Abstract
Treatment of 4a-acetyl-8a-hydroxydecahydroquinazoline-2-thione with NaH in acetonitrile leads to its isomerization into 1-hydroxy-1-methyl-3-thioxo-2,4-diazaspiro[5.5]undecan-7-one followed by the C1-C6 bond cleavage to give N-acetyl-N’-[(2-oxocyclohexyl)methyl]thiourea. The starting compound as a single diastereomer was prepared by the reaction between the K-enolate of 2-acetylcyclohexanone and N-(tosylmethyl)thiourea or N-(azidomethyl)thiourea.
1. Introduction
Macroheterocycles are the objects of intensive research in modern organic chemistry [1,2,3]. We have recently become interested in the synthesis of macrocyclic ureas and thioureas 1 (Scheme 1), which until now have been practically unknown. We assumed that these heterocycles can be obtained using pyrimidine ring expansion by cleavage of the zero-bridge in 4,5-polymethylenehexahydropyrimidin-2-ones(thiones) 2. Obviously, such cleavage will proceed especially smoothly if the carbon atoms forming this bridge contain a donor and acceptor substituent.
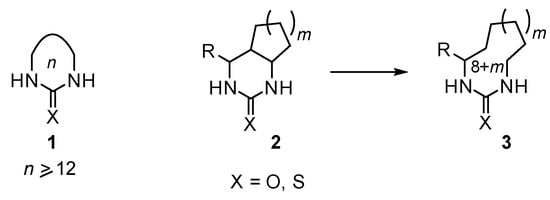
Scheme 1.
Synthesis of macrocyclic ureas and thioureas 3 using pyrimidine ring expansion in 4,5-polymethylenehexahydropyrimidin-2-ones(thiones) 2.
Previously, we developed a general convenient approach to 5-acyl-substituted 4-hydroxyhexahydropyrimidin-2-ones(thiones) 4 based on (thio)ureidoalkylation of enolates of 1,3-dicarbonyl compounds [4,5,6,7] (Scheme 2). The structural feature of pyrimidines 4 is the presence of an acceptor acyl group at the carbon C5 and a donor OH group at the carbon C4, which suggests a high tendency for the C4-C5 bond to break. Indeed, we found that, in the presence of a strong base (KOH, NaH, NaOH, etc.) in an aprotic solvent, these compounds undergo a rearrangement proceeding with the cleavage of the C4-C5 bond to give N-acyl-substituted N’-(γ-oxoalkyl)ureas and -thioureas [8,9] (Scheme 2).
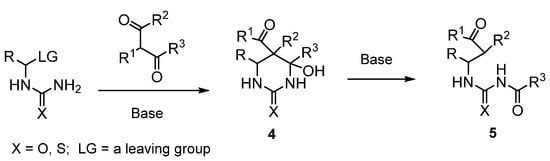
Scheme 2.
Synthesis of 5-acyl-substituted 4-hydroxyhexahydropyrimidin-2-ones(thiones) 4 and base-promoted C4-C5 bond cleavage in 4 to give (thio)ureides 5.
We suggested that such a rearrangement could be used in the synthesis of functionally substituted macrocyclic ureas and thioureas. Indeed, the formation of these compounds should have been expected during the rearrangement of bicyclic pyrimidines 5 with R2 + R3 = (CH2)n or R1 + R3 = (CH2)n, etc.
Here, we report the reaction of N-(tosylmethyl)- and N-(azidomethyl)thiourea with 2-acetylcyclohexanone in the presence of KOH to afford the expected bicyclic 4a-acetyl-8a-hydroxydecahydroquinazoline-2-thione. We also describe a base-promoted cascade transformation of this compound to give N-acetyl-N’-[(2-oxocyclohexyl)methyl]- thiourea instead of the expected 10-membered cyclic thioureide.
2. Results and Discussion
Readily available (azidomethyl)thiourea (6a) and (tosylmethyl)thiourea (6b) served as a starting thioureidomethylation reagents. We found that thiourea 6a reacts smoothly with the K-enolate of 2-acetylcyclohexanone (EtOH, room temperature, 6.35 h) generated by the reaction of the corresponding CH-acid 7 with an equivalent amount of KOH to give a chromatographically pure product in 79% yield with the empirical formula of C10H16N2O2S. We also prepared the same compound in 72% yield by reacting thiourea 6b with the K-enolate of 2-acetylcyclohexanone in EtOH. The structure of the obtained product established by 1D and 2D NMR spectroscopy corresponds to 4а-acetyl- 8а-hydroxydecahydroquinazolin-2-thione (8) (Scheme 3). Another possible product of the above reaction, 1-hydroxy-1-methyl-3-thioxo-2,4-diazaspiro[5.5]undecan-7-one (9), is not formed.
