A Randomized Crossover Study Comparing the Effects of Diabetes-Specific Formula with Common Asian Breakfasts on Glycemic Control and Satiety in Adults with Type 2 Diabetes Mellitus
Abstract
1. Introduction
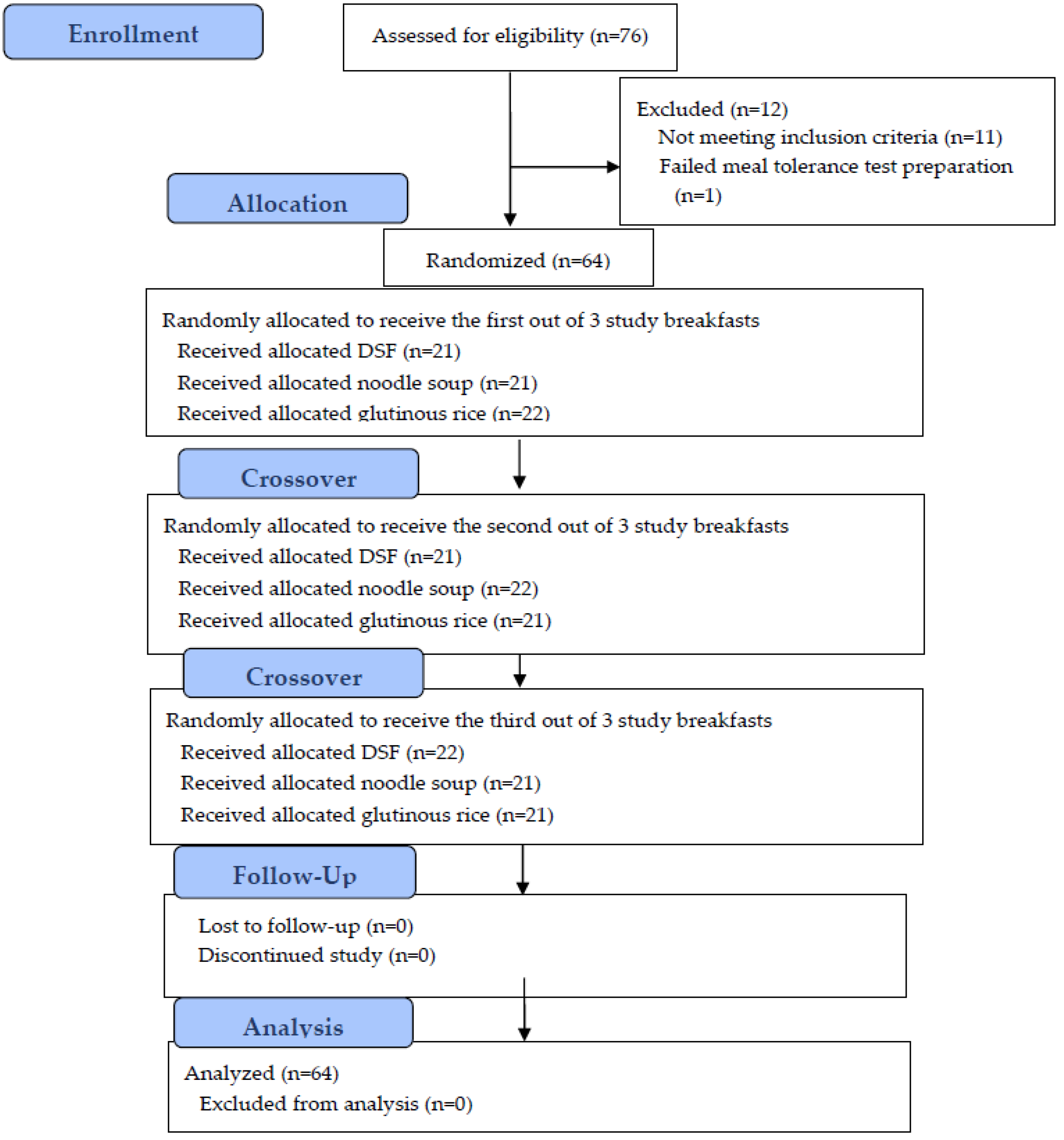
2. Materials and Methods
2.1. Study Population
