In Vitro and In Silico Investigations of Natural Compounds with Predicted Activity against Neuroblastomas †
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeed, M.E.M.; Yücer, R.; Dawood, M.; Hegazy, M.E.F.; Drif, A.; Ooko, E.; Kadioglu, O.; Seo, E.J.; Kamounah, F.S.; Titinchi, S.J.; et al. In Silico and In Vitro Screening of 50 Curcumin Compounds as EGFR and NF-κB Inhibitor. Int. J. Mol. Sci. 2022, 23, 3966. [Google Scholar] [CrossRef] [PubMed]
- Sidhar, H.; Giri, R.K. Induction of Bex Genes by Curcumin Is Associated with Apoptosis and Activation of P53 in N2a Neuroblastoma. Cells Sci. Rep. 2017, 7, 41420. [Google Scholar] [CrossRef]
- Chairez-Ramirez, M.H.; de la Cruz-Lopez, K.G.; Garcia-Carranca, A. Polyphenols as Antitumor Agents Targeting Key Players in Cancer-Driving Signaling Pathways. Front. Pharm. 2021, 12, 710304. [Google Scholar] [CrossRef] [PubMed]
- Piletz, J.E.; Mao, Y.; Roy, D.; Qizilbash, B.; Nkamssi, E.; Weir, E.; Graham, J.; Emmanuel, M.; Iqbal, S.; Brue, K.; et al. Transepithelial Anti-Neuroblastoma Response to Kale among Four Vegetable Juices Using In Vitro Model Co-Culture System. Nutrients 2021, 13, 488. [Google Scholar] [CrossRef] [PubMed]
