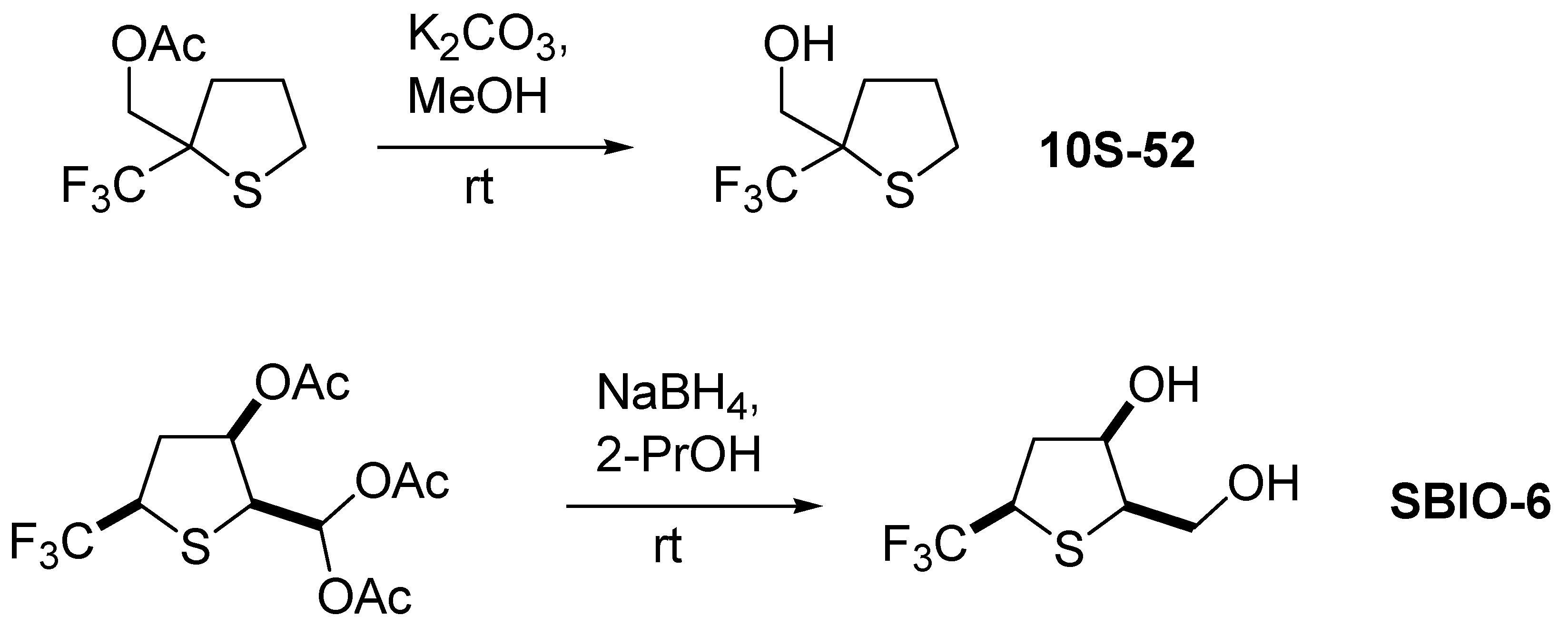
2.1. Structure of the Compounds
2.2. Virus and Cells
2.3. Cytotoxicity Assays
2.4. Antiviral Assay
2.5. Statistical Analysis
3. Results and Discussion
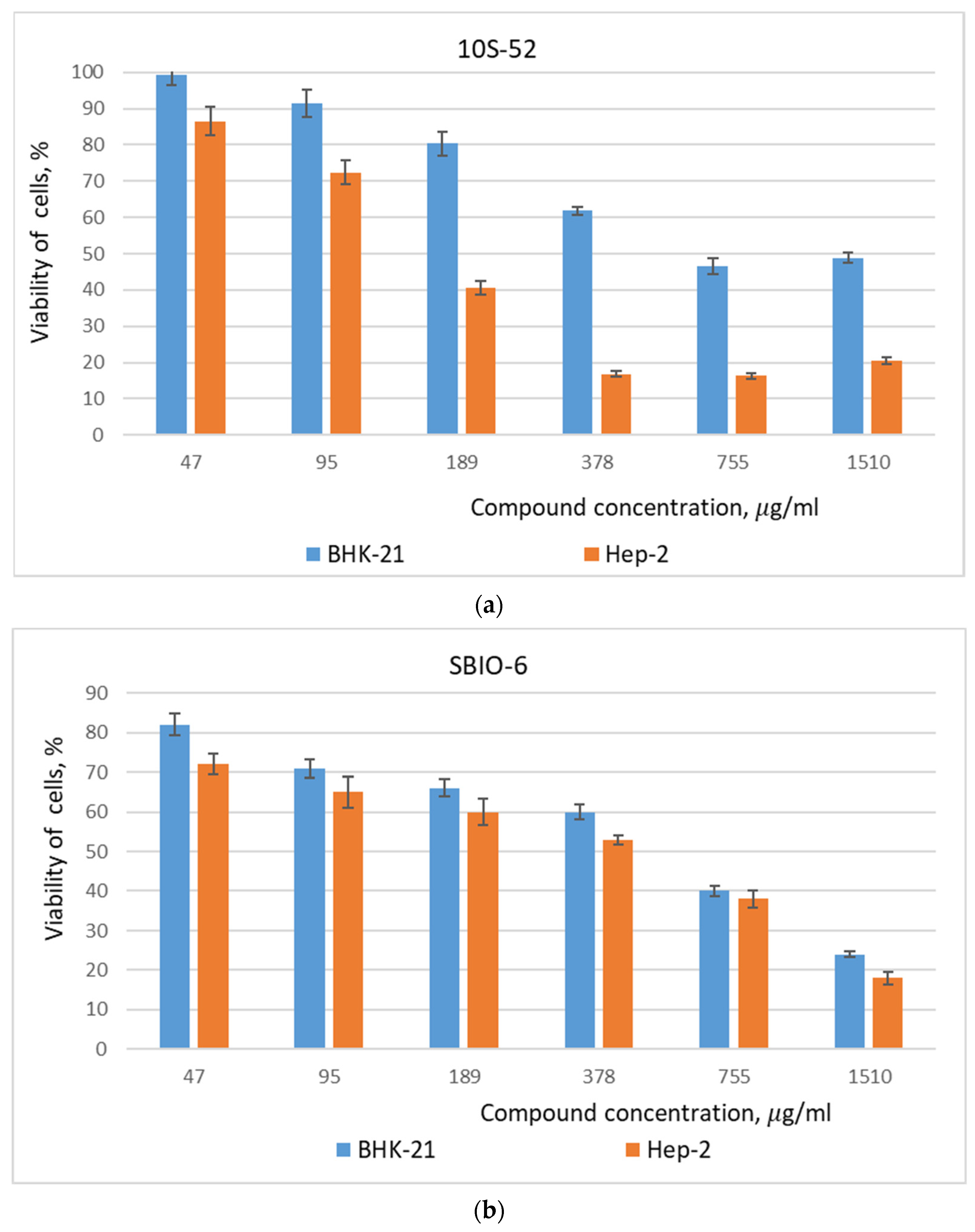
3.1. Cell Viability
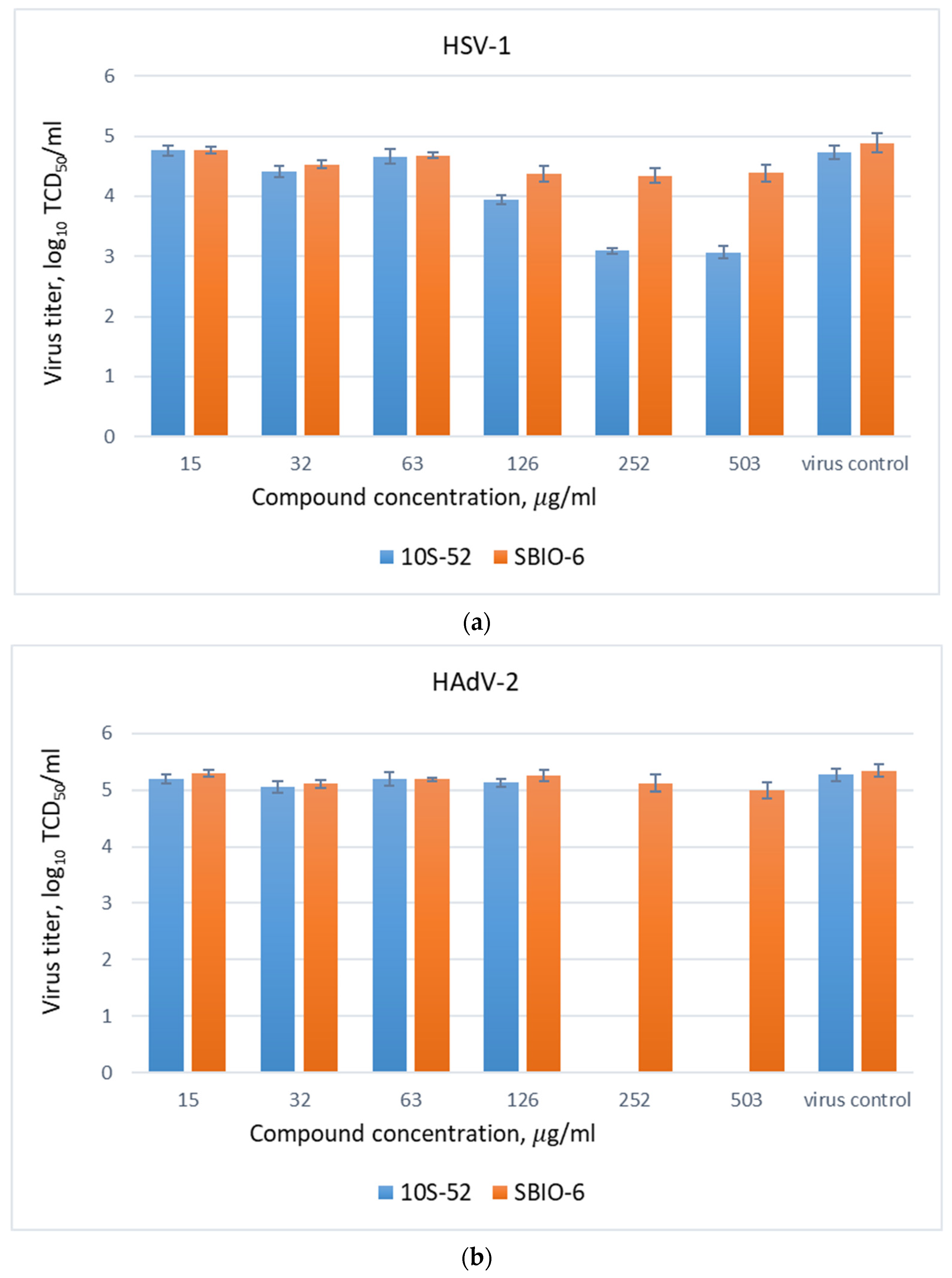
3.2. Influence of Trifluoromethylthiolane Derivatives on Human Viruses’ Reproduction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nickbakhsh, S.; Mair, C.; Matthews, L.; Reeve, R.; Johnson, P.; Thorburn, F. Virus-virus interactions impact the population dynamics of influenza and the common cold. Proc. Natl. Acad. Sci. USA 2019, 116, 27142–27150. [Google Scholar] [CrossRef]
- Bernstein, D.I.; Bellamy, A.R.; Hook, E.W.; Levin, M.J.; Wald, A.; Ewell, M.G. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013, 56, 344–351. [Google Scholar] [CrossRef]
- Kaufman, H.E. Adenovirus advances: New diagnostic and therapeutic options. Curr. Opin. Ophthalmol. 2011, 22, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Meyding-Lamadé, U.; Strank, C. Herpesvirus infections of the central nervous system in immunocompromised patients. Ther. Adv. Neurol. Disord. 2012, 5, 279–296. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E.; Li, G. Approved Antiviral Drugs over the Past 50 Years. Clin. Microbiol. Rev 2016, 29, 695–747. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, D.M.; Castillo, E.; Duarte, L.F.; Arriagada, J.; Corrales, N.; Farías, M.A. Current Antivirals and Novel Botanical Molecules Interfering With Herpes Simplex Virus Infection. Front. Microbiol. 2020, 11, 139. [Google Scholar] [CrossRef]