2.2. Study Procedures
2.3. Study Treatments
2.4. Outcomes
2.4.1. Glucose and Insulin Response
2.4.2. Subjective Appetite
2.4.3. Adverse Events
2.4.4. Sensory and Hedonic Ratings
2.5. Analytical Method
2.6. Statistical Analyses
3. Results
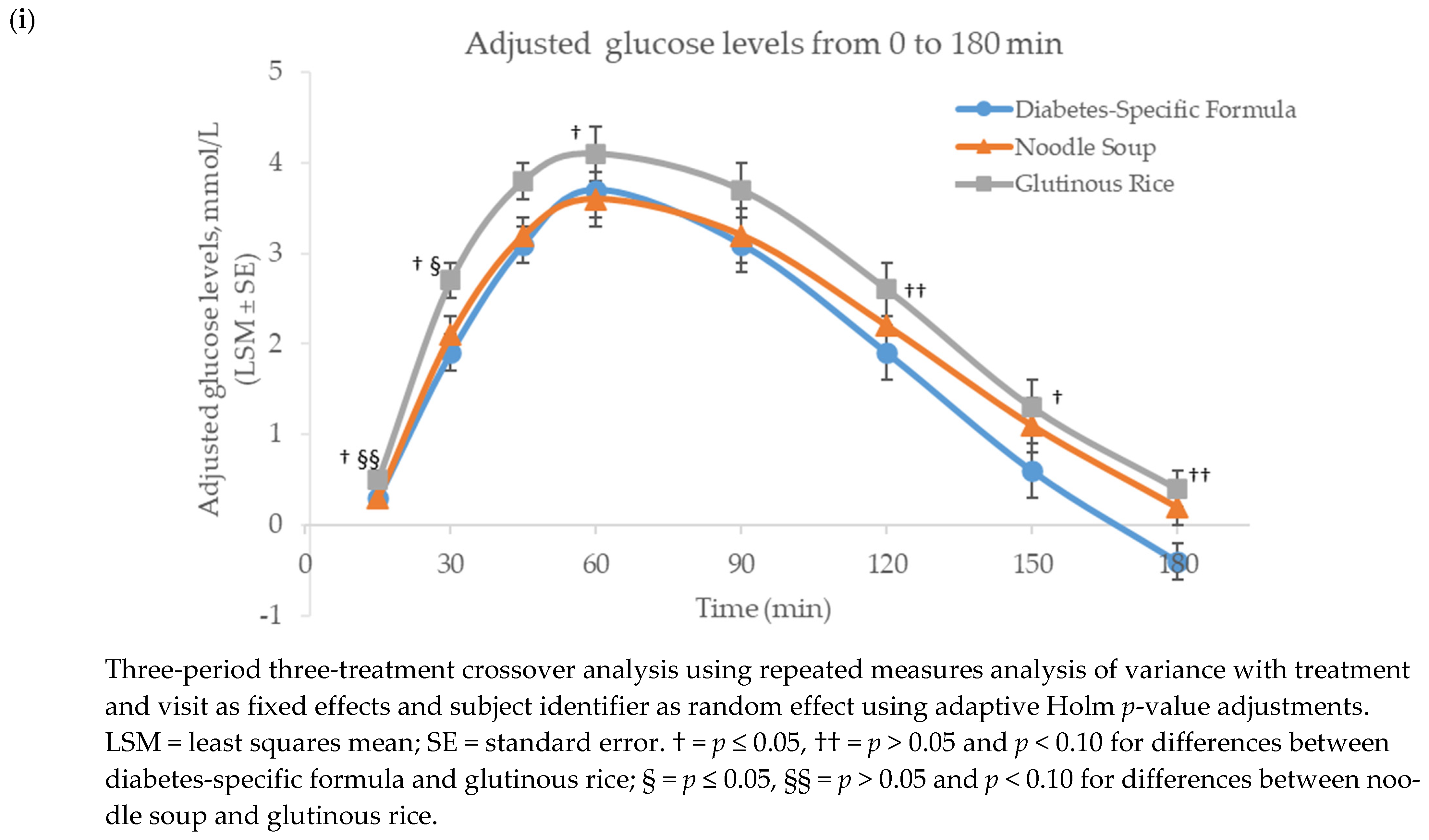
