Diabetic Retinopathy: An Overview on Mechanisms, Pathophysiology and Pharmacotherapy
Abstract
1. Introduction
2. Materials and Methods
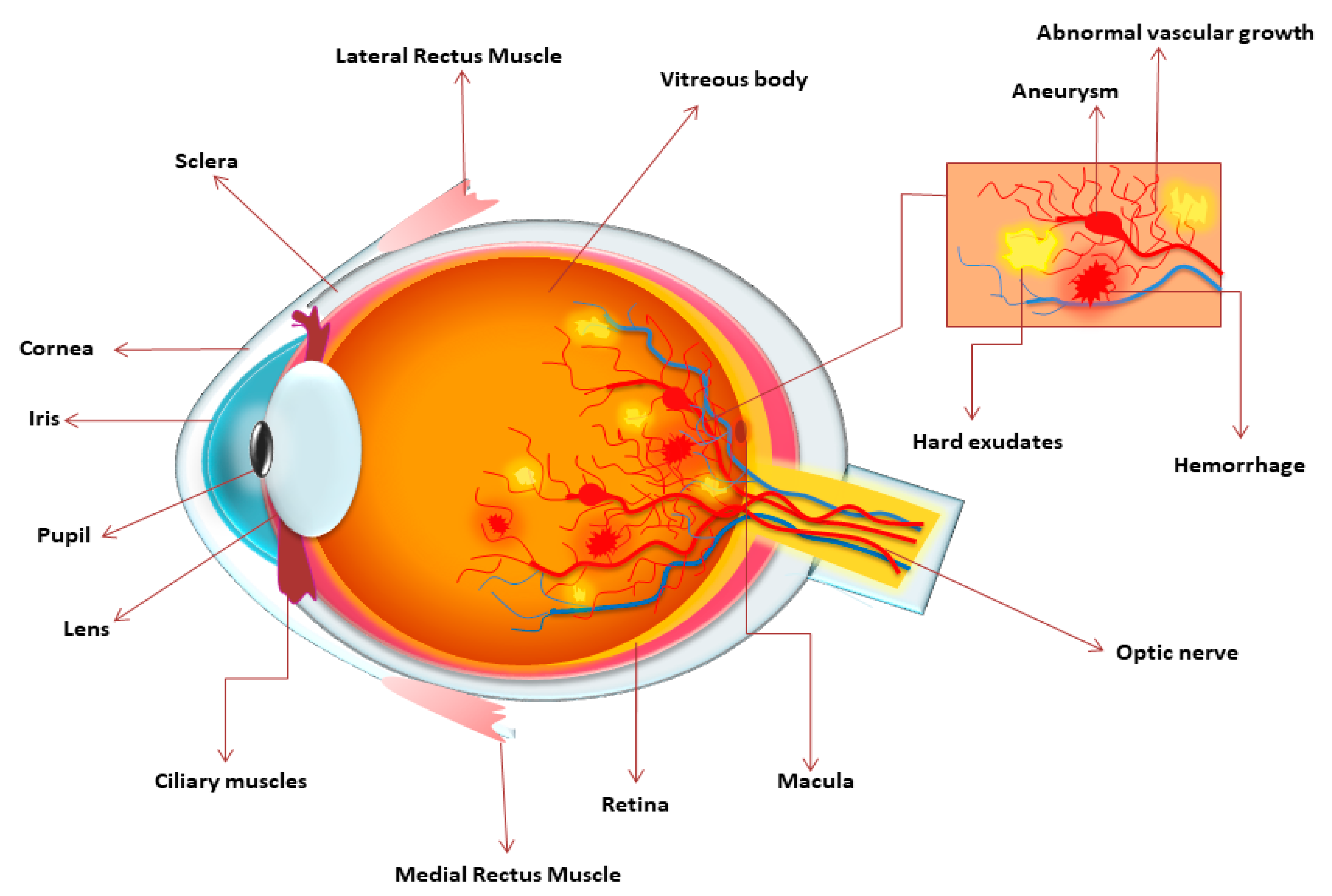
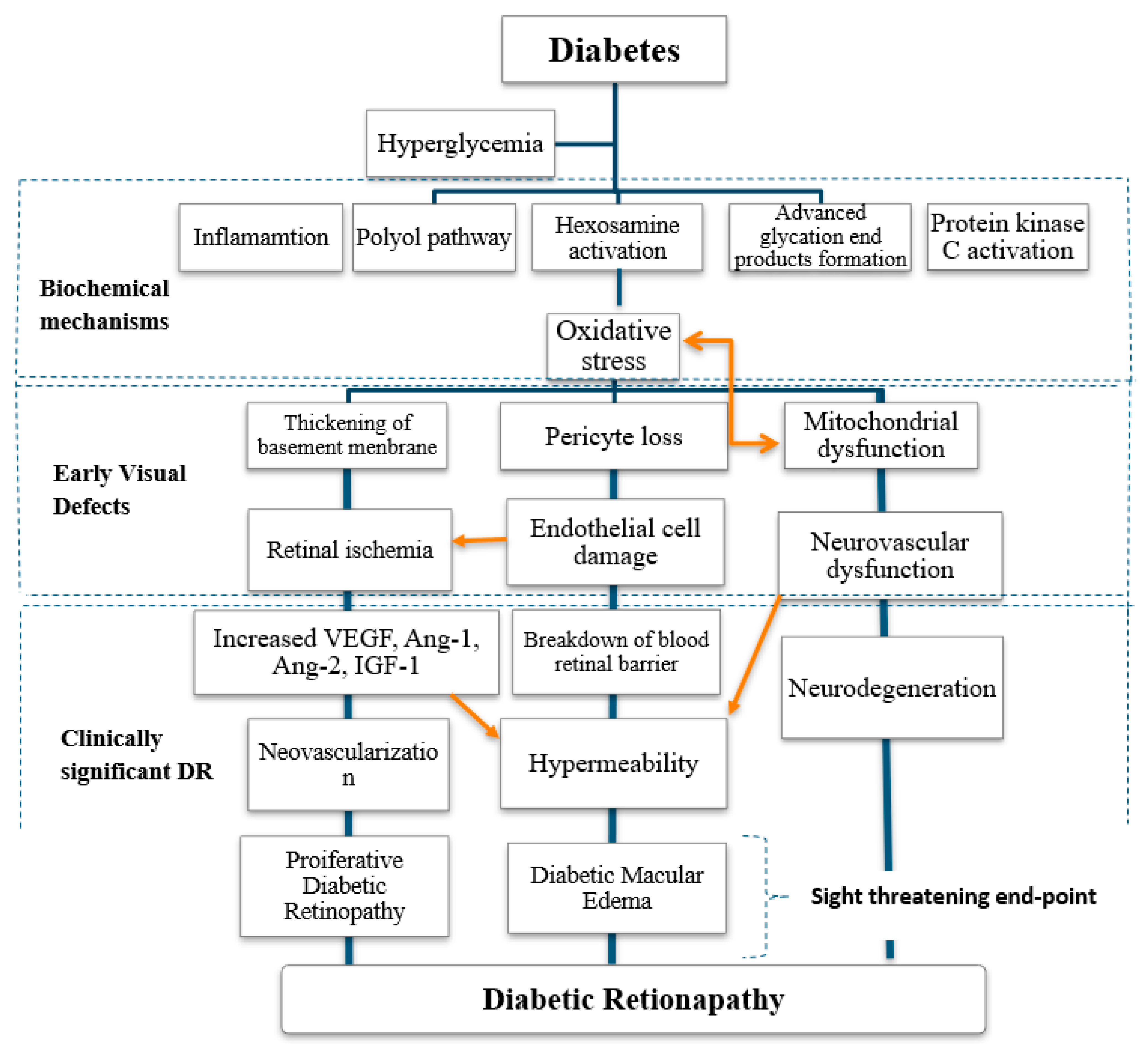
3. Mechanism of Diabetic Retinopathy and Classification
3.1. Hyperglycaemia in Diabetic Retinopathy
3.2. Malfunction of Insulin Signaling in Retinopathy
4. Pathophysiology
5. Epidemiology
6. Risk Factors of Diabetic Retinopathy
6.1. Genetic Risk Factors of Retinopathy
6.2. Other Risk Factors
7. Diagnosis and Management of Diabetic Retinopathy
7.1. Diagnosis
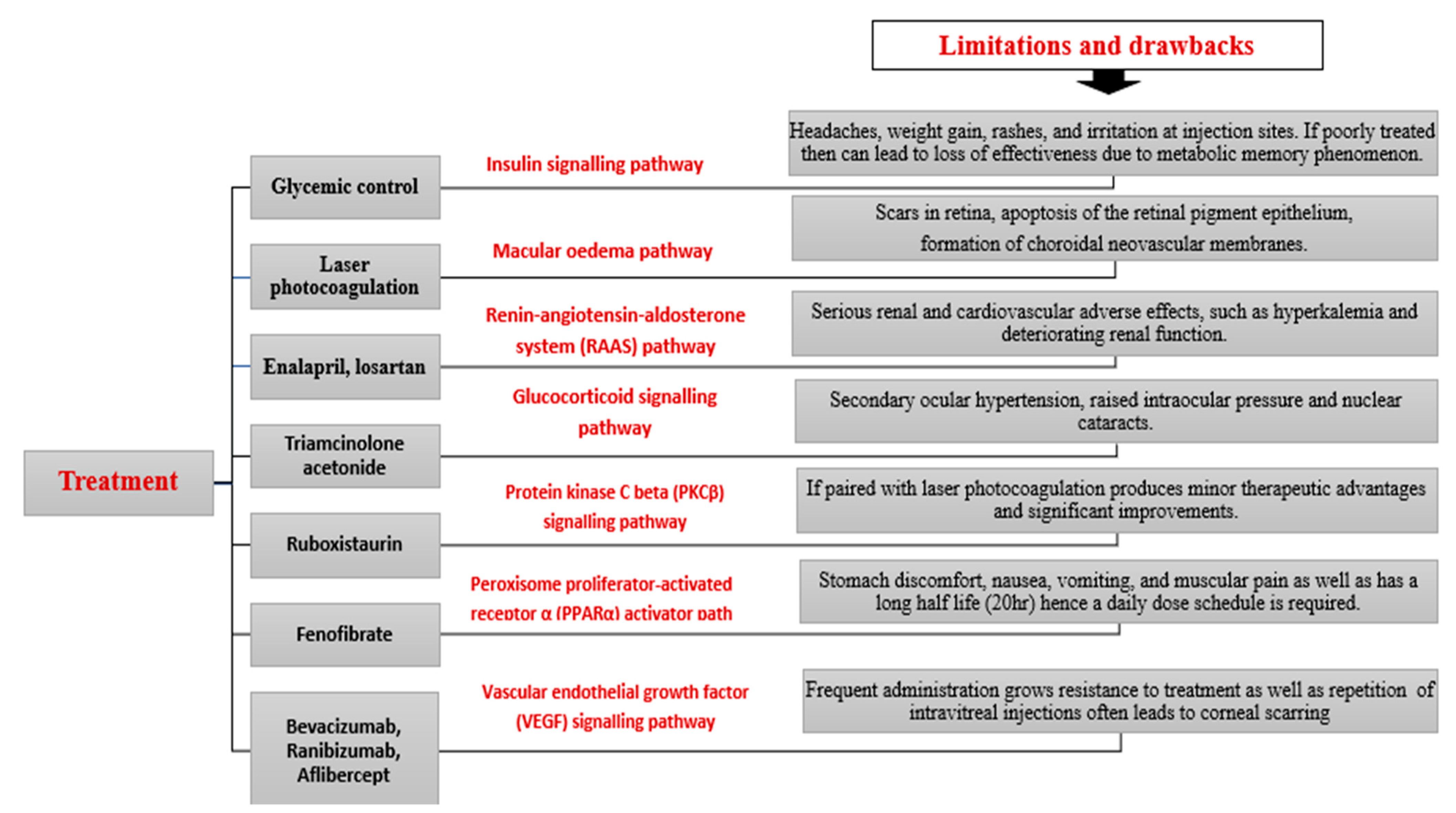
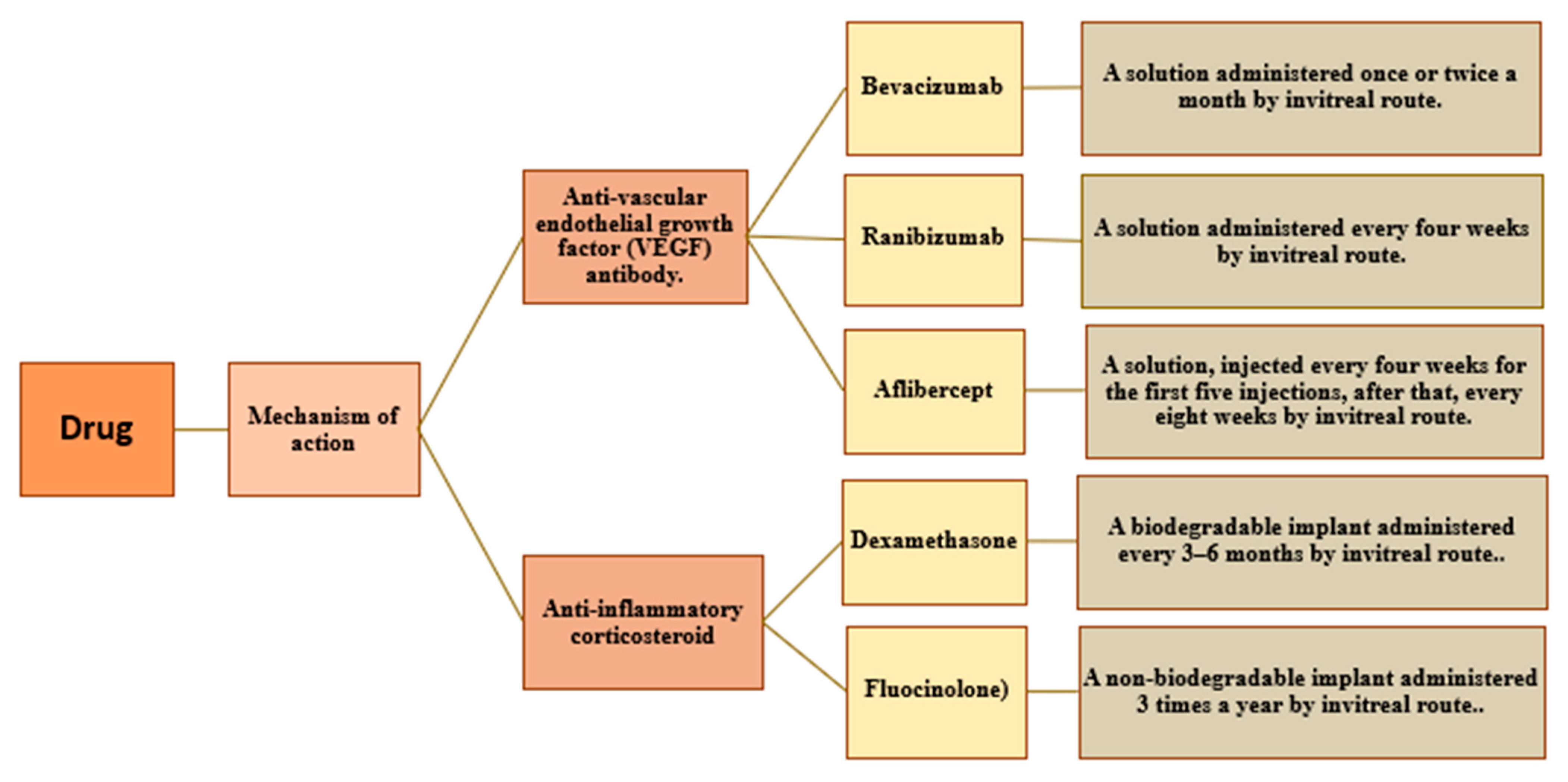
7.2. Management
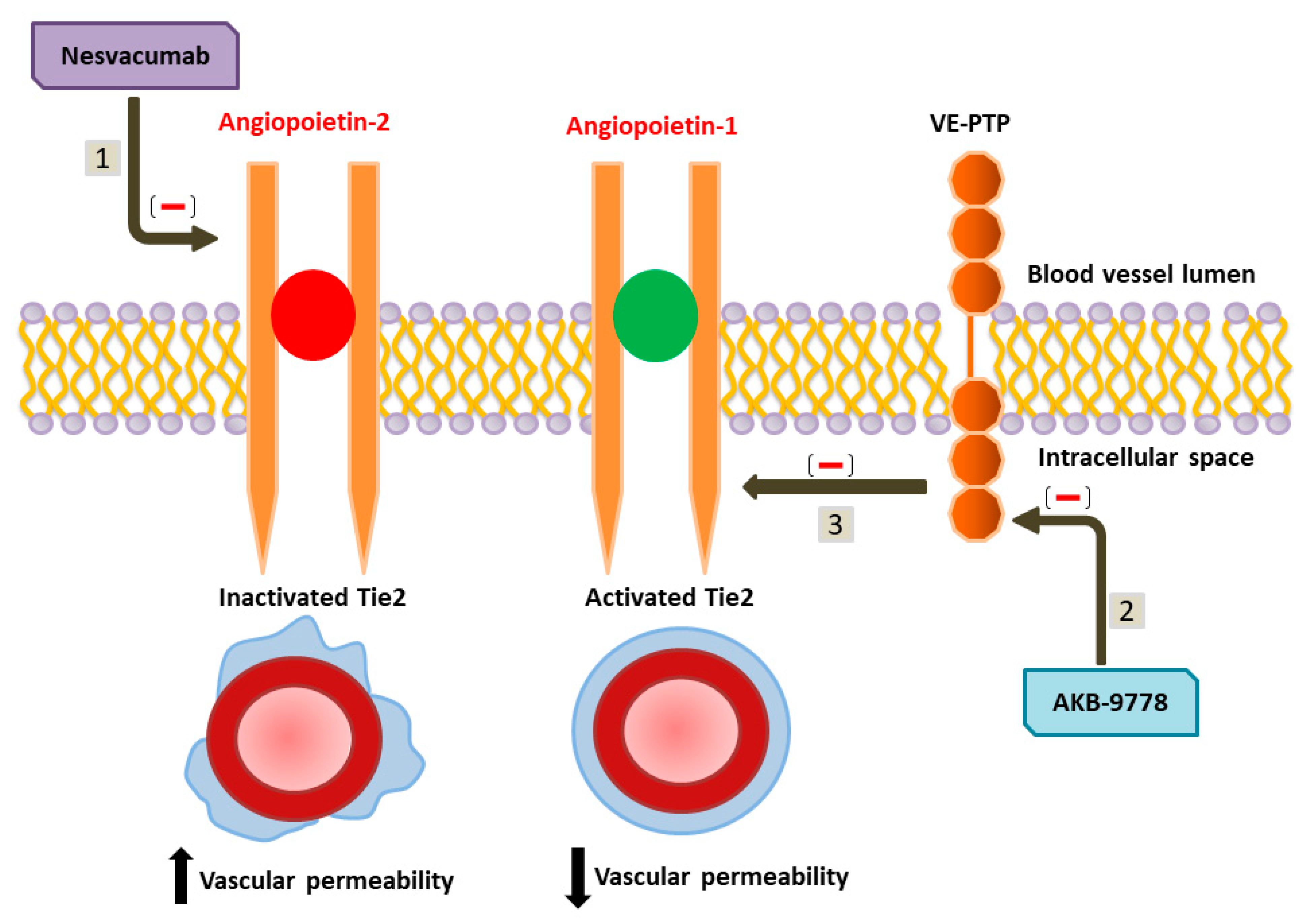
8. Pharmacological Targets and Future Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maurya, R.P. Diabetic retinopathy: My brief synopsis. Indian J. Clin. Exp. Ophthalmol. 2015, 1, 189–190. [Google Scholar]
- Song, P.; Yu, J.; Chan, K.Y.; Theodoratou, E.; Rudan, I. Prevalence, risk factors and burden of diabetic retinopathy in China: A systematic review and meta-analysis. J. Glob. Health 2018, 8, 010803. [Google Scholar] [CrossRef] [PubMed]
- Watkins, P.J. Retinopathy. Br. Med. J. 2003, 326, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Fowler, M.J. Microvascular and macrovascular complications of diabetes. Clin. Diabetes 2008, 26, 77–82. [Google Scholar] [CrossRef]
- Porta, M.; Bandello, F. Diabetic retinopathy. Diabetologia 2002, 45, 1617–1634. [Google Scholar] [CrossRef]
- Fong, D.S.; Aiello, L.; Gardner, T.W.; King, G.L.; Blankenship, G.; Cavallerano, J.D.; Ferris, F.L.; Klein, R. Retinopathy in diabetes. Diabetes Care 2004, 27, s84–s87. [Google Scholar] [CrossRef]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.P.; Simo, R.; et al. The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.; Gella, L.; Srinivasan, S.; Sharma, T. Diabetic retinopathy: An epidemic at home and around the world. Indian J. Ophthalmol. 2016, 64, 69. [Google Scholar] [CrossRef]
- Aiello, L.M. Perspectives on diabetic retinopathy. Am. J. Ophthalmol. 2003, 136, 122–135. [Google Scholar] [CrossRef]
- Wang, W.; Lo, A. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef]