- Askarizadeh, A.; Barreto, G.; Henney, N.C.; Majeed, M.; Sahebkar, A. Neuroprotection by curcumin: A review on brain delivery strategies. Int. J. Pharm. 2020, 585, 119476. [Google Scholar] [CrossRef] [PubMed]
- Neagu, G.; Stefaniu, A.; Albulescu, A.; Pintilie, L.; Pirvu, L. Antiproliferative activity of Stokesialaevis ethanolic extract in combination with several food-related bioactive compounds; in vitro (CacO2) and in silico docking (TNKS1 and TNKS2) studies. Appl. Sci. 2021, 11, 9944. [Google Scholar] [CrossRef]
- Protocols & Applications Guide. Available online: www.promega.com (accessed on 2 August 2021).
- Bruncko, M.; Oost, T.K.; Belli, B.A.; Ding, H.; Joseph, M.K.; Kunzer, A.; Martineau, D.; McClellan, W.J.; Mitten, M.; Ng, S.C.; et al. Solution structure of the anti-apoptotic protein Bcl-2 in complex with an acyl-sulfonamide-based ligand. J. Med. Chem. 2007, 50, 641–662. [Google Scholar] [CrossRef] [PubMed]
- Pirvu, L.; Stefaniu, A.; Neagu, G.; Albu, B.; Pintilie, L. In Vitro Cytotoxic and Antiproliferative Activity of Cydonia oblonga flower petals, leaf and fruit pellet ethanolic extracts. Docking simulation of the active flavonoids on anti-apoptotic protein Bcl-2. Open Chem. 2018, 16, 591–604. [Google Scholar] [CrossRef]
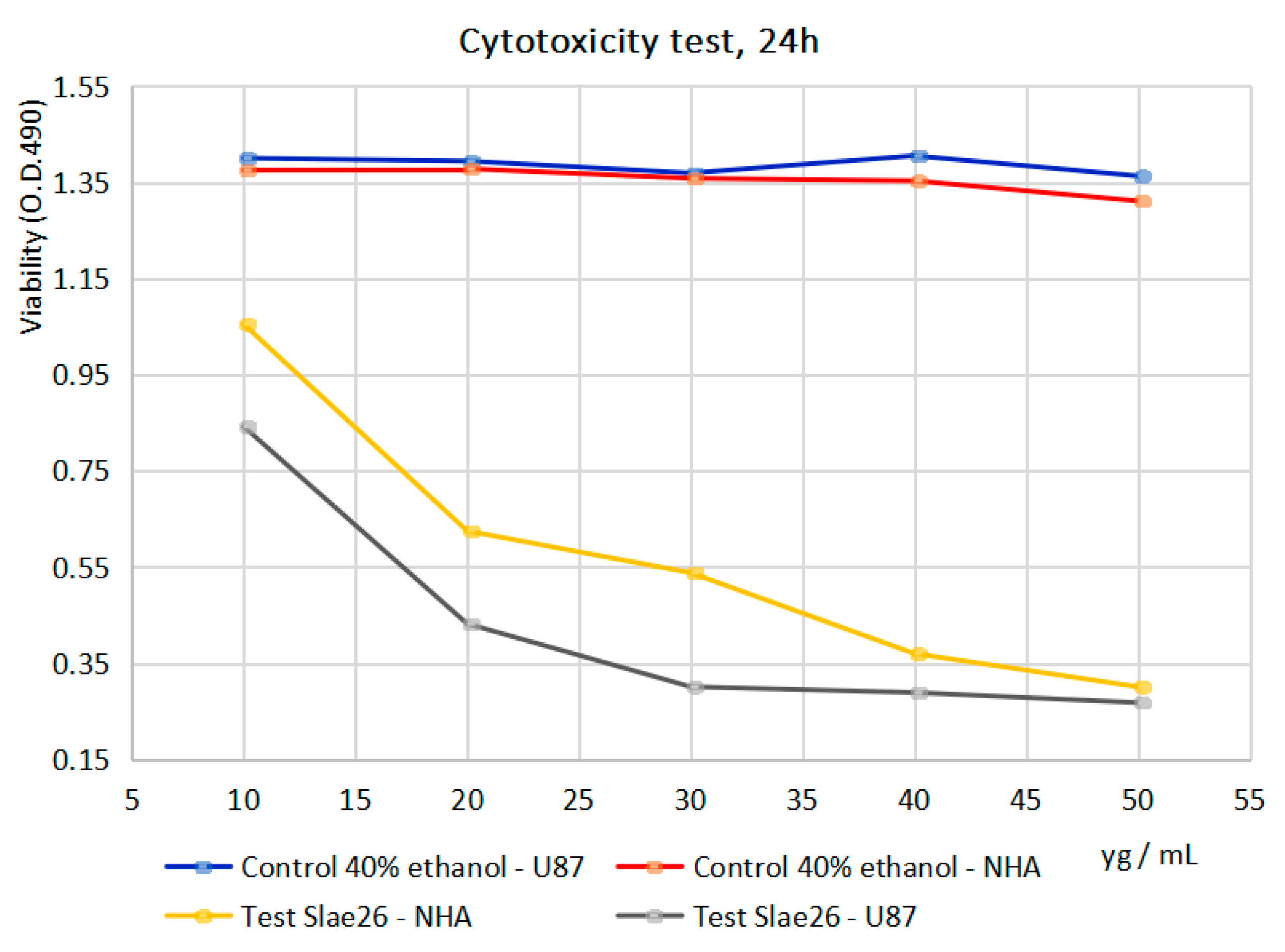
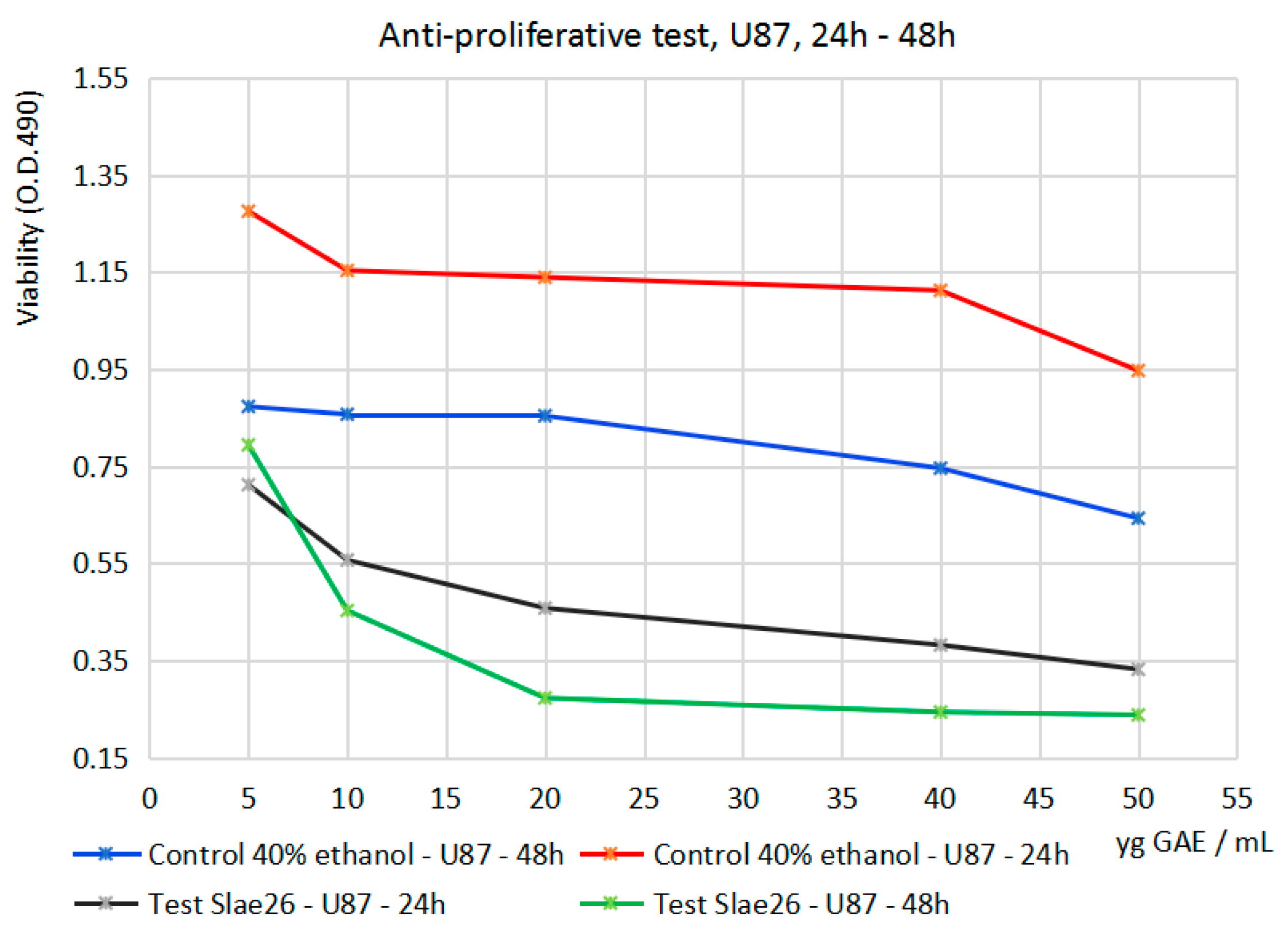

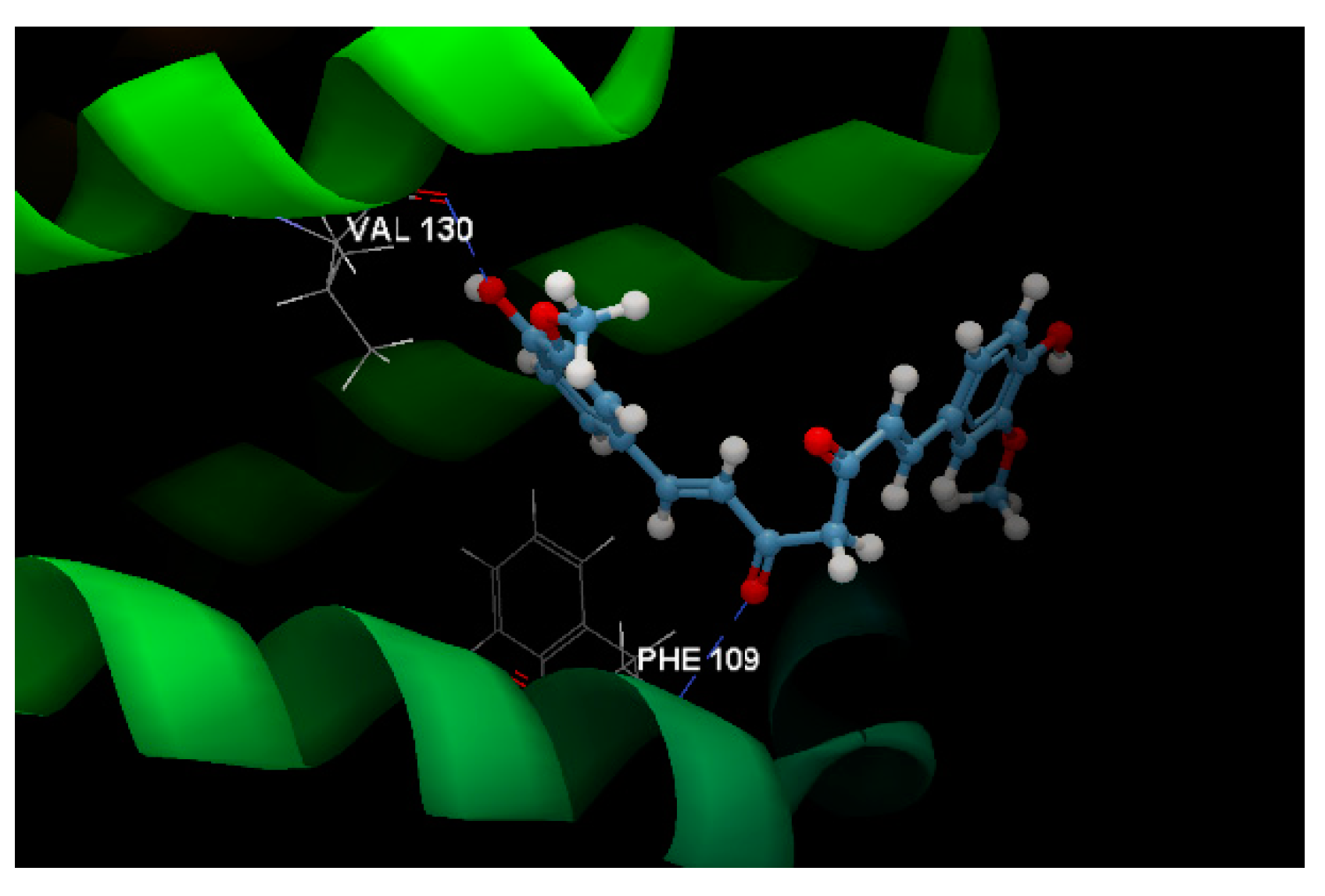
| Cytotoxicity Study/Cell Line | Inhibitory Activity (%) of Slae26 against the Dilution Series (10–50 μg of [GAE]/mL of Sample) | ||||
|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | |
| NHA | 23.27 | 54.64 | 60.38 | 72.65 | 77.04 |
| U87 | 39.86 | 69.01 | 78.01 | 79.43 | 80.33 |
| Anti-Proliferative Study at 24 h and 8 h, Resp. | Inhibitory Activity (%) of Slae26 against the Dilution Series (μg of [GAE]/mL of Sample) | ||||
|---|---|---|---|---|---|
| 5 | 10 | 20 | 40 | 50 | |
| U87–24 h | 18.44 | 35.01 | 46.37 | 48.79 | 48.36 |