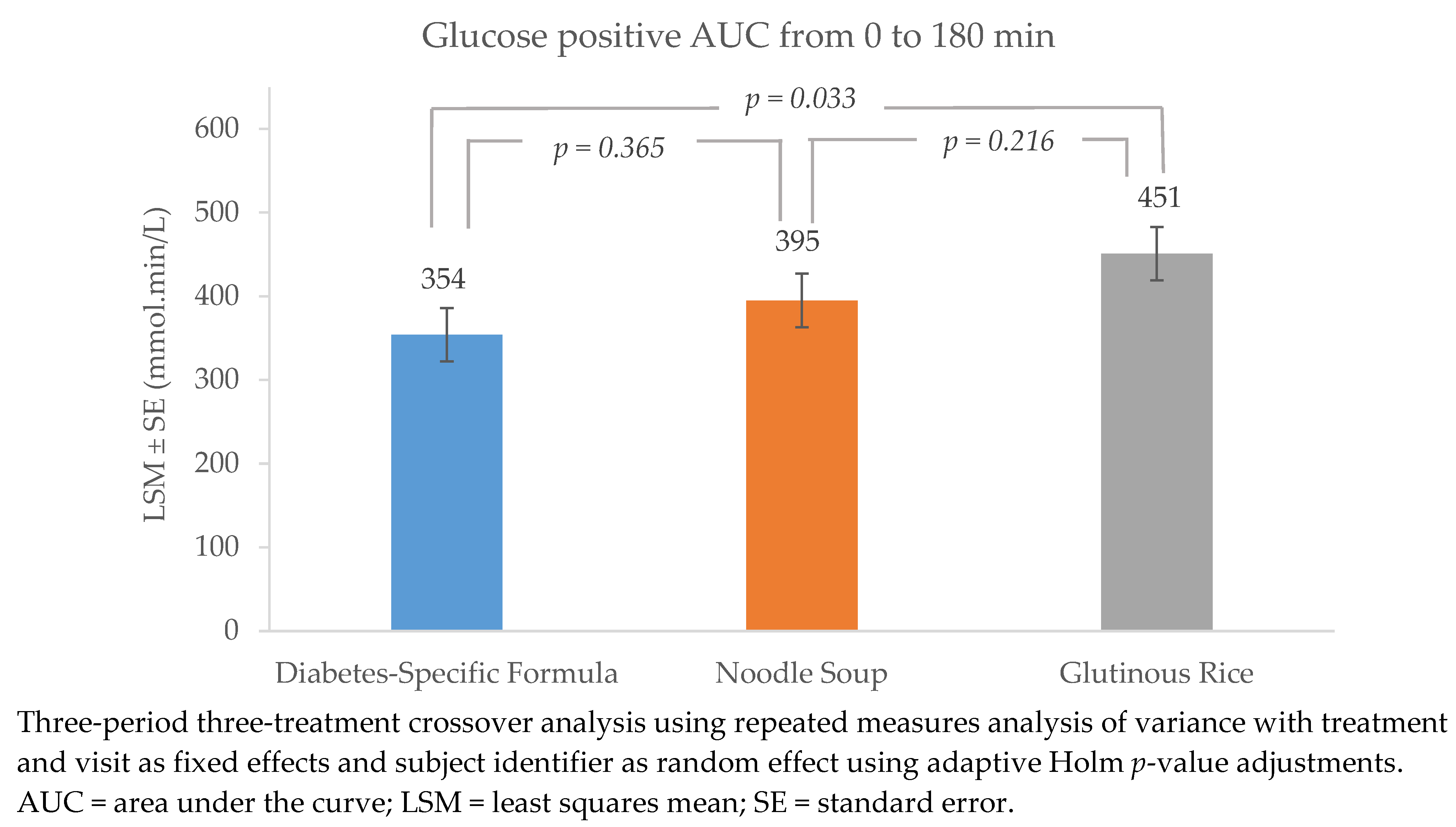
3.1. Glucose
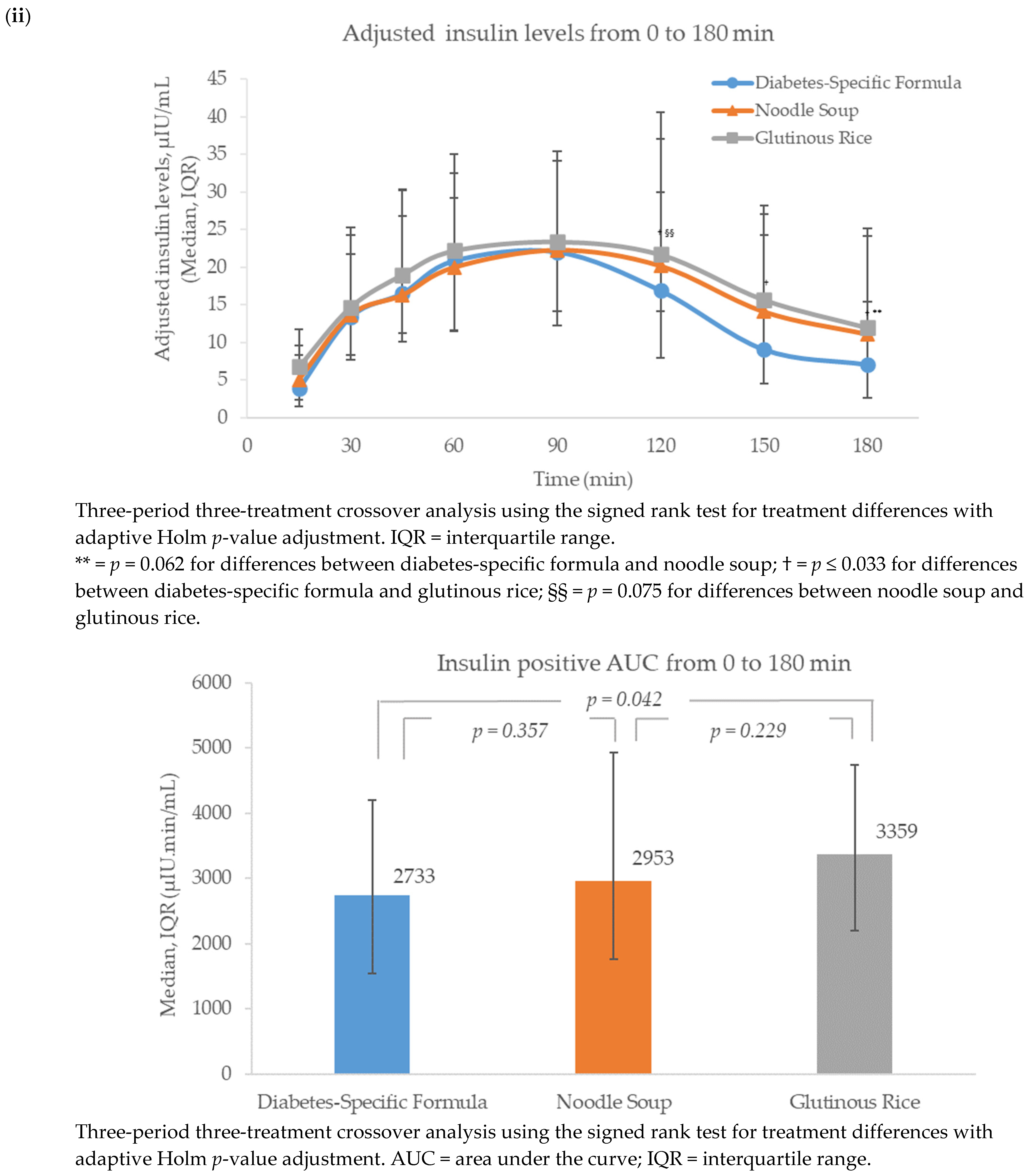
3.2. Insulin
Insulinogenic Index
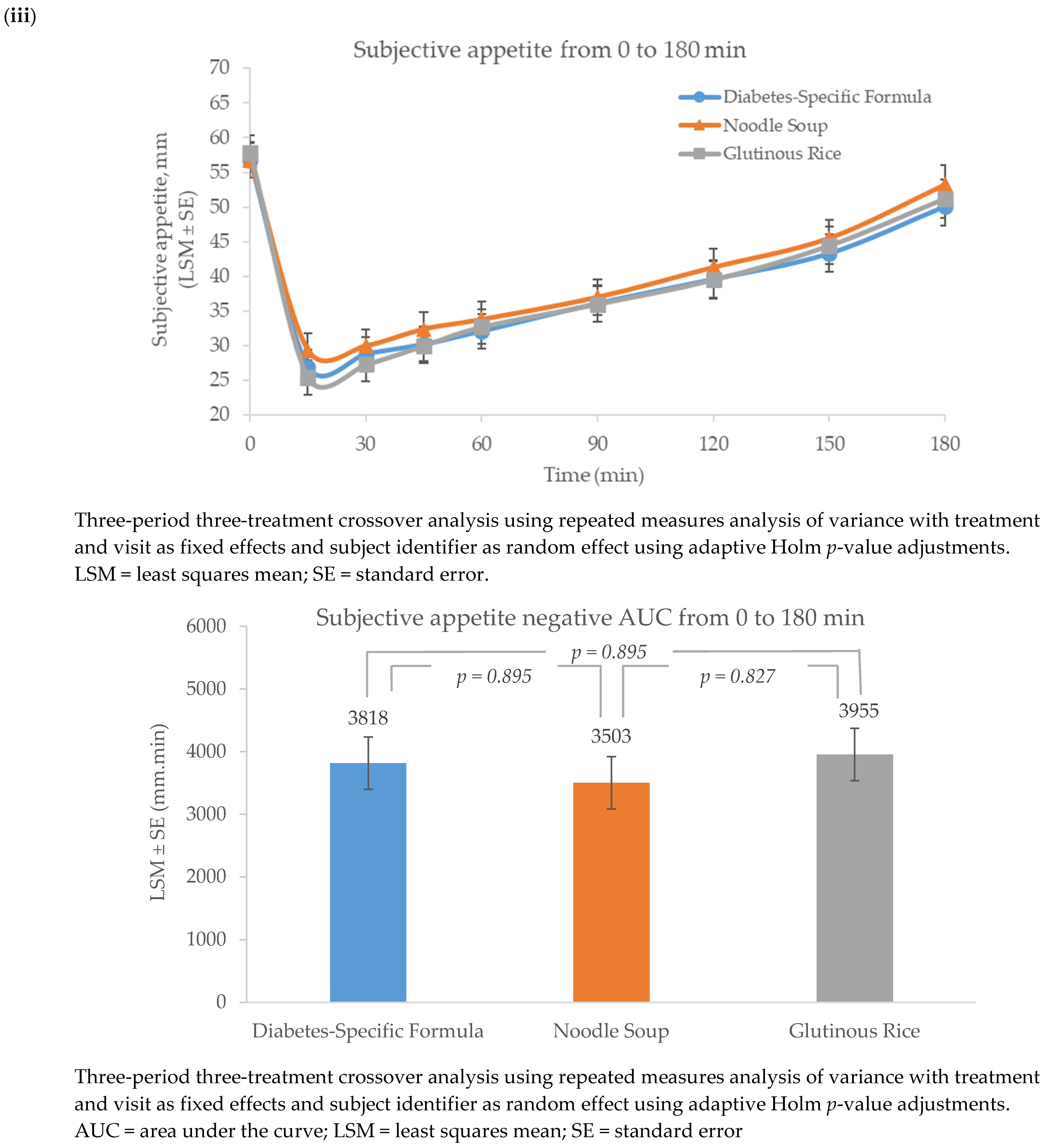
3.3. Appetite
3.4. Sensory and Hedonic Ratings
3.5. Adverse Events and Serious Adverse Events
4. Discussion
4.1. DSF and Glutinous Rice
4.2. DSF and Noodle Soup
4.3. Glutinous Rice and Noodle Soup
4.4. Sensory and Hedonic Ratings and AEs
4.5. Strength and Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas 10th Edition. Available online: https://www.diabetesatlas.org (accessed on 19 January 2024).