- Madsen-Bouterse, S.A.; Kowluru, R.A. Oxidative stress and diabetic retinopathy: Pathophysiological mechanisms and treatment perspectives. Rev. Endocr. Metab. Disord. 2008, 9, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. J. Clin. Investig. 2017, 2, e93751. [Google Scholar] [CrossRef] [PubMed]
- Shani, M.; Eviatar, T.; Komaneshter, D.; Vinker, S. Diabetic Retinopathy –Incidence and Risk Factors in A Community Setting- A Longitudinal Study. Scand. J. Prim. Health Care 2018, 36, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.A.; Ciulla, T.A.; McGill, J.B.; Kles, K.A.; Anderson, P.W. How the diabetic eye loses vision. Endocrine 2007, 32, 107–116. [Google Scholar] [CrossRef]
- Ola, M.S.; Nawaz, M.I.; Siddiquei, M.M.; Al-Amro, S.; Abu El-Asrar, A.M. Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. J. Diabetes Complicat. 2012, 26, 56–64. [Google Scholar] [CrossRef]
- Yamagishi, S.; Matsui, T.; Inoue, H. Inhibition by advanced glycation end products (AGEs) of pigment epithelium-derived factor (PEDF) gene expression in microvascular endothelial cells. Drugs Exp. Clin. Res. 2005, 31, 227–232. [Google Scholar]
- Yamagishi, S.; Matsui, T.; Nakamura, K.; Takeuchi, M.; Imaizumi, T. Pigment epithelium-derived factor (PEDF) prevents diabetes- or advanced glycation end products (AGE)-elicited retinal leukocytosis. Microvasc. Res. 2006, 72, 86–90. [Google Scholar] [CrossRef]
- Yamagishi, S.; Matsui, T. Advanced Glycation End Products (AGEs), Oxidative Stress and Diabetic Retinopathy. Curr. Pharm. Biotechnol. 2011, 12, 362–368. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; El-Remessy, A.B.; Matragoon, S.; Zhang, W.; Patel, Y.; Khan, S.; Al Gayyer, M.M.; El-Shishtawy, M.M.; Liou, G.I. Retinal Microglial Activation and Inflammation Induced by Amadori-Glycated Albumin in a Rat Model of Diabetes. Diabetes 2011, 60, 1122–1133. [Google Scholar] [CrossRef]
- Calderon, G.; Juarez, O.; Hernandez, G.; Punzo, S.; De la Cruz, Z. Oxidative stress and diabetic retinopathy: Development and treatment. Eye 2007, 31, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, M. The Polyol Pathway as a Mechanism for Diabetic Retinopathy: Attractive, Elusive, and Resilient. Exp. Diabetes Res. 2007, 2007, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Van den Enden, M.K.; Nyengaard, J.R.; Ostrow, E.; Burgan, J.H.; Williamson, J.R. Elevated glucose levels increase retinal glycolysis and sorbitol pathway metabolism, Implications for diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1675–1685. [Google Scholar]
