Modified Algar–Flynn–Oyamada Reaction for the Synthesis of 3-Hydroxy-2-styryl-chromen-4-ones under Solvent-Free Conditions †
Abstract
:1. Introduction
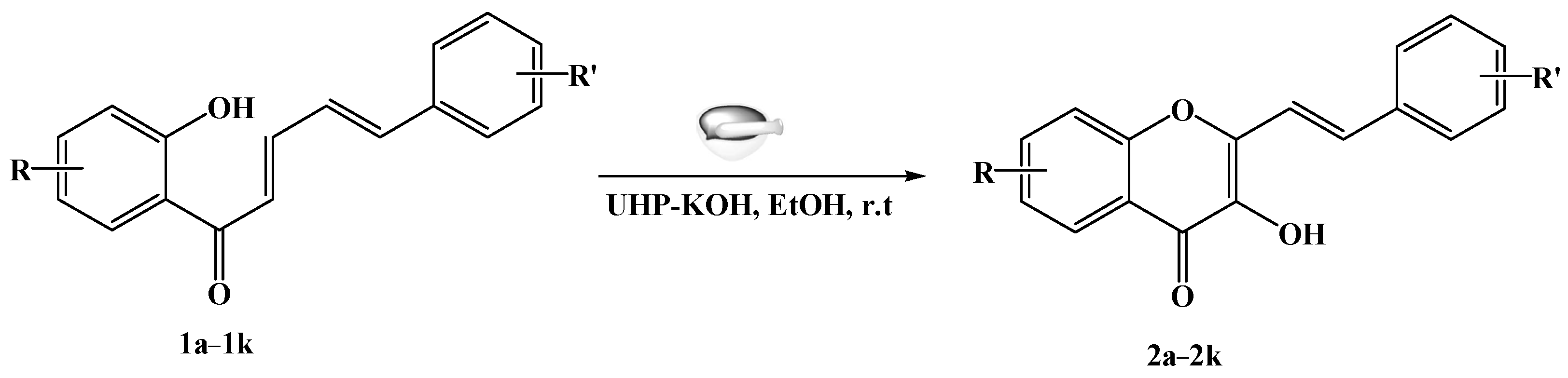
2. Results and Discussion
3. Experimental Section
General Procedure for the Synthesis of 3-Hydroxy-2-styrylchromones 2a–2k
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sugita, Y.; Takao, K.; Uesava, Y.; Nagai, J.; Lijima, Y.; Sano, M.; Sakagami, H. Development of newly synthesized chromone derivatives with high tumor specificity against human oral squamous cell carcinoma. Medicines 2020, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Takao, K.; Endo, S.; Nagai, J.; Kamauchi, H.; Takemura, Y.; Uesawa, Y.; Sugita, Y. 2-Styrylchromone derivatives as potent and selective monoamine oxidase B inhibitors. Bioorg. Chem. 2019, 92, 103285. [Google Scholar] [CrossRef] [PubMed]
- Uesawa, Y.; Nagai, J.; Shi, H.; Sakagami, H.; Bandow, K.; Tomomura, A.; Tomomura, M.; Endo, S.; Takao, K.; Sugita, Y. Quantitative structure–cytotoxicity relationship of 2-styrylchromones. Anticancer Res. 2019, 39, 6489–6498. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Freitas, M.; Fernandes, E.; Lima, J.L.F.C. Biological activities of 2-styrylchromones. Mini Rev. Med. Chem. 2010, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Budagumpi, S.; Pai, R.K.; Balakrishna, R.G. Chromones as a privileged scaffold in drug discovery: A review. Eur. J. Med. Chem. 2014, 78, 340–374. [Google Scholar] [CrossRef]
- Gaspar, A.; Matos, M.J.; Garrido, J.; Uriarte, E.; Borges, F. Chromone: A valid scaffold in medicinal chemistry. Chem. Rev. 2014, 114, 4960–4992. [Google Scholar] [CrossRef]
- Sharma, K.S.; Kumar, S.; Chand, K.; Kathuria, A.; Gupta, A.; Jain, R. An update on natural occurrence and biological activity of chromones. Curr. Med. Chem. 2011, 18, 3825–3852. [Google Scholar] [CrossRef]
- Santos, C.M.M.; Silva, A.M.S. An overview of 2-styrylchromones: Natural occurrence, synthesis, reactivity and biological properties. Eur. J. Org. Chem. 2017, 2017, 3115–3133. [Google Scholar] [CrossRef]
- Gerwick, W.H.; Lopez, A.; Van Duyne, G.D.; Clardy, J.; Ortiz, W.; Baez, A. Hormothamnione, a novel cytotoxic styrylchromone from the marine cyanophyte hormothamnion enteromorphoides grunow. Tetrahedron Lett. 1986, 27, 1979–1982. [Google Scholar] [CrossRef]
- Doria, G.; Romeo, C.; Forgione, A.; Saberze, P.; Tibolla, N.; Corno, M.L.; Cruzzola, G.; Cadelli, G. Synthesis and antiallergic activity of 2-styrylchromones. Eur. J. Med. Chem. Chim. Ther. 1979, 14, 347–351. [Google Scholar]