| U87–48 h | 37.73 | 60.71 | 76.03 | 78.06 | 78.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirvu, L.C.; Neagu, G.; Çığ, Ö.; Albulescu, A.; Pintilie, L.; Stefaniu, A. In Vitro and In Silico Investigations of Natural Compounds with Predicted Activity against Neuroblastomas. Chem. Proc. 2022, 12, 2. https://doi.org/10.3390/ecsoc-26-13541
Pirvu LC, Neagu G, Çığ Ö, Albulescu A, Pintilie L, Stefaniu A. In Vitro and In Silico Investigations of Natural Compounds with Predicted Activity against Neuroblastomas. Chemistry Proceedings. 2022; 12(1):2. https://doi.org/10.3390/ecsoc-26-13541
Chicago/Turabian StylePirvu, Lucia Camelia, Georgeta Neagu, Özge Çığ, Adrian Albulescu, Lucia Pintilie, and Amalia Stefaniu. 2022. "In Vitro and In Silico Investigations of Natural Compounds with Predicted Activity against Neuroblastomas" Chemistry Proceedings 12, no. 1: 2. https://doi.org/10.3390/ecsoc-26-13541
APA StylePirvu, L. C., Neagu, G., Çığ, Ö., Albulescu, A., Pintilie, L., & Stefaniu, A. (2022). In Vitro and In Silico Investigations of Natural Compounds with Predicted Activity against Neuroblastomas. Chemistry Proceedings, 12(1), 2. https://doi.org/10.3390/ecsoc-26-13541