- Steinmetz, P.R.; Balko, C.; Gabbay, K.H. The Sorbitol Pathway and the Complications of Diabetes. N. Engl. J. Med. 1973, 288, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Szwergold, B.; Kappler, F.; Brown, T. Identification of fructose 3-phosphate in the lens of diabetic rats. Science 1990, 247, 451–454. [Google Scholar] [CrossRef]
- Gonzalez, R.G.; Miglior, S.; Von Saltza, I.; Buckley, L.; Neuringer, J.; Cheng, H.-M. 31P NMR studies of the diabetic lens. Magn. Reson. Med. 1988, 435–444. [Google Scholar] [CrossRef]
- Ido, Y.; Kilo, C.; Williamson, J.R. Cytosolic NADH/NAD+, free radicals, and vascular dysfunction in early diabetes mellitus. Diabetologia 1997, 40, S115–S117. [Google Scholar] [CrossRef]
- Drel, V.R.; Xu, W.; Zhang, J.; Kador, P.F.; Ali, T.K.; Shin, J.; Julius, U.; Slusher, B.; El-Remessy, A.B.; Obrosova, I.G. Poly (ADP-Ribose) Polymerase Inhibition Counteracts Cataract Formation and Early Retinal Changes in Streptozotocin-Diabetic Rats. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1778. [Google Scholar] [CrossRef]
- Engerman, R.L.; Kern, T.S. Hyperglycemia as a cause of diabetic retinopathy. Metabolism 1986, 35, 20–23. [Google Scholar] [CrossRef]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A.W. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef]
- Reiter, C. Functions of insulin and insulin receptor signaling in retina: Possible implications for diabetic retinopathy. Prog. Retin. Eye Res. 2003, 22, 545–562. [Google Scholar] [CrossRef]
- Ferguson, T.A.; Griffith, T.S. A vision of cell death: Insights into immune privilege. Immunol. Rev. 1997, 156, 167–184. [Google Scholar] [CrossRef]
- James, C.R.; Cotlier, E. Fate of insulin in the retina: An autoradiographic study. Br. J. Ophthalmol. 1983, 67, 80–88. [Google Scholar] [CrossRef]
- Folli, F.; Bonfanti, L.; Renard, E.; Kahn, C.; Merighi, A. Insulin receptor substrate-1 (IRS-1) distribution in the rat central nervous system. J. Neurosci. 1994, 14, 6412–6422. [Google Scholar] [CrossRef]