- Brion, J.D.; Le Baut, G.; Zammattio, F.; Pierre, A.; Atassi, G.; Belachmi, L. New Heterocyclic Compounds: 2-Styryl-4h-1-Benzopyran-4-ones Process for Their Preparation and Pharmaceutical Compositions Containing Them. CA2041163A1, 28 October 1991. [Google Scholar]
- Desideri, N.; Conti, C.; Mastromarino, P.; Mastropaolo, F. Synthesis and Anti-Rhinovirus Activity of 2-Styrylchromones. Antivir. Chem. Chemother. 2000, 11, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, F.; Barros, A.I.R.N.A.; Silva, A.M.S. Interactions of a new 2-styrylchromone with mitochondrial oxidative phosphorylation. J. Biochem. Mol. Toxicol. 2002, 16, 220–226. [Google Scholar] [CrossRef]
- Fernandes, E.; Carvalho, F.; Silva, A.M.S.; Santos, C.M.M.; Pinto, D.C.G.A.; Cavaleiro, J.A.S.; Bastos, M. de L. 2-Styrylchromones As Novel Inhibitors of Xanthine Oxidase. A Structure-activity Study. J. Enzym. Inhib. Med. Chem. 2002, 17, 45–48. [Google Scholar] [CrossRef]
- Shaw, A.Y.; Chang, C.-Y.; Liau, H.-H.; Lu, P.-J.; Chen, H.-L.; Yang, C.-N.; Li, H.-Y. Synthesis of 2-styrylchromones as a novel class of antiproliferative agents targeting carcinoma cells. Eur. J. Med. Chem. 2009, 44, 2552–2562. [Google Scholar] [CrossRef]
- Ono, M.; Maya, Y.; Haratake, M.; Nakayama, M. Synthesis and characterization of styrylchromone derivatives as β-amyloid imaging agents. Bioorganic Med. Chem. 2007, 15, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Fernandes, E.; Silva, A.M.S.; Pinto, D.C.G.A.; Santos, C.M.M.; Cavaleiro, J.A.S.; Lima, J.L.F.C. Anti-inflammatory potential of 2-styrylchromones regarding their interference with arachidonic acid metabolic pathways. Biochem. Pharmacol. 2009, 78, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Schonberg, A.; Mustafa, A.; Aziz, G. Diels-Alder Reaction. II. Experiments with 2-Styrylchromones. On the Nature of the Dimer of 1,3-Diphenylisobenzofuran. J. Am. Chem. Soc. 1954, 76, 4576–4577. [Google Scholar] [CrossRef]
- Mustafa, A.; Ali, M.I. 2-Styrylchromones in the Diene Synthesis. J. Org. Chem. 1956, 21, 849–851. [Google Scholar] [CrossRef]
- Robinson, R.; Shinoda, J. CCLXV.-The synthesis of certain 2-styrylchromonol derivatives. J. Chem. Soc. Trans. 1925, 127, 1973–1980. [Google Scholar] [CrossRef]
- Baker, W. Molecular rearrangement of some o-acyloxyacetophenones and the mechanism of the production of 3-acylchromones. J. Chem. Soc. 1933, 1381–1389. [Google Scholar] [CrossRef]