- Saha, B.; Parks, R.J. Recent Advances in Novel Antiviral Therapies against Human Adenovirus. Microorganisms 2020, 8, 1284. [Google Scholar] [CrossRef]
- Dodge, M.J.; MacNeil, K.M.; Tessier, T.M.; Weinberg, J.B.; Mymryk, J.S. Emerging antiviral therapeutics for human adenovirus infection: Recent developments and novel strategies. Antivir. Res. 2021, 188, 105034. [Google Scholar] [CrossRef]
- Seley-Radtke, K.L.; Yates, M.K. The evolution of nucleoside analogue antivirals: A review for chemists and non-chemists. Part 1: Early structural modifications to the nucleoside scaffold. Antivir. Res. 2018, 154, 66–86. [Google Scholar] [CrossRef]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.J.; Meanwell, N.A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef]
- Liu, P.; Sharon, A.; Chu, C.K. Fluorinated nucleosides: Synthesis and biological implication. J. Fluor. Chem. 2008, 129, 743–766. [Google Scholar] [CrossRef]
- Derudas, M.; Meneghesso, S. The role of fluorine in antiviral drug discovery. Future Science Ltd.: London, UK, 2015. [CrossRef]
- Zheng, F.; Qiu, X.-L.; Qing, F.-L. Synthesis and Antiviral, Antitumour Activities of Fluorinated Sugar Nucleosides. Mol. Med. Med. Chem. 2012, 6, 241–297. [Google Scholar] [CrossRef]