- Baskin, D.G.; Sipols, A.J.; Schwartz, M.W.; White, M.F. Immunocytochemical detection of insulin receptor substrate-1 (IRS-1) in rat brain: Colocalization with phosphotyrosine. Regul. Pept. 1993, 48, 257–266. [Google Scholar] [CrossRef]
- Zetterstrom, C.; Benjamin, A.; Rosenzweig, S.A. Differential Expression of Retinal Insulin Receptors in STZ-Induced Diabetic Rats. Diabetes 1992, 41, 818–825. [Google Scholar] [CrossRef]
- Gardner, T.W.; Antonetti, D.A.; Barber, A.J.; Lanoue, K.F.; Nakamura, M. New insights into the pathophysiology of diabetic retinopathy: Potential cell-specific therapeutic targets. Diabetes Technol. Ther. 2000, 2, 601–608. [Google Scholar] [CrossRef]
- Lechner, J.; O’Leary, O.E.; Stitt, A.W. The pathology associated with diabetic retinopathy. Vis. Res. 2017, 139, 7–14. [Google Scholar] [CrossRef]
- Ciulla, T.A.; Amador, A.G.; Zinman, B. Diabetic retinopathy and diabetic macular edema: Pathophysiology, screening, and novel therapies. Diabetes Care 2003, 26, 2653–2664. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33, S62–S69. [Google Scholar] [CrossRef]
- Jonas, J.B.; Sabanayagam, C. Epidemiology and Risk Factors for Diabetic Retinopathy. Front. Diabetes 2019, 27, 20–37. [Google Scholar] [CrossRef]
- Chua, J.; Lim, C.X.Y.; Wong, T.Y.; Sabanayagam, C. Diabetic retinopathy in the Asia-Pacific. Asia-Pac. J. Ophthalmol. 2018, 7, 3–16. [Google Scholar] [CrossRef]
- Ding, J.; Wong, T.Y. Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr. Diabetes Rep. 2012, 12, 346–354. [Google Scholar] [CrossRef]
- Klein, R. The epidemiology of diabetic retinopathy: Findings from the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Int. Ophthalmol. Clin. 1987, 27, 230–238. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.; Moss, S.E.; Davis, M.D.; DeMets, D.L. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Achieves Ophthalmol. 1984, 102, 520–526. [Google Scholar] [CrossRef]
- Sabanayagam, C.; Banu, R.; Chee, M.L.; Lee, R.; Wang, Y.X.; Tan, G.; Jonas, J.B.; Lamoureux, E.L.; Cheng, C.Y.; Klein, B.E.; et al. Incidence and progression of diabetic retinopathy: A systematic review. Lancet Diabetes Endocrinol. 2019, 7, 140–149. [Google Scholar] [CrossRef]
- Zhang, X.; Saaddine, J.B.; Chou, C.F.; Cotch, M.F.; Cheng, Y.J.; Geiss, L.S.; Gregg, E.W.; Albright, A.L.; Klein, B.E.; Klein, R. Prevalence of diabetic retinopathy in the United States, 2005–2008. J. Am. Med. Assoc. 2010, 304, 649–656. [Google Scholar] [CrossRef]