- Obrecht, D. Acid-Catalyzed Cyclization Reactions of Substituted Acetylenic Ketones: A new Approach for the Synthesis of 3-Halofurans, Flavones, and Styrylchromones. HCA 1989, 72, 447–456. [Google Scholar] [CrossRef]
- Zammattio, F.; Brion, J.D.; Ducrey, P.; Le Baut, G. New route to styrylchromones via [1-(2-hydroxybenzoyl) alkylidene] triphenylphosphoranes. Synthesis 1992, 4, 375–376. [Google Scholar] [CrossRef]
- Helibron, I.M.; Barnes, H.; Morton, R.A. CCXCIV.-Chemical reactivity and conjugation: The reactivity of the 2-methyl group in 2:3-dimethylchromone. J. Chem. Soc., Trans. 1923, 123, 2559–2570. [Google Scholar] [CrossRef]
- Cheema, U.S.; Gulati, K.C.; Venkataraman, K. Synthetical experiments in the chromone group. Part VI. 2-Styrylchromones. J. Chem. Soc. 1932, 925–933. [Google Scholar] [CrossRef]
- Desideri, N.; Mastromarino, P.; Conti, C. Synthesis and evaluation of antirhinovirus activity of 3-hydroxy and 3-methoxy 2-styrylchromones. Antivir. Chem. Chemother. 2003, 14, 195–203. [Google Scholar] [CrossRef]
- Gupta, S.C.; Yusuf, M.; Sharma, S.; Saini, A.; Arora, S.; Kamboj, R.C. Phototransformations of some 3-alkoxy-2-styrylchromones: Type II cyclisations of 1,4- and 1,6-biradicals. Tetrahedron 2004, 60, 8445–8454. [Google Scholar] [CrossRef]
- Jain, N.; Gambhir, G.; Krishnamurty, H.G. Synthesis of hormothamnione and 6-desmethoxyhormothamnione. Indian J. Chem. 2001, 40, 278–283. [Google Scholar]
- Conti, C.; Mastromarino, P.; Goldoni, P.; Portalone, G.; Desideri, N. Synthesis and Anti-Rhinovirus Properties of Fluoro-Substituted Flavonoids. Antivir. Chem. Chemother. 2005, 16, 267–276. [Google Scholar] [CrossRef]
- Marini-Bettòlo, G.B. Synthesis of flavonoids. Gazzeta 1942, 72, 201–205. [Google Scholar]
- Dean, F.M.; Podimuang, V. The course of the Algar–Flynn–Oyamada (A.F.O.) reaction. J. Chem. Soc. 1965, 3978–3987. [Google Scholar] [CrossRef]
- Dhoubhadel, S.P.; Tuladhar, S.M.; Tuladhar, S.M.; Wagley, P.P. Synthesis of some 3-methoxyflavones and chromones. Indian J. Chem. Sect. B-Org. Chem. Incl. Med. Chem. 1981, 20, 511–512. [Google Scholar]
- Bennett, M.; Burke, A.J.; O’Sullivan, W.I. Aspects of the Algar-Flynn-Oyamada (AFO) reaction. Tetrahedron 1996, 52, 7163–7178. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Hatua, K. Computational insight of the mechanism of Algar–Flynn–Oyamada (AFO) reaction. RSC Adv. 2014, 4, 18702–18709. [Google Scholar] [CrossRef]