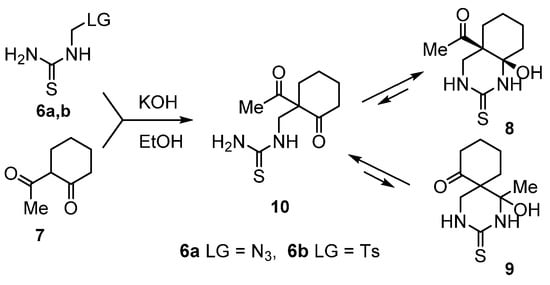
Scheme 3.
Synthesis of 4а-acetyl-8а-hydroxydecahydroquinazolin-2-thione (8).
It should be noted that compound 8 is formed as a single diastereomer, structure of which was determined using 1Н,1Н-NOESY. This diastereomer has (4aR*,8aR*)-configuration and conformation with the axial orientation of the hydroxyl group and the equatorial orientation of the acetyl substituent.
We expected that quinazolin-2-thione 8 in the presence of a strong base would give 10-membered cyclic thioureide 11 as a result of cleavage of the zero-bridge (Scheme 4). We found that treatment of compound 8 with NaH (1.5 equivalents) in dry MeCN at room temperature for 5 h 40 min resulted in a new substance, which was isolated from the reaction mixture in an 88% yield.
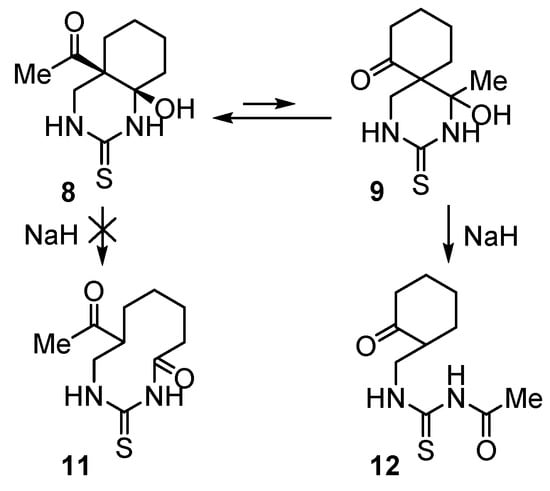
Scheme 4.
Base-promoted cascade transformation of 4а-acetyl-8а-hydroxydecahydroquinazolin- 2-thione (8) into N-acetyl-N’-[(2-oxocyclohexyl)methyl]thiourea (12).
However, to our surprise, the structure of the obtained product established by 1D and 2D NMR spectroscopy corresponded to N-acetyl-N’-[(2-oxocyclohexyl)methyl]- thiourea (12), and not the expected thioureide 11 (Scheme 4).
An independent confirmation of the structure of the obtained product followed from the result of its alkaline hydrolysis (Scheme 5).
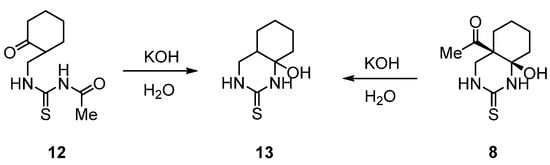
Scheme 5.
Transformation of thioureide 12 and quinazolin-2-thione 8 into compound 13 under the action of aqueous KOH.
Indeed, when thioureide 12 was treated with aqueous KOH at room temperature, bicyclic hydroxypyrimidine 13 was obtained in 64% yield. The formation of this compound is explained by deacetylation of 12, followed by cyclization of the resulting intermediate into 13. The latter compound was also obtained from pyrimidine 8 under similar conditions (aqueous KOH, room temperature). This transformation apparently proceeds through the formation of the acyclic isomeric form 10, followed by the removal of the acetyl group via Claisen retro-condensation, and further recyclization.
The transformation of quinazoline 8 into thioureide 12 was also observed under other reaction conditions, but with lower yields. For example, treatment of 8 with 3 equivalents of NaH at room temperature gave compound 12 in a 56% yield. The reaction of 8 with NaH (1.51 equivalents) in dry tetrahydrofuran (THF) or with 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (3 equivalents) in MeCN resulted in thioureide 12 in 79% and 56% yield, respectively.
Thus, treatment of quinazolin-2-thione 8 with a strong base in an aprotic solvent leads to its rapid isomerization into 1-hydroxy-1-methyl-3-thioxo-2,4-diazaspiro[5.5]-undecan-7-one (9) followed by the C1-C6 bond cleavage to give the final compound 12. Clearly, under strong basic conditions, the rate of this cascade transformation (8→9→12) is much higher than the rate of cleavage of the zero-bridge in 8 to give 11. It can be explained by the dramatic effect of stereoelectronic factors associated with the relative configuration and conformation of compound 8 compared to compound 9.