- Dwyer, M.S.; Melton, L.J.; Ballard, D.J.; Palumbo, P.J.; Trautmann, J.C.; Chu, C.P. Incidence of diabetic retinopathy and blindness: A population-based study in Rochester, Minnesota. Diabetes Care 1985, 8, 316–322. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.; Moss, S.E. Epidemiology of proliferative diabetic retinopathy. Diabetes Care 1992, 15, 1875–1891. [Google Scholar] [CrossRef]
- Sivaprasad, S.; Gupta, B.; Crosby-Nwaobi, R.; Evans, J. Prevalence of diabetic retinopathy in various ethnic groups: A worldwide perspective. Surv. Ophthalmol. 2012, 57, 347–370. [Google Scholar] [CrossRef]
- Sabanayagam, C.; Yip, W.; Ting, D.S.; Tan, G.; Wong, T.Y. Ten emerging trends in the epidemiology of diabetic retinopathy. Ophthalmic Epidemiol. 2016, 23, 209–222. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Tao, L.; Lv, H.; Jiang, X.; Zhang, M.; Li, X. Risk factors of diabetic retinopathy and sight-threatening diabetic retinopathy: A cross-sectional study of 13,473 patients with type 2 diabetes mellitus in mainland China. Br. Med. J. 2017, 7, e016280. [Google Scholar] [CrossRef]
- Matuszewski, W.; Stefanowicz-Rutkowska, M.M.; Szychlińska, M.; Bandurska-Stankiewicz, E. Differences in Risk Factors for Diabetic Retinopathy in Type 1 and Type 2 Diabetes Mellitus Patients in North-East Poland. Medicine 2020, 56, 177. [Google Scholar] [CrossRef]
- Vujosevic, S.; Aldington, S.J.; Silva, P.; Hernández, C.; Scanlon, P.; Peto, T.; Simó, R. Screening for diabetic retinopathy: New perspectives and challenges. Lancet Diabetes Endocrinol. 2020, 8, 337–347. [Google Scholar] [CrossRef]
- McNair, P.; Christiansen, C.; Madsbad, S.; Lauritzen, E.; Faber, O.; Binder, C.; Transbøl, I. Hypomagnesemia, a Risk Factor in Diabetic Retinopathy. Diabetes 1978, 27, 1075–1077. [Google Scholar] [CrossRef]
- Rodríguez, M.L.; Pérez, S.; Mena-Mollá, S.; Desco, M.C.; Ortega, Á.L. Oxidative Stress and Microvascular Alterations in Diabetic Retinopathy: Future Therapies. Oxidative Med. Cell. Longev. 2019, 2019, 1–18. [Google Scholar] [CrossRef]
- Young, R.J.; McCulloch, D.K.; Prescott, R.J.; Clarke, B.F. Alcohol: Another risk factor for diabetic retinopathy. Br. Med. J. 1984, 288, 1035–1037. [Google Scholar] [CrossRef]