- Seshadri, S.; Trivedi, P. Reactions of Nitrohydroxychalcones. Oxidation by Hydrogen Peroxide in Alkaline Medium. J. Org. Chem. 1960, 25, 841–843. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, S.; Makrandi, J.K. Aqueous-mediated green synthesis of 3-carboxyxoumarins using grinding technique. Green Chem. Lett. Rev. 2015, 8, 21–25. [Google Scholar] [CrossRef]
- Sharma, D.K.; Kumar, S. A facile synthesis of 3-chloro-2-phenyl-4H-chromen-4-ones using grinding technique at room temperature. Bulg. Chem. Commun. 2017, 49, 309–312. [Google Scholar]
- Bose, A.K.; Pednekar, S.; Ganguly, S.N.; Chakraborty, G.; Manhas, M.S. A simplified green chemistry approach to the Biginelli reaction using ‘Grindstone Chemistry’. Tetrahedron Lett. 2004, 45, 8351–8353. [Google Scholar] [CrossRef]
- Sharma, D. An efficient and eco-friendly synthesis of quinolinyl chalcones under solvent-free conditions at room temperature. Res. Chem. Intermed. 2015, 41, 927–933. [Google Scholar] [CrossRef]
- Lu, C.S.; Hughes, E.W.; Giguère, P.A. The Crystal Structure of the Urea-Hydrogen Peroxide Addition Compound CO(NH2)2·H2O2. J. Am. Chem. Soc. 1941, 63, 1507–1513. [Google Scholar] [CrossRef]
| Compound | R | R′ | Time (min) (a + b) | Yield c (%) | Mp d (°C) |
|---|---|---|---|---|---|
| 2a | H | H | 5 + 5 | 92 | 188–190 |
| 2b | H | 4′-OCH3 | 5 + 5 | 92 | 217–220 |
| 2c | H | 4′-Cl | 5 + 5 | 90 | 220–222 |
| 2d | H | 4′-NO2 | 5 + 10 | 88 | 222–225 |
| 2e | 6-Cl | H | 5 + 10 | 90 | 220–222 |
| 2f | 6-Cl | 4′-OCH3 | 5 + 10 | 85 | 218–222 |
| 2g | 6-Cl | 4′-Cl | 5 + 10 | 88 | 220–222 |
| 2h | 6-Cl | 4′-NO2 | 5 + 5 | 90 | 222–225 |
| 2i | 6-F | H | 5 + 10 | 90 | 225–228 |
| 2j | 6-CH3 | H | 5 + 10 | 88 | 229–230 |
| 2k | 5,7-CH3 | H | 5 + 10 | 85 | 194–196 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, D. Modified Algar–Flynn–Oyamada Reaction for the Synthesis of 3-Hydroxy-2-styryl-chromen-4-ones under Solvent-Free Conditions. Chem. Proc. 2022, 12, 27. https://doi.org/10.3390/ecsoc-26-13558
Kumar D. Modified Algar–Flynn–Oyamada Reaction for the Synthesis of 3-Hydroxy-2-styryl-chromen-4-ones under Solvent-Free Conditions. Chemistry Proceedings. 2022; 12(1):27. https://doi.org/10.3390/ecsoc-26-13558
Chicago/Turabian StyleKumar, Dinesh. 2022. "Modified Algar–Flynn–Oyamada Reaction for the Synthesis of 3-Hydroxy-2-styryl-chromen-4-ones under Solvent-Free Conditions" Chemistry Proceedings 12, no. 1: 27. https://doi.org/10.3390/ecsoc-26-13558
APA StyleKumar, D. (2022). Modified Algar–Flynn–Oyamada Reaction for the Synthesis of 3-Hydroxy-2-styryl-chromen-4-ones under Solvent-Free Conditions. Chemistry Proceedings, 12(1), 27. https://doi.org/10.3390/ecsoc-26-13558