- Weyer, C.; Bogardus, C.; Mott, D.M.; Pratley, R.E. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J. Clin. Investig. 1999, 104, 787–794. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 6. Glycemic goals and hypoglycemia: Standards of care in diabetes—2024. Diabetes Care 2024, 47, S111–S125. [Google Scholar] [CrossRef] [PubMed]
- Blevins, T. Control of postprandial glucose levels with insulin in type 2 diabetes. Postgrad. Med. 2011, 123, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Hiyoshi, T.; Fujiwara, M.; Yao, Z. Postprandial hyperglycemia and postprandial hypertriglyceridemia in type 2 diabetes. J. Biomed. Res. 2017, 33, 1–16. [Google Scholar] [CrossRef]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition therapy for adults with diabetes or prediabetes: A consensus report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef] [PubMed]
- Dyson, P.A.; Twenefour, D.; Breen, C.; Duncan, A.; Elvin, E.; Goff, L.; Hill, A.; Kalsi, P.; Marsland, N.; McArdle, P.; et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet. Med. 2018, 35, 541–547. [Google Scholar] [CrossRef]
- Samson, S.L.; Vellanki, P.; Blonde, L.; Christofides, E.A.; Galindo, R.J.; Hirsch, I.B.; Isaacs, S.D.; Izuora, K.E.; Wang, C.C.L.; Twining, C.L.; et al. American Association of Clinical Endocrinology Consensus Statement: Comprehensive type 2 diabetes management algorithm—2023 update. Endocr. Pract. 2023, 29, 305–340. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 5. Facilitating positive health behaviors and well-being to improve health outcomes: Standards of care in diabetes—2024. Diabetes Care 2024, 47, S77–S110. [Google Scholar] [CrossRef] [PubMed]
- Mechanick, J.I.; Marchetti, A.; Hegazi, R.; Hamdy, O. Diabetes-specific nutrition formulas in the management of patients with diabetes and cardiometabolic risk. Nutrients 2020, 12, 3616. [Google Scholar] [CrossRef]
- Noronha, J.C.; Mechanick, J.I. Is there a role for diabetes-specific nutrition formulas as meal replacements in type 2 diabetes? Front. Endocrinol. 2022, 13, 874968. [Google Scholar] [CrossRef]
- Peng, J.; Lu, J.; Ma, X.; Ying, L.; Lu, W.; Zhu, W.; Bao, Y.; Zhou, J. Breakfast replacement with a liquid formula improves glycaemic variability in patients with type 2 diabetes: A randomised clinical trial. Br. J. Nutr. 2019, 121, 560–566. [Google Scholar] [CrossRef]
- Tey, S.L.; Chee, W.S.S.; Deerochanawong, C.; Berde, Y.; Lim, L.-L.; Boonyavarakul, A.; Wakefield, B.; Baggs, G.; Huynh, D.T.T. Diabetes-specific formula with standard of care improves glycemic control, body composition, and cardiometabolic risk factors in overweight and obese adults with type 2 diabetes: Results from a randomized controlled trial. Front. Nutr. 2024, 11, 1400580. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.M.; Agudo, F.R.; Medina, J.A.L.; Paris, A.S.; Santabalbina, F.T.; Pascual, J.R.D.; Penabad, L.L.; Barriuso, R.S. Effectiveness of an oral diabetes-specific supplement on nutritional status, metabolic control, quality or life, and functional status in elderly patients. A multicentre study. Clin. Nutr. 2019, 38, 1253–1261. [Google Scholar] [CrossRef]
- Sanz-Paris, A.; Boj-Carceller, D.; Lardies-Sanchez, B.; Perez-Fernandez, L.; Cruz-Jentoft, A.J. Health-care costs, glycemic control and nutritional status in malnourished older diabetics treated with a hypercaloric diabetes-specific enteral nutritional formula. Nutrients 2016, 8, 153. [Google Scholar] [CrossRef]
- Miller, V.; Jenkins, D.A.; Dehghan, M.; Srichaikul, K.; Rangarajan, S.; Mente, A.; Mohan, V.; Swaminathan, S.; Ismail, R.; Diaz, M.L.; et al. Associations of the glycaemic index and the glycaemic load with risk of type 2 diabetes in 127,594 people from 20 countries (PURE): A prospective cohort study. Lancet Diabetes Endocrinol. 2024, 12, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Camps, S.G.; Lim, J.; Koh, M.X.N.; Henry, C.J. The glycaemic and insulinaemic response of pasta in Chinese and Indians compared to Asian carbohydrate staples: Taking spaghetti back to Asia. Nutrients 2021, 13, 451. [Google Scholar] [CrossRef]