- Katušić, D.; Tomić, M.; Jukić, T.; Kordić, R.; Šikić, J.; Vukojević, N.; Šarić, B. Obesity—A risk factor for diabetic retinopathy in type 2 diabetes. Coll. Antropol. 2005, 29, 47–50. [Google Scholar]
- Yau, J.W.Y.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global Prevalence and Major Risk Factors of Diabetic Retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef]
- Cho, H.; Sobrin, L. Genetics of diabetic retinopathy. Curr. Diabetes Rep. 2014, 14, 515. [Google Scholar] [CrossRef]
- Leslie, R.D.; Pyke, D.A. Diabetic retinopathy in identical twins. Diabetes 1982, 31, 19–21. [Google Scholar] [CrossRef]
- Wong, T.Y.; Klein, R.; Islam, F.A.; Cotch, M.F.; Folsom, A.R.; Klein, B.E.; Sharrett, A.R.; Shea, S. Multi-Ethnic Study of Atherosclerosis (MESA. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am. J. Ophthalmol. 2006, 141, 446–455. [Google Scholar] [CrossRef]
- Liew, G.; Klein, R.; Wong, T.Y. The role of genetics in susceptibility to diabetic retinopathy. Int. Ophthalmol. Clin. 2009, 49, 35. [Google Scholar] [CrossRef]
- Simó-Servat, O.; Hernández, C.; Simo, R. Genetics in diabetic retinopathy: Current concepts and new insights. Curr. Genom. 2013, 14, 289–299. [Google Scholar] [CrossRef]
- Dirani., M.; Crowston, J.G.; Wijngaarden, P. Physical inactivity as a risk factor for diabetic retinopathy. A review. Clin. Exp. Ophthalmol. 2014, 42, 574–581. [Google Scholar] [CrossRef]
- Sharma, T.; Rani, P.; Raman, R.; Chandrakantan, A.; Pal, S.; Perumal, G. Risk factors for diabetic retinopathy in self-reported rural population with diabetes. J. Postgrad. Med. 2009, 55, 92. [Google Scholar] [CrossRef]
- Dascalu, A.M.; Serban, D.; Papanas, N.; Kempler, P.; Rizzo, M.; Stana, D.; Roman, G.; Pantea-Stoian, A. Type 2 Diabetes—From Pathophysiology to Cyber Systems; IntechOpen: London, UK, 2021; Chapter 10; p. 249. [Google Scholar] [CrossRef]
- Fong, D.S.; Aiello, L.P.; Ferris, F.L.; Klein, R. Diabetic Retinopathy. Diabetes Care 2004, 27, 2540–2553. [Google Scholar] [CrossRef]
- Song, S.J.; Wong, T.Y. Current Concepts in Diabetic Retinopathy. Diabetes Metab. J. 2014, 38, 416. [Google Scholar] [CrossRef][Green Version]
- Whitehead, M.; Wickremasinghe, S.; Osborne, A.; Van Wijngaarden, P.; Martin, K.R. Diabetic retinopathy: A complex pathophysiology requiring novel therapeutic strategies. Expert Opin. Biol. Ther. 2018, 18, 1257–1270. [Google Scholar] [CrossRef]