- Sofia, M.J.; Bao, D.; Chang, W. Discovery of β-D-2′-deoxy-2′-α-fluoro-2′-β-C-methyluridine nucleotide prodrug (PSI-7977) for the treatment of hepatitis C virus. J. Med. Chem. 2010, 53, 7202–7218. [Google Scholar] [CrossRef] [PubMed]
- Wojtowicz-Rajchel, H. Synthesis and applications of fluorinated nucleoside analogues. J Fluor. Chem 2012, 143, 11–48. [Google Scholar] [CrossRef]
- Siry, S.A.; Timoshenko, V.M.; Vlasenko, Y.G.; Baranova, G.V.; Zagorodnya, S.D.; Nesterova, N.V. Pummerer Reactions of Thiopyran Derivatives as a Method for the Preparation of Trifluoro-Methyl-Substituted Thiolanes with Antiviral Activity. Chem. Heterocycl. Compd. 2014, 50, 467–478. [Google Scholar] [CrossRef]
- Siry, S.A.; Timoshenko Rusanov, E.B.; Schermolovich, Y.G. Synthesis and oxidative transformations of 2-functionalized 2-trifluoromethyltetrahydrothiophenes. J. Fluor. Chem. 2022, 261–262, 109999. [Google Scholar] [CrossRef]
- Satyavani, K.; Gurudeeban, S.; Ramanathan, T.; Balasubramanian, T. Toxicity Study of Silver Nanoparticles Synthesized from Suaeda monoica on Hep-2 Cell Line. Avicenna J. Med. Biotechnol. 2012, 4, 35–39. [Google Scholar]
- Salem, S.S.; EL-Belely, E.F.; Niedbała, G.; Alnoman, M.M.; Hassan, S.E.-D.; Eid, A.M. Bactericidal and In-Vitro Cytotoxic Efficacy of Silver Nanoparticles (Ag-NPs) Fabricated by Endophytic Actinomycetes and Their Use as Coating for the Textile Fabrics. Nanomaterials 2020, 10, 2082. [Google Scholar] [CrossRef]