- Tan, W.S.K.; Tan, W.J.K.; Ponnalagu, S.D.; Koecher, K.; Menon, R.; Tan, S.Y.; Henry, C.J. The glycaemic index and insulinaemic index of commercially available breakfast and snack foods in an Asian population. Br. J. Nutr. 2018, 119, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Sridonpai, P.; Prachansuwan, A.; Praengam, K.; Tuntipopipat, S.; Kriengsinyos, W. Postprandial effects of a whey protein-based multi-ingredient nutritional drink compared with a normal breakfast on glucose, insulin, and active GLP-1 response among type 2 diabetic subjects: A crossover randomised controlled trial. J. Nutr. Sci. 2021, 10, e49. [Google Scholar] [CrossRef]
- Devitt, A.A.; Oliver, J.S.; Hegazi, R.A.; Mustad, V.A. Glycemia targeted specialized nutrition (GTSN) improves postprandial glycemia and GLP-1 with similar appetitive responses compared to a healthful whole food breakfast in persons with type 2 diabetes: A randomized, controlled trial. J. Diabetes Res. Clin. Metab. 2012, 1, 20. [Google Scholar] [CrossRef]
- Mottalib, A.; Mohd-Yusof, B.N.; Shehabeldin, M.; Pober, D.M.; Mitri, J.; Hamdy, O. Impact of diabetes-specific nutritional formulas versus oatmeal on postprandial glucose, insulin, GLP-1 and postprandial lipidemia. Nutrients 2016, 8, 443. [Google Scholar] [CrossRef]
- Gulati, S.; Misra, A.; Nanda, K.; Pandey, R.M.; Garg, V.; Ganguly, S.; Cheung, L. Efficacy and tolerance of a diabetes specific formula in patients with type 2 diabetes mellitus: An open label, randomized, crossover study. Diabetes Metab. Syndr. 2015, 9, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Voss, A.C.; Mustad, V.A.; Ivanova, L.; Morugova, T.; Alexeeva, E.; Ruyatkina, L.; Suplotova, L. Four-hour evaluation of a medical food in subjects with type 2 diabetes receiving oral hypoglycemic medication. J. Diabetes Mellit. 2012, 2, 214–220. [Google Scholar] [CrossRef][Green Version]
- Sanz-París, A.; Matía-Martín, P.; Martín-Palmero, Á.; Gómez-Candela, C.; Camprubi Robles, M. Diabetes-specific formulas high in monounsaturated fatty acids and metabolic outcomes in patients with diabetes or hyperglycaemia. A systematic review and meta-analysis. Clin. Nutr. 2020, 39, 3273–3282. [Google Scholar] [CrossRef]
- Ojo, O.; Weldon, S.M.; Thompson, T.; Crockett, R.; Wang, X.H. The effect of diabetes-specific enteral nutrition formula on cardiometabolic parameters in patients with type 2 diabetes: A systematic review and meta-analysis of randomised controlled trials. Nutrients 2019, 11, 1905. [Google Scholar] [CrossRef]
- Phillips, D.I.; Clark, P.M.; Hales, C.N.; Osmond, C. Understanding oral glucose tolerance: Comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet. Med. 1994, 11, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Anderson, G.H.; Catherine, N.L.; Woodend, D.M.; Wolever, T.M. Inverse association between the effect of carbohydrates on blood glucose and subsequent short-term food intake in young men. Am. J. Clin. Nutr. 2002, 76, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Song, C.Q.; Kuznetsova, O.M. Fitting Gompertz nonlinear mixed model to infancy growth data with SAS version 8 procedure NLMIXED. In Proceedings of the SAS Conference Proceedings: PharmaSUG, Boston, MA, USA, 20–23 May 2001. [Google Scholar]
- Juliano, B.O.; Perez, C.M.; Komindr, S.; Banphotkasem, S. Properties of Thai cooked rice and noodles differing in glycemic index in noninsulin-dependent diabetics. Plant Foods Hum. Nutr. 1989, 39, 369–374. [Google Scholar] [CrossRef]
- Chen, Y.J.; Sun, F.H.; Wong, S.H.; Huang, Y.J. Glycemic index and glycemic load of selected Chinese traditional foods. World J. Gastroenterol. 2010, 16, 1512–1517. [Google Scholar] [CrossRef]
- Vinoy, S.; Meynier, A.; Goux, A.; Jourdan-Salloum, N.; Normand, S.; Rabasa-Lhoret, R.; Brack, O.; Nazare, J.-A.; Péronnet, F.; Laville, M. The effect of a breakfast rich in slowly digestible starch on glucose metabolism: A statistical meta-analysis of randomized controlled trials. Nutrients 2017, 9, 318. [Google Scholar] [CrossRef]
- Miñambres, I.; Cuixart, G.; Gonçalves, A.; Corcoy, R. Effects of inositol on glucose homeostasis: Systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2019, 38, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef] [PubMed]