- Mustafi, D.; Saraf, S.S.; Shang, Q.; Olmos de Koo, L.C. New developments in angiography for the diagnosis and management of diabetic retinopathy. Diabetes Res. Clin. Pract. 2020, 167, 108361. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Garcia-Arumi, J.; Bandello, F.; Berg, K.; Chakravarthy, U.; Gerendas, B.S.; Jonas, J.; Larsen, M.; Tadayoni, R.; Loewenstein, A. Guidelines for the Management of Diabetic Macular Edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica 2017, 237, 185–222. [Google Scholar] [CrossRef]
- Ellis, D.; Burgess, P.I.; Kayange, P. Management of Diabetic Retinopathy. Malawi Med. J. 2013, 25, 116–120. [Google Scholar]
- Zarbin, M.A.; Smiddy, W.E. Diabetic Retinopathy Management. Surg. Retin. 2012, 2, 1–34. [Google Scholar] [CrossRef]
- El Rami, H.; Barham, R.; Sun, J.K.; Silva, P.S. Evidence-Based Treatment of Diabetic Retinopathy. Semin. Ophthalmol. 2016, 32, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Honasoge, A.; Nudleman, E.; Smith, M.; Rajagopal, R. Emerging Insights and Interventions for Diabetic Retinopathy. Curr. Diabetes Rep. 2019, 19, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, Q.; Gillies, M.C.; Wong, T.Y. Management of Diabetic Retinopathy. J. Am. Med. Assoc. 2007, 298, 902. [Google Scholar] [CrossRef]
- Deissler, H.L.; Lang, G.E. The Protein Kinase C Inhibitor: Ruboxistaurin. Dev. Ophthalmol. 2016, 55, 295–301. [Google Scholar] [CrossRef]
- Wong, T.Y.; Sun, J.; Kawasaki, R.; Ruamviboonsuk, P.; Gupta, N.; Lansingh, V.; Maia, M.; Mathenge, W.; Moreker, S.; Muqit, M.M.K.; et al. Guidelines on Diabetic Eye Care The International Council of Ophthalmology Recommendations for Screening, Follow-up, Referral, and Treatment Based on Resource Settings. Ophthalmology 2018, 125, 1608–1622. [Google Scholar] [CrossRef]
- Lang, G.E. Pharmacological Treatment of Diabetic Retinopathy. Ophthalmologica 2007, 221, 112–117. [Google Scholar] [CrossRef]
- Dulull, N.; Kwa, F.; Osman, N.; Rai, U.; Shaikh, B.; Thrimawithana, T.R. Recent advances in the management of diabetic retinopathy. Drug Discov. Today 2019, 24, 1499–1509. [Google Scholar] [CrossRef]