- Cheng, H.; Lin, T.; Yang, C.; Wang, K.; Lin, L.; Li, C. Putranjivain A from Euphorbia jolkini inhibits both virus entry and late stage replication of herpes simplex virus type 2 in vitro. J. Antimicrob. Chem. 2014, 53, 577–583. [Google Scholar] [CrossRef]
- Hu, R.L.; Li, S.R.; Kong, F.J.; Hou, R.J.; Guan, X.L.; Guo, F. Inhibition effect of silver nanoparticles on herpes simplex virus 2. Genet. Mol. Res. 2014, 13, 7022–7028. [Google Scholar] [CrossRef]
- Mloston, G.; Kowalczyk, M.; Celeda, M.; Jasinski, M.; Denel-Bobrowska, M.; Olejniczak, A.B. Fluorinated Analogues of Lepidilines A and C: Synthesis and Screening of Their Anticancer and Antiviral Activity. Molecules 2022, 27, 3524. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, A.; Probst, K.C.; Westwell, A.D.; Slusarczyk, M. Fluorinated nucleosides as an important class of anticancer and antiviral agents. Future Med. Chem. 2017, 9, 1809–1833. [Google Scholar] [CrossRef] [PubMed]
- Stuyver, L.J.; McBrayer, T.R.; Whitaker, T. Inhibition of the subgenomic hepatitis C virus replicon in huh-7 cells by 2′-deoxy-2′-fluorocytidine. Antimicrob Agents Chemother 2004, 48, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.K.; Ma, T.; Shanmuganathan, K. Use of 2′-fluoro-5-methylbeta-L-arabinofuranosyluracil as a novel antiviral agent for hepatitis B virus and Epstein–Barr virus. Antimicrob Agents Chemother 1995, 39, 979–981. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, S.J.; Otto, M.J.; Schinazi, R.F. Comparative pharmacokinetics of Racivir, (+/−)-β-2′,3′-dideoxy-5-fluoro-3′- thiacytidine in rats, rabbits, dogs, monkeys and HIV-infected humans. Antivir. Chem. Chemother. 2005, 16, 117–127. [Google Scholar] [CrossRef]
- Park, B.; Kitteringham, N.; O’Neill, P. Metabolism of fluorine-containing drugs. Annu. Rev. Pharm. Toxicol. 2001, 41, 443–470. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artiukh, L.; Povnitsa, O.; Shermolovich, Y.; Siry, S.; Zahorodnia, S. The Antiviral Activity of Trifluoromethylthiolane Derivatives. Chem. Proc. 2022, 12, 41. https://doi.org/10.3390/ecsoc-26-13643
Artiukh L, Povnitsa O, Shermolovich Y, Siry S, Zahorodnia S. The Antiviral Activity of Trifluoromethylthiolane Derivatives. Chemistry Proceedings. 2022; 12(1):41. https://doi.org/10.3390/ecsoc-26-13643
Chicago/Turabian StyleArtiukh, Liubov, Olga Povnitsa, Yuriy Shermolovich, Sergiy Siry, and Svitlana Zahorodnia. 2022. "The Antiviral Activity of Trifluoromethylthiolane Derivatives" Chemistry Proceedings 12, no. 1: 41. https://doi.org/10.3390/ecsoc-26-13643
APA StyleArtiukh, L., Povnitsa, O., Shermolovich, Y., Siry, S., & Zahorodnia, S. (2022). The Antiviral Activity of Trifluoromethylthiolane Derivatives. Chemistry Proceedings, 12(1), 41. https://doi.org/10.3390/ecsoc-26-13643