- Buck, A.W. Resistant maltodextrin overview. In Dietary Fiber and Health; CRC Press: Boca Raton, FL, USA, 2012; pp. 279–292. [Google Scholar]
- Singh, B.; Saxena, A. Surrogate markers of insulin resistance: A review. World J. Diabetes 2010, 1, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.K.; Lee, J.H.; Choi, I.Y.; Kwon, H.S.; Shin, J.A.; Jeong, S.H.; Lee, S.-H.; Cho, J.H.; Son, H.Y.; Yoon, K.H. The insulin resistance but not the insulin secretion parameters have changed in the Korean population during the last decade. Diabetes Metab. J. 2015, 39, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Cubeddu, L.X.; Hoffmann, I.S. Impact of traits of metabolic syndrome on β-cell function and insulin resistance in normal fasting, normal glucose tolerant subjects. Metab. Syndr. Relat. Disord. 2012, 10, 344–350. [Google Scholar] [CrossRef]
- Bynoe, K.; Unwin, N.; Taylor, C.; Murphy, M.M.; Bartholomew, L.; Greenidge, A.; Abed, M.; Jeyaseelan, S.; Cobelli, C.; Man, C.D.; et al. Inducing remission of type 2 diabetes in the Caribbean: Findings from a mixed methods feasibility study of a low-calorie liquid diet-based intervention in Barbados. Diabet. Med. 2020, 37, 1816–1824. [Google Scholar] [CrossRef]
- Skytte, M.J.; Samkani, A.; Petersen, A.D.; Thomsen, M.N.; Astrup, A.; Chabanova, E.; Frystyk, J.; Holst, J.J.; Thomsen, H.S.; Madsbad, S.; et al. A carbohydrate-reduced high-protein diet improves HbA1c and liver fat content in weight stable participants with type 2 diabetes: A randomised controlled trial. Diabetologia 2019, 62, 2066–2078. [Google Scholar] [CrossRef]
- Bruce, D.G.; Chisholm, D.J.; Storlien, L.H.; Kraegen, E.W. Physiological importance of deficiency in early prandial insulin secretion in non-insulin-dependent diabetes. Diabetes 1988, 37, 736–744. [Google Scholar] [CrossRef]
- Feng, L.; Chen, C.; Guo, Q.; Chen, L.; Yang, W. Improvement of early-phase insulin secretion is an independent factor for achieving glycaemic control: A pooled analysis of SEED and DAWN study. Diabetes Obes. Metab. 2024, 26, 745–753. [Google Scholar] [CrossRef]
- Schiller, J.M.; Chanphengxay, M.B.; Linquist, B.; Appa Rao, S. Rice in Laos; International Rice Research Institute: Los Banos, Phillipines, 2006; 457p. [Google Scholar]
- García-Rodríguez, C.E.; Mesa, M.D.; Olza, J.; Buccianti, G.; Pérez, M.; Moreno-Torres, R.; de la Cruz, A.P.; Gil, A. Postprandial glucose, insulin and gastrointestinal hormones in healthy and diabetic subjects fed a fructose-free and resistant starch type IV-enriched enteral formula. Eur. J. Nutr. 2013, 52, 1569–1578. [Google Scholar] [CrossRef]
- Angarita Dávila, L.; Bermúdez, V.; Aparicio, D.; Céspedes, V.; Escobar, M.C.; Durán-Agüero, S.; Cisternas, S.; Costa, J.d.A.; Rojas-Gómez, D.; Reyna, N.; et al. Effect of oral nutritional supplements with sucromalt and isomaltulose versus standard formula on glycaemic index, entero-insular axis peptides and subjective appetite in patients with type 2 diabetes: A randomised cross-over study. Nutrients 2019, 11, 1477. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Lin, J. Effects of storage temperature and time on the glycemic response of white rice. Chiang Mai J. Sci. 2018, 45, 1439–1448. [Google Scholar]
- Burton, P.; Lightowler, H.J. The impact of freezing and toasting on the glycaemic response of white bread. Eur. J. Clin. Nutr. 2008, 62, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Carreira, M.C.; Lajolo, F.M.; Menezes, E.W.d. Glycemic index: Effect of food storage under low temperature. Braz. Arch. Biol. Technol. 2004, 47, 569–574. [Google Scholar] [CrossRef]
- Russell, W.R.; Baka, A.; Bjorck, I.; Delzenne, N.; Gao, D.; Griffiths, H.R.; Hadjilucas, E.; Juvonen, K.; Lahtinen, S.; Lansink, M.; et al. Impact of diet composition on blood glucose regulation. Crit. Rev. Food Sci. Nutr. 2016, 56, 541–590. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Sodium Intake for Adults and Children; World Health Organization (WHO): Geneva, Switzerland, 2012. [Google Scholar]
- The Committee and Working Group Improve the Daily Nutritional Requirements for Thai People. Dietary Reference Intake for Thais 2020. Available online: https://www.thaidietetics.org/wp-content/uploads/2020/04/dri2563.pdf (accessed on 9 July 2024).