- Waisbourd, M.; Goldstein, M.; Loewenstein, A. Treatment of diabetic retinopathy with anti-VEGF drugs. Acta Ophthalmol. 2010, 89, 203–207. [Google Scholar] [CrossRef]
- Neubauer, A.S.; Ulbig, M.W. Laser Treatment in Diabetic Retinopathy. Ophthalmologica 2007, 221, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.P.; Lent-Schochet, D.; Lo, T.; Yiu, G. Emerging Concepts in the Treatment of Diabetic Retinopathy. Curr. Diabetes Rep. 2019, 19, 137. [Google Scholar] [CrossRef] [PubMed]
- DeLacruz, J.P.G.; Correa, J.A.G. Pharmacological approach to diabetic retinopathy. Diabetes Metab. Res. Rev. 2004, 20, 91r113. [Google Scholar]
- Leal, E.; Santiago, A.; Ambrosio, A. Old and New Drug Targets in Diabetic Retinopathy: From Biochemical Changes to Inflammation and Neurodegeneration. Curr. Drug Target-CNS Neurol. Disord. 2005, 4, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Barot, M.; Gokulgandhi, M.R.; Patel, S.; Mitra, A.K. Microvascular complications and diabetic retinopathy: Recent advances and future implications. Future Med. Chem. 2013, 5, 301–314. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansari, P.; Tabasumma, N.; Snigdha, N.N.; Siam, N.H.; Panduru, R.V.N.R.S.; Azam, S.; Hannan, J.M.A.; Abdel-Wahab, Y.H.A. Diabetic Retinopathy: An Overview on Mechanisms, Pathophysiology and Pharmacotherapy. Diabetology 2022, 3, 159-175. https://doi.org/10.3390/diabetology3010011
Ansari P, Tabasumma N, Snigdha NN, Siam NH, Panduru RVNRS, Azam S, Hannan JMA, Abdel-Wahab YHA. Diabetic Retinopathy: An Overview on Mechanisms, Pathophysiology and Pharmacotherapy. Diabetology. 2022; 3(1):159-175. https://doi.org/10.3390/diabetology3010011
Chicago/Turabian StyleAnsari, Prawej, Noushin Tabasumma, Nayla Nuren Snigdha, Nawfal Hasan Siam, Rachana V. N. R. S. Panduru, Shofiul Azam, J. M. A. Hannan, and Yasser H. A. Abdel-Wahab. 2022. "Diabetic Retinopathy: An Overview on Mechanisms, Pathophysiology and Pharmacotherapy" Diabetology 3, no. 1: 159-175. https://doi.org/10.3390/diabetology3010011
APA StyleAnsari, P., Tabasumma, N., Snigdha, N. N., Siam, N. H., Panduru, R. V. N. R. S., Azam, S., Hannan, J. M. A., & Abdel-Wahab, Y. H. A. (2022). Diabetic Retinopathy: An Overview on Mechanisms, Pathophysiology and Pharmacotherapy. Diabetology, 3(1), 159-175. https://doi.org/10.3390/diabetology3010011