Since the preparation of 10-membered cyclic thioureide 11 from quinazolin-2-thione 8 failed due to the rapid isomerization of 8 into 9 under basic conditions, we tested the methodology of macrocyclic (thio)ureas synthesis (see, Introduction) using a symmetric cyclic 1,3-diketone, cyclotetradecane-1,3-dione (14), for pyrimidine ring construction (Scheme 6).
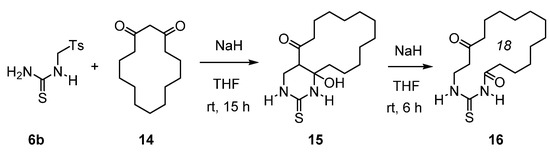
Scheme 6.
Synthesis of 18-membered cyclic thioureide 16.
Thus, the reaction of sulfone 6b with the Na-enolate of 14 in dry THF gave pyrimidine 15 in a 94% yield. Treatment of the latter with NaH (1.50 equivalents) in THF led to 18-membered cyclic thioureide 16 in a 36% isolated yield. Work is in progress to improve the yield of 16 and to synthesize other macrocyclic (thio)ureas.
3. Conclusions
In summary, we have shown that the reaction of N-(tosylmethyl)thiourea and N-(azidomethyl)thiourea with the K-enolate of 2-acetylcyclohexanone in EtOH proceeds exclusively regio- and stereoselectively to give (4aR*,8aR*)-4a-acetyl-8a-hydroxy-decahydroquinazoline-2-thione. Treatment of this compound with NaH in MeCN leads to its isomerization into 1-hydroxy-1-methyl-3-thioxo-2,4-diazaspiro[5.5]undecan-7-one followed by the C1-C6 bond cleavage to form N-acetyl-N’-[(2-oxocyclohexyl)methyl]- thiourea. The expected cleavage of the zero-bridge to form 10-membered cyclic thioureide does not occur due to the effect of stereoelectronic factors. Synthesis of an 18-membered cyclic thioureide was carried out using (tosylmethyl)thiourea and cyclotetradecane-1,3-dione as starting materials.
Author Contributions
Methodology, synthetic investigation, software, writing—original draft preparation, A.D.S.; synthetic investigation, writing—original draft preparation, A.A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Foundation for Basic Research, grant number 20-03-00928.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fitzpatrick, D.W.; Ulrich, H.J. (Eds.) Macrocyclic Chemistry: New Research Developments; Nova Science Publishers, Inc.: New York, NY, USA, 2010. [Google Scholar]
- Hermann, P.; Kotek, J. Ten-membered Rings or Larger with One or More Nitrogen Atoms. In Comprehensive Heterocyclic Chemistry III; Elsevier: Amsterdam, The Netherlands, 2008; Volume 14, pp. 613–665. [Google Scholar]
- Gloe, K. (Ed.) Macrocyclic Chemistry: Current Trends and Future Perspectives; Springer: New York, NY, USA, 2005. [Google Scholar]
- Solovyev, P.A.; Fesenko, A.A.; Shutalev, A.D. A new synthesis of 4- or/and 6-CF3-containing hexahydro- and 1,2,3,4-tetrahydropyrimidin-2-ones. J. Fluor. Chem. 2016, 182, 28–33. [Google Scholar] [CrossRef]
- Shutalev, A.D.; Kuksa, V.A. Utilization of the amidoalkylation reaction in the synthesis of hydrogenated pyrimidine-2-thiones. Chem. Heterocycl. Compd. 1997, 33, 91–95. [Google Scholar] [CrossRef]
- Fesenko, A.A.; Grigoriev, M.S.; Shutalev, A.D. Nucleophile-Mediated Ring Expansion of 5-Acyl-substituted 4-Mesyloxymethyl-1,2,3,4-tetrahydropyrimidin-2-ones in the Synthesis of 7-Membered Analogues of Biginelli Compounds and Related Heterocycles. J. Org. Chem. 2017, 82, 8085–8110. [Google Scholar] [CrossRef] [PubMed]
- Shutalev, A.D.; Kuksa, V.A. Novel method for synthesis of 4-hydroxyhexahydropyrimidine-2-thiones. Chem. Heterocycl. Compd. 1995, 31, 86–91. [Google Scholar] [CrossRef]
- Shutalev, A.D.; Kishko, E.A.; Alekseeva, S.G. Unexpected 5-acetyl-4-hydroxy-4,5-dimethylhexahydropyrimidine-2-thione ring cleavage by the action of bases. Chem. Heterocycl. Compd. 1999, 35, 750–751. [Google Scholar] [CrossRef]
- Shutalev, A.D.; Zhukhlistova, N.E.; Gurskaya, G.V. Acyl C→N migration in the thioureidomethylation of 2-(4-methylbenzoyl)cyclohexanone. Mendeleev Commun. 2004, 14, 31–33. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).