- He, F.J.; Li, J.; Macgregor, G.A. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ 2013, 346, f1325. [Google Scholar] [CrossRef]
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; Bittner, V.; Brewer, L.C.; Demeter, S.H.; Dixon, D.L.; Fearon, W.F.; Hess, B.; Johnson, H.M.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the management of patients with chronic coronary disease: A report of the American Heart Association/American College of Cardiology Joint Committee on clinical practice guidelines. Circulation 2023, 148, e9–e119. [Google Scholar] [CrossRef]
- Stallings, V.A.; Harrison, M.; Oria, M. (Eds.) Dietary Reference Intakes for Sodium and Potassium; The National Academies Press: Washington, DC, USA, 2019. [Google Scholar]
- Li, H.; Liu, B.; Bess, K.; Wang, Z.; Liang, M.; Zhang, Y.; Wu, Q.; Yang, L. Impact of low-temperature storage on the microstructure, digestibility, and absorption capacity of cooked rice. Foods 2022, 11, 1642. [Google Scholar] [CrossRef] [PubMed]
- Dalgaard, L.B.; Kruse, D.Z.; Norup, K.; Andersen, B.V.; Hansen, M. A dairy-based protein-rich breakfast enhances satiety and cognitive concentration before lunch in young females with overweight to obesity: A randomized controlled cross-over study. J. Dairy Sci. 2024, 107, 2653–2667. [Google Scholar] [CrossRef]
- Gwin, J.A.; Leidy, H.J. Breakfast consumption augments appetite, eating behavior, and exploratory markers of sleep quality compared with skipping breakfast in healthy young adults. Curr. Dev. Nutr. 2018, 2, nzy074. [Google Scholar] [CrossRef]
- Buranapin, S.; Siangruangsang, S.; Chantapanich, V.; Hengjeerajarus, N. The comparative study of diabetic specific formula and standard formula on postprandial plasma glucose control in type 2 DM patients. J. Med. Assoc. Thail. 2014, 97, 582–588. [Google Scholar]
- Chaiyakul, S.; Ketkham, N.; Chaichana, C.; Khumkhana, N.; Deekum, W.; Wongshaya, P.; Suwanmalai, T.; Hutchinson, C.; Pramyothin, P. Effects of a novel rice-based diabetes-specific formula on postprandial glucose and gastrointestinal hormones: A double-blinded multi-arm randomized crossover trial. Front. Endocrinol. 2023, 14, 1141497. [Google Scholar] [CrossRef] [PubMed]
- DeTora, L.M.; Toroser, D.; Sykes, A.; Vanderlinden, C.; Plunkett, F.J.; Lane, T.; Hanekamp, E.; Dormer, L.; DiBiasi, F.; Bridges, D.; et al. Good Publication Practice (GPP) Guidelines for Company-Sponsored Biomedical Research: 2022 Update. Ann. Intern. Med. 2022, 175, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total (n = 64) |
|---|---|
| Gender, n (%) | |
| Male | 23 (35.9%) |
| Female | 41 (64.1%) |
| Ethnic group, n (%) | |
| Thai | 64 (100%) |
| Age, years | 54.5 ± 1.0 |
| Capillary HbA1c, % | 7.75 ± 0.08 |
| Body weight, kg | 72.15 ± 1.62 |
| Height, cm | 161.33 ± 1.11 |
| Body mass index, kg/m2 | 27.63 ± 0.48 |
| Hip circumference, cm | 100.85 ± 1.17 |
| Waist circumference, cm | 92.43 ± 1.28 |
| Diabetes duration, years | 7.9 ± 0.7 |
| HOMA index | |
| HOMA-β | 58.05 ± 6.31 |
| HOMA-IR | 3.05 ± 0.31 |
| Co-morbidities, n (%) | |
| Hyperlipidemia | 58 (90.6%) |
| Hypertension | 43 (67.2%) |
| Others | 21 (32.8%) |
| Number of glucose-lowering medications, n (%) | |
| One | 22 (34%) |
| Two | 25 (39%) |
| Three | 10 (16%) |
| Four or more | 7 (11%) |
| Medications, n (%) | |
| Glucose-lowering medication | 64 (100%) |
| Metformin | 61 (95%) |
| Sulphonylureas | 32 (50%) |
| Thiazolidinediones | 14 (22%) |
| Dipeptidyl peptidase 4 (DPP-4) inhibitors | 13 (20%) |
| Sodium-glucose co-transporter 2 (SGLT2) inhibitors | 11 (17%) |
| Blood pressure-lowering medication | 37 (58%) |
| Lipid-modifying agents | 57 (89%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, S.T.; Huynh, D.T.T.; Srivanichakorn, W.; Khovidhunkit, W.; Washirasaksiri, C.; Sitasuwan, T.; Huang, C.; Paunikar, S.; Yalawar, M.; Tey, S.L. A Randomized Crossover Study Comparing the Effects of Diabetes-Specific Formula with Common Asian Breakfasts on Glycemic Control and Satiety in Adults with Type 2 Diabetes Mellitus. Diabetology 2024, 5, 447-463. https://doi.org/10.3390/diabetology5040033
Kong ST, Huynh DTT, Srivanichakorn W, Khovidhunkit W, Washirasaksiri C, Sitasuwan T, Huang C, Paunikar S, Yalawar M, Tey SL. A Randomized Crossover Study Comparing the Effects of Diabetes-Specific Formula with Common Asian Breakfasts on Glycemic Control and Satiety in Adults with Type 2 Diabetes Mellitus. Diabetology. 2024; 5(4):447-463. https://doi.org/10.3390/diabetology5040033
Chicago/Turabian StyleKong, Sing Teang, Dieu Thi Thu Huynh, Weerachai Srivanichakorn, Weerapan Khovidhunkit, Chaiwat Washirasaksiri, Tullaya Sitasuwan, Chengrong Huang, Swapnil Paunikar, Menaka Yalawar, and Siew Ling Tey. 2024. "A Randomized Crossover Study Comparing the Effects of Diabetes-Specific Formula with Common Asian Breakfasts on Glycemic Control and Satiety in Adults with Type 2 Diabetes Mellitus" Diabetology 5, no. 4: 447-463. https://doi.org/10.3390/diabetology5040033
APA StyleKong, S. T., Huynh, D. T. T., Srivanichakorn, W., Khovidhunkit, W., Washirasaksiri, C., Sitasuwan, T., Huang, C., Paunikar, S., Yalawar, M., & Tey, S. L. (2024). A Randomized Crossover Study Comparing the Effects of Diabetes-Specific Formula with Common Asian Breakfasts on Glycemic Control and Satiety in Adults with Type 2 Diabetes Mellitus. Diabetology, 5(4), 447-463. https://doi.org/10.3390/diabetology5040033